INTRODUCTION
Viruses are intracellular obligate pathogens that uses its host machinery and metabolism to multiply and assemble to manifest an infection. Viral infections have posed a major threat worldwide. The number of people affected with Hepatitis C virus is fourfold greater than the ones affected by human immunodeficiency virus (HIV). 67% of population is estimated to have HSV-1 infection. 32 million deaths from HIV, sickness of 50–100 million per year from dengue and severe acute respiratory syndrome infection in more than 8,000 people along with 770 deaths in 2 years are estimated (Bryan-Marrugo et al., 2015; De Clercq and Li, 2016). The dawn of globalization has highlighted the need for potent drugs to combat such infections and maintain a healthy population. Every virus has its own distinct characters, so are the diseases. Hence, there are several categories of antiviral agents used to fight the virus which are classified into: A. general antiviral drugs which are sub-categorized based on their mechanism of action into nucleoside analogs, nucleotide analogs, antisense drugs, pyrimidines, interferon alpha and combinations, tropical immuno modulators, ion channel function inhibitors, and inhibitors of Mg2+ proton, Neuraminidase inhibitors. B. antiretroviral means that comprise nucleoside analogues with reverse transcriptase activity, the non-nucleoside reverse transcriptase inhibitors, protease inhibitors, and miscellaneous agents like maraviroc which constrains binding to T-cell receptor, fusion inhibitor, and integrase inhibitors that block the integrase enzyme (Mohammadi Pour et al., 2019).
However, such effective drugs come along with certain side effects. Studies have shown that repeated use and higher dosages of these drugs could potentially alter intra-cellular nucleoside pool which affects the erythropoiesis. Few other studies also observe that these drugs influence T-cell activity and exert their impact on dividing cells (Heagy et al., 1991). Therefore, a lot of patients prefer to consume herbal supplements that perhaps aid in enhancing innate response of the host to infections and ameliorate the side effects (Surendran et al., 2017; Wang et al., 2018; Yan et al., 2019). Herbs like elderberries (Sambucus nigra L.), ginger (Zingiber officinale), neem (Azadirachta indica) and others have antiviral effects (viricidal) or help in reinforcing the immune system of the host (Arora et al., 2011; Krawitz et al., 2011; Tiwari et al., 2010). The host defence mechanism includes chemical messengers like cytokines and tumour necrosis factor alpha (TNF-α) which regulate the innate and adaptive immune systems and have pleiotropic functions, majorly inhibiting the viral replication (Bryan-Marrugo et al., 2015). Immusante is one such formulation comprising Symplocos recemosa and Prosopis glandulosa extracts which helps in augmenting expressions of cytokines and increases the productions of TNF-α and nitric oxide due to their rich phytopharmaceutical constituents like oleanolic acid, betulic acid, β-sitosterol, mesquitol, quercetin, and apigenin (Firashathulla et al., 2016). A higher apprehension of probable pharmacodynamic or pharmacokinetic interactions must be noted to diminish the potentially dangerous side effects and/ or reduced benefits from the medications. Here we have shown the beneficial effect of the Immusante as an adjuvant. This is a validated liquid chromatography tandem mass spectrometry (LC-MS/MS) based evidence to prove that the pharmacokinetics of analogue of nucleoside, antiviral drug acyclovir which inhibits the activity of polymerase viral DNA and also replication of DNA of several herpesvirus, lamivudine a nucleoside reverse transcriptase inhibitor (NRTI) with an action against HIV-Type 1 and HBV to interrupt viral DNA production and Amantadine- a drug that interferes with a viral protein, M2 (1) and the herbal supplement Immusante are mutually exclusive (King, 1988; O’Brien and Campoli-Richards, 1989; Van Leeuwen et al., 1992). A selective multiple reaction monitor (MRM) in ESI-positive ion mode was used for quantitative bioanalysis of acyclovir (m/z 226.10 > 151.90), amantadine (m/z 152.00 > 134.90) and lamivudine (m/z 230.00 > 111.90). The separation was achieved through Phenomenex Kinetex Polar C18 column.
MATERIALS AND METHODS
Chemical and reagents
Reference standards of Acyclovir (ACV) (98%), Amantadine (AMT) (98%) and Lamivudine (3TC) (98%) were purchased from TCI Chemicals (Tokyo, Japan). All the internal standards (ISs) ACV-D4 (isotopic purity: 97.19%), AMT-D6 (isotopic purity: 99.85%) and 3TC13C15N2 (isotopic purity: 97.69%) were procured from Clearsynth Labs Ltd. (Mumbai, India). LC-MS grade, acetic acid and other solvents like water and methanol were obtained from Thermo Fisher (Hanover Park, IL). Amantrel (Amantadine-100 mg), Lamivir (Lamivudine-150 mg), and Zovirax (Acyclovir-400 mg) manufactured by Cipla Ltd. and GSK Pharmaceuticals Ltd., respectively were purchased from a local medical store of Bengaluru.
Experimental animals
The experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC) of Himalaya Wellness Company, Bengaluru, (Protocol no. 165/16) and the animals received humane care as per the guidelines prescribed by the Committee for Control and Supervision on Experiments on Animals (CPCSEA).
Instrumentation and LC-MS/MS conditions
A mass spectrophotometer, API 2,000 from Applied Biosystems/MDS SCIEX, (AB Sciex, Foster, CA), which is united with Electron Spray Ionization, ESI source and a chromatographic organization. Batch attainment and handling of data were processed by Analyst 1.6.3 software. Analytes detections were accomplished in positive as well as negative ionization modes, however, positive mode was found to give relatively higher signal response and hence the same mode was chosen for further analysis and validation. The final MS/MS optimized parameters values are as follows: 20 V of declustering potential, 400 V of focusing potential, 10 V of entrance potential, 30 psi curtain gas, 420ºC source temperature, 50 and 60 psi source gas, ion spray voltage 5,500 V and collision energy was optimized to the respective MRM transition state. The separation was attained via Phenomenex Kinetex Polar C18 (50 × 4.6 mm, 2.6 μm) column. With an aid of column oven, Shimadzu CTO-10 AS VP, the column temperature was maintained to be 40ºC. The mobile phase was delivered at a set flow rate of 1.0 ml/minute through the Shimadzu LC-20 AD series binary gradient pump with Shimadzu DGU-20A3 degasser. The mobile phase composed of solvent-A which contains 100% water and solvent-B as Methanol with 0.05% acetic acid. Solvent B was linearly ramped from 20% to 40% in 2 minutes and then ramped to 60% in the next 1 minute. After 1 minute, the solvent B was further ramped to 80% and held for 1 minute before decreasing back to initial concentration of 20%. It was then re-equilibrated at 20% for an additional 2 minutes. The injection volume 5 μl was injected through Shimadzu SIL-HTC autosampler and 8 minutes was the total analysis run time. Final optimized MRM parameters are summarized in Table 1.
Preparation of standard solutions, calibration standards and quality control samples
The stock solution having a known concentration of 1 mg/ml is made by the dissolution of an adequate quantity of analyte and IS which were individually dissolved in methanol and later maintained at 4ºC prior to utilization. To a tube, a small aliquot 5 μl of standard solution, to the same vial IS of 5 µl as functional solution were added to residual sample. Furthermore, freshly prepared sample was blended thoroughly by the addition of 50 µl of blank rat plasma spiked to attain calibration standard to a particular concentration scale of 0.057–5 µg/ml. The quality controls (QCs), including less (LQC), moderate (MQC), and higher (HQC) concentration range, were obtained separately by the above preparation method at 150, 2,750 and 4,400 ng/ml for ACV, AMT and 3TC, respectively.
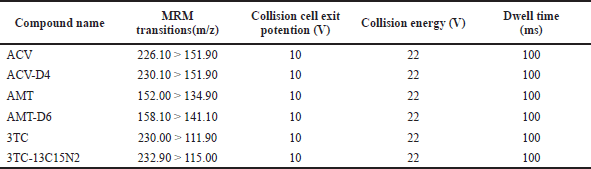 | Table 1. Optimized MRM parameters for analytes and corresponding ISs. [Click here to view] |
Sample preparation
Various samples such as rat plasma sample, calibration standard, and QC sample were prepared by following the below procedure:
Initially, about 100 µl plasma sample of rat should be mixed for 300 µl water followed by fortification with 10 µl IS cocktail (AcylovirD4, Amantadine D6 and Lamivudine 13C15N2,) and subjected to vortex for few seconds followed by treatment with 0.1 M sodium hydrogen phosphate and extracted with 0.2% ammonia solution in methanol. Finally, the obtained samples were further centrifuged for 8 minutes maintained at 5ºC at 6,000 rotations per minute (rpm), further, the supernatant was completely evaporated to dryness under nitrogen and reconstituted with water and analyzed in a single high-throughput LC-MS/MS run (Farid et al., 2022; Shalaby et al., 2022).
Bioanalytical method validation
Fundamental specifications as a new bioanalytical technique of characterization which is confirmed by a particular biological matrix was carried out in accordance with the US Food and Drug Administration (US-FDA), as well as the Guidance for the Industry in the year 2018 from Bioanalytical method validation (FDA and CDER, 2018) which specifies the parameters such as accuracy, precision, calibration curve, reproducibility, selectivity, stability and sensitivity.
Selectivity, specificity, and carryover
Selectivity was estimated by correlating blank with rat plasma belonging to six independent animals, samples spiked with ACV, AMT, and 3TC at lower limit of quantification (LLOQ) and in the rat’s plasma sample. In comparison, the analyte reaction in blank below 20% LLOQ of sample was spiked. The contamination in the samples were assessed by determining the blank samples soon after the upper limit of quantification samples.
Calibration curve
Calibration curves for ACV, AMT and 3TC were estimated for three successive days, consisting of blank and IS as zero calibrator, seven nonzero calibrators and also contains blank as without IS and analyte. Linear measure of each calibration curve was attained based on estimation of concentration dependent-correlation (i.e., peak-area ratio of analytes divided by IS verses concentration of analyte) utilizing weighted (1/×2) least squares linear regression. The nonzero calibrator must be ±15% of the theoretical concentration except for LLOQ (±20%).
Accuracy and precision
While assessing accuracy and precision in determination range that involves analyzing the effect of ACV, AMT and 3TC at LLOQ in the calibration curve, less, MQC and higher QCs particularly in five replicates per QC level in three individualistic performances. whereas precision between and within run (relative standard deviation, RSD%) should be less than 15%, excluding 20% at LLOQ. Whereas accuracy (relative error, RE) it should not deviate ±15% of theoretical concentration, excluding ±20% at LLOQ.
Extraction recovery and matrix effect
Recovery of ACV, AMT, and 3TC are determined by considering peak area between post-extracted and extracted spiked samples at less, MQC, and higher QC concentrations. Whereas matrix effect was calculated on the basis of peak area ratio between water-substituted samples at three QC levels compared with post extraction samples.
Stability
Stability study of QC samples were carried out at three replicates with lesser as well as at higher concentration levels that includes different stability which mentioned as follows, post-preparation, stock-solution, long-term, bench-top, auto-sampler as well as freeze-thaw stability. This technique is stable when accuracy (% nominal) at each level was ±15%.
Procedure for herb-drug interaction study
The interaction of antiviral drugs with Immusante® in rat plasma was analyzed by a validated LC-MS/MS method. Twelve Wistar rats (280 ± 20 g) of 24 weeks were distributed into two groups of six animals each. Group-I rats received cocktail of anti-viral drugs which include Acyclovir (80 mg/kg), Lamivudine (30 mg/kg) and Amantadine (20 mg/kg) through intra-gastric administration. Group-II rats received Immusante® (250 mg/kg of body weight) along with the antiviral drugs cocktail as mentioned above. The influence of Immusante® on the metabolism of antiviral drugs was evaluated by the co-administration of Immusante® with antiviral drugs for 7 days. The drugs were suspended in demineralized water and administered orally. On day 8, all the animals received respective treatments and the blood samples were collected in heparinized vials at different time points like 0, 0.25, 0.50, 1, 2, 4, 8, 12, and 24 hours. The samples collected were subjected for analysis using LC-MS/MS. The pharmacokinetic parameters like drug maximum concentration (Cmax), concentration peak time (tmax), area under the concentration-time curve (AUC), clearance (CL), half-life (t1/2), and volume of distribution (Vd) were evaluated. The drug plasma concentration was log transformed and plotted on the Y-axis and time was plotted on the X-axis.
Statistical analysis
The pharmacokinetic parameter values were expressed in terms of mean ± standard error mean. The results were statistically analyzed by Student’s t-test using Prism GraphPad version 6.07 software (GraphPad Software Inc., San Diego, CA). A p value <0.05 was considered statistically significant.
RESULTS
LC-MS/MS method development
Particularly, in positive ESI mode the [M + H] + protonated molecular ion of ACV (m/z 226.10), AMT (m/z 152.00) and 3TC (m/z 230.00) reveals prominent mass response in water as well as in 0.05% of acetic acid in methanol (Fig. 1). ACV, AMT, and 3TC produced the stable and prominent product ion of m/z 151.90, 111.90, and 134.90, sequentially. In mass spectroscopy, to improve the sensitivity and to attain good performance the parameters like manual optimization was controlled by Flow injection analysis. Particularly, in a positive ESI mode, the methanol with 0.05% acetic acid will produces better ionization efficiency compared to 100% methanol or 100% acetonitrile. Herein, acetic acid in methanol was necessary for the formation of precursor ions and peak shape of lamivudine. It is intended to study the effect of concentration as (0.01%, 0.05%, and 0.1%) of acetic acid whereas, the mobile phase consists of methanol and water having 0.05% of acetic acid below gradient elution that should produce the correct retention time and better sensitivity (Fig. 1).
Bioanalytical method validation
The retention times of ACV, AMT, and 3TC were 2.40, 2.87, and 4.90 respectively. These results exhibit that the analytical method is independent of potentially interfering substances and there is no carryover in the activity.
The calibration curves for ACV, AMT, and 3TC executed good linearity with r2 > 0.992 in the quantification range of 57–5,000 ng/ml. The corresponding calibration equations were found to be y = 0.926x + 0.00704 for ACV, y = 2.630x + 0.0199 (r2 = 0.9992) for AMT and y = 1.620x + 0.0179 for 3TC. In which, y refers ratio of peak area, x refers analyte concentration in plasma and r2 refers to correlation coefficient.
Accuracy and precision for QC samples (LQC, HQC, MQC, and for LLQC) at four different concentration levels were showed in Table 2. Where inter-batch precision (% RSD) is less than 7.47 and intra- batch precision (% RSD) is 9.28, whereas the accuracies covered in the range from 86.66% to 110.72%. By analyzing these results, we can conclude that this technique is repeatable and reliable.
The percent recovery for extraction are 100.57% ± 2.13% and 102.72% ± 1.18% for ACV, 100.60% ± 3.44% and 101.00% ± 3.22% for AMT and 103.98% ± 3.67% and 99.20% ± 2.24% for 3TC at LQC and HQC levels, respectively. The matrix effect for ACV was measured to be 107.34 ± 2.22 and 103.52 ± 2.69 for AMT it is 95.65 ± 1.18 and 96.58 ± 4.44 and for 3TC it is 95.41 ± 4.78 and 105.57 ± 3.22 at LQC and HQC levels sequentially.
As displayed in Table 3, a sequence of stability studies was carried out that consist of preparation of sample under different conditions. Initially, for 18 hours at room temperature (RT), later for 18 hours in an auto sampler, finally in three freeze-thaw at −20ºC. the results reveals that both IS and analytes displays better stability results without much loss in the concentration during the stability study.
Method application
The above analytical technique/method was used to study the herbal-drug interactions of Immusante with frequently used anti-viral drugs (ACV, AMT and 3TC). As shown in Table 4, there were no significant interactions found (as per USFDA guidelines) between Immusante® and the anti-viral drugs (Fig. 2a–c) (Mahmoud et al., 2022).
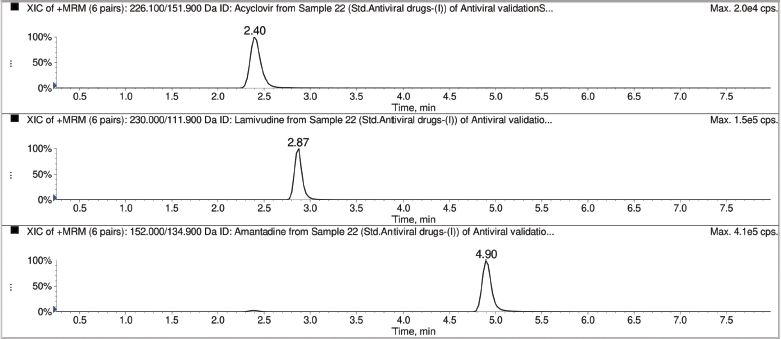 | Figure 1. Representative MRM chromatograms of acyclovir, lamivudine and amantadine. [Click here to view] |
DISCUSSION
Immusante® is a proprietary herbal formulation of Himalaya Wellness Company, Bengaluru, which is recommended as an immunotherapeutic supplement to support immune-compromised patients, especially when patients combat viral diseases to strengthen the immune function, decrease disease manifestation, and improve resistance to diseases. With the growing understanding of plant based complementary or alternative medicine, it’s beneficial to assess the herb-drug interaction in drug development process. Aiming to improve the information on herbal drug interactions, further clinical research is necessary. The interactions are either in the form of inhibition or in the form of induction of hepatic and intestinal metabolic enzymes especially in CYP enzyme family. Besides to that, a related effect on efflux proteins and drug transporters specially the p-glycoproteins in the intestines. Lamivudine metabolism is a minor pathway for the elimination. Whereas in man, trans-sulfoxide is the only known metabolite of lamivudine. In which the biotransformation is catalyzed by the sulfotransferase’s metabolite. By the secretion of active organic cation, the greater portion of lamivudine is eradicated and remains unchanged in the urine. In urine a total of 5.2% ± 1.4% of dose was ejected as trans-sulfoxide metabolite. The antiviral drug Acyclovir is <15% which oxidizes to 9-carboxymethoxymethylguanine by aldehyde and alcohol dehydrogenase. Further, aldehyde oxidase converts 1% 8-hydroxylate into 8-hydroxy-acyclovir. By the influence of viral thymidine kinase, acyclovir is metabolizing into acyclovir monophosphate furthermore, guanylate kinase converts monophosphate to diphosphate and finally other kinase such as nucleoside diphosphate, pyruvate, creatine, phosphoglycerate, phosphoenolpyruvate carboxy, and synthetase such as succinyl-CoA and adenylosuccinate converts acyclovir diphosphate to triphosphate. Amantadine metabolism and excretion: neither a substrate nor an inhibitor of CYP1A2, 3A4, 3A5, 2B6, 2C8, 2C9, 2C19, 2D6 and 2E1 based on in-vitro studies. Reported to be a poor substrate to MATE1 based on in-vitro studies, while, in-vivo amantadine clearance increased by 33% in the presence of quinidine, OCT2 inhibitor. excreted initially remains constant in the urine by tubular secretion and glomerular filtration.
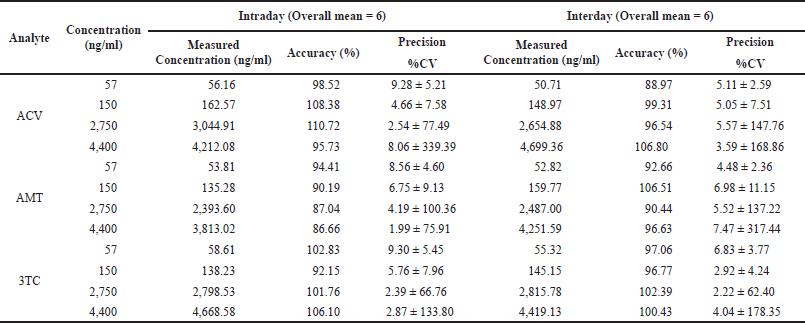 | Table 2. Intra-day and inter-day accuracy and precision for ACV, AMT and 3TC. [Click here to view] |
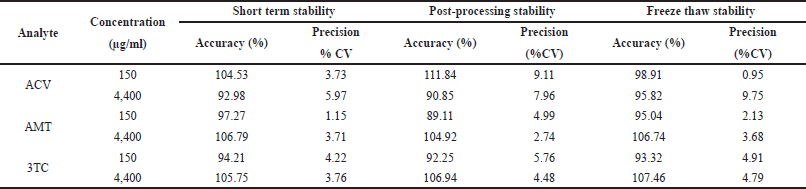 | Table 3. Stability study results of ACV, AMT and 3TC in rat plasma sample during the process of different storage conditions (n = 6). [Click here to view] |
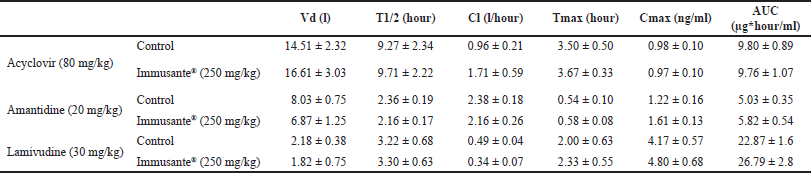 | Table 4. Effect of Immusante® on pharmacokinetic parameters of anti-viral drugs. [Click here to view] |
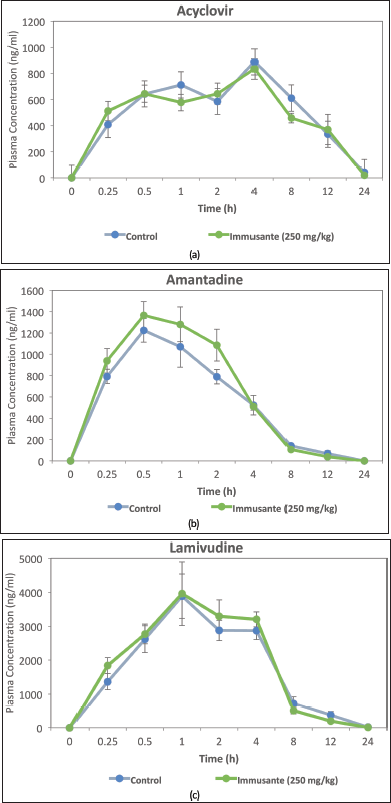 | Figure 2. Effect of Immusante® on plasma concentration of anti-viral drugs. (a) Plasma concentration of acyclovir and acylovir with immusante (250 mg/kg) at different timepoints. (b) Plasma concentration of amantadine and amantadine with immusante (250 mg/kg) at different timepoints. (c) Plasma concentration of lamivudine and lamivudine with immusante (250 mg/kg) at different timepoints. [Click here to view] |
The objective of the study was to develop a sensitive and rapid method to evaluate herb-drug interaction using LC-MS/MS and also to evaluate the interaction of Immusante® with a cocktail of anti-viral drugs using this hyphenated technique. Few interactions were documented among the drugs Acyclovir, Lamivudine and Amantadine. Acyclovir decreases the ejection rate of both Amantadine and Lamivudine that results in high serum level. When Amantadine and Lamivudine are co-administered, it increases the serum concentration of Lamivudine (Drug Bank Amantadine, 2023; Drug Bank Lamivudine, 2023; Morris, 1994). But none of these interactions were found in the current study.
CONCLUSION
This robust and validated LC-MS/MS technique is used for the simultaneous determination of the amount of ACV, AMT, and 3TC (cocktail of anti-viral drugs) present in rat plasma and to determine its interaction with Immusante®. This technique can be a useful tool for evaluating herb-drug interactions in short span of time. The study also showed that Immusante® does not effect or alter the pharmacokinetic profile of anti-viral drugs when co administered. Thus, Immusante® may be recommended as an adjuvant in various viral infections and can be co-administered with anti-viral drugs.
ACKNOWLEDGMENTS
The authors thank “Himalaya Wellness Company” for providing facility to carry out the research and Ms. Keerthi Priya for her inputs in drafting the manuscript.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research work was funded by Himalaya Wellness Company, Bengaluru, India.
CONFLICTS OF INTEREST
Some of the authors are the employees of Himalaya Wellness Company, Bengaluru. The authors declare no other conflict of interest.
ETHICAL APPROVALS
The experimental protocols were approved by the Institutional Animal Ethics Committee (IAEC) of Himalaya Wellness Company, Bengaluru, (Protocol no. 165/16) and the animals received humane care as per the guidelines prescribed by the Committee for Control and Supervision on Experiments on Animals (CPCSEA).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Arora R, Chawla R, Marwah R, Arora P, Sharma RK, Kaushik V, Goel R, Kaur A, Silambarasan M, Tripathi RP, Bhardwaj JR. Potential of complementary and alternative medicine in preventive management of novel H1N1 Flu (Swine Flu) pandemic: thwarting potential disasters in the bud. Evid Based Complement Alternat Med, 2011; 586506; doi:10.1155/2011/586506. CrossRef
Bioanalytical method validation, guidance for industry. Available via https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (Accessed 04 Noveber 2022).
Bryan-Marrugo OL, Ramos-Jiménez J, Barrera-Saldaña H, Rojas-Martínez A, Vidaltamayo R, Rivas-Estilla AM. History and progress of antiviral drugs: from acyclovir to direct-acting antiviral agents (DAAs) for Hepatitis C. Med Univ, 2015; 17(68):165–74. CrossRef
De Clercq E, Li G. Approved antiviral drugs over the past 50 years. Clin Microbiol Rev, 2016; 29(3):695–747; doi:10.1128/CMR.00102-15. CrossRef
Drug Bank Amantadine. 2023. Available via https://go.drugbank.com/drugs/DB00915
Drug Bank Lamivudine. 2023. Available via https://go.drugbank.com/drugs/DB00709
Drug Bank Online. Lamivudine. 2011. Available via https://go.drugbank.com/drugs/DB00709 (Accessed 12 January 2023).
Farid EH, Nada S. Abdelwahab, a new HPLC methodology for the analysis of metronidazole and dexibuprofen: application to pharmacokinetic study and comparative greenness assessment. Microchem J, 2022; 183:108048. CrossRef
FDA, CDER. Bioanalytical method validation. 2018. Available via https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf
Firashathulla S, Inamdar MN, Rafiq M, Viswanatha GL, Sharath Kumar LM, Babu UV, Ramakrishnan S, Paramesh R. IM-133N—a useful herbal combination for eradicating disease-triggering pathogens in mice via immunotherapeutic mechanisms. J Pharmacopuncture, 2016; 19(1):21–7; doi:10.3831/KPI.2016.19.003. CrossRef
Heagy W, Crumpacker C, Lopez PA, Finberg RW. Inhibition of immune functions by antiviral drugs. J Clin Invest, 1991; 87(6):1916–24; doi:10.1172/JCI115217. CrossRef
King DH. History, pharmacokinetics, and pharmacology of acyclovir. J Am Acad Dermatol, 1988; 18(1 Pt 2):176–9; doi:10.1016/s0190-9622(88)70022-5. CrossRef
Krawitz C, Mraheil MA, Stein M, Imirzalioglu C, Domann E, Pleschka S, Hain T. Inhibitory activity of a standardized elderberry liquid extract against clinically-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Complement Altern Med, 2011; 11:16; doi:10.1186/1472-6882-11-16. CrossRef
Mohammadi Pour P, Fakhri S, Asgary S, Farzaei MH, Echeverría J. The signaling pathways, and therapeutic targets of antiviral agents: focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front Pharmacol, 2019; 10:1207; doi:10.3389/fphar.2019.01207. CrossRef
Mahmoud ST, Marwa A. Moffid, Rawda M. Sayed, Eman A. Mostafa. Core shell stationary phase for a novel separation of some COVID-19 used drugs by UPLC-MS/MS method: study of grapefruit consumption impact on their pharmacokinetics in rats. Microchem J, 2022; 181:107769. CrossRef
Morris DJ. Adverse effects and drug interactions of clinical importance with antiviral drugs. Drug Saf, 1994; 10(4):281–291. CrossRef
O’Brien JJ, Campoli-Richards DM. Acyclovir. An updated review of its antiviral activity, pharmacokinetic properties and therapeutic efficacy. Drugs, 1989; 37(3):233–309. CrossRef
Shalaby K, Alghamdi S, Gamal M, Elhalim LMA, Tony RM. A validated LC–MS/MS method for analysis of Cabergoline in human plasma with its implementation in a bioequivalent study: Investigation of method greenness. BMC Chem, 2022; 16(1):71. CrossRef
Surendran S, Paul D, Sushmita R, Krishna L, Tiwari NK, Giri S, Satheeshkumar N. A validated LC–MS/MS method for the estimation of glimepiride and pitavastatin in rat plasma: application to drug interaction studies. J Chromatogr B Analyt Technol Biomed Life Sci, 2017; 1046:218–5. CrossRef
Tiwari V, Darmani NA, Yue BY, Shukla D. In vitro antiviral activity of neem (Azardirachta indica L.) bark extract against herpes simplex virus type-1 infection. Phytother Res, 2010; 24(8):1132–40; doi:10.1002/ptr.3085 CrossRef
Van Leeuwen R, Lange JM, Hussey EK, Donn KH, Hall ST, Harker AJ, Jonker P, Danner SA. The safety and pharmacokinetics of a reverse transcriptase inhibitor, 3TC, in patients with HIV infection: a phase I study. AIDS, 1992; 6(12):1471–5. CrossRef
Wang L, Zhao J, Zhang R, Mi L, Shen X, Zhou N, Feng T, Jing J, Liu X, Zhang S. Drug–drug interactions between PA-824 and darunavir based on pharmacokinetics in rats by LC–MS-MS. J Chromatogr Sci, 2018; 56(4):327–35. CrossRef
Yan L, Wang S, Zhao L, Qiu J, Zhou L, Wang W, Xu X, Wang D, Qiu X, Qin D. The herb-drug pharmacokinetic interaction of fluoxetine and its metabolite norfluoxetine with a traditional Chinese medicine in rats by LC-MS/MS. EvidBased Complement Alternat Med, 2019; 2471870; doi: 10.1155/2019/2471870 CrossRef