INTRODUCTION
Epilepsy is a common neurological disorder caused by imbalanced excitatory and inhibitory stimuli. In humans, it expresses itself as epileptic syndromes and convulsions, determined by genetic and ambient factors (Stewart et al., 2012).
Experimental animal models are long used to study epilepsy, showing remarkable resemblance with clinical phenotypes (Stewart et al., 2012). Rodents are classically used for convulsion assessment, either induced by electrical kindling, as proposed by Racine et al. (1972), or induced by drugs such as kainate, pilocarpine, and pentylenetetrazol (Morimoto et al., 2004). However, zebrafish (Danio rerio) have gained space in drug screening due to several favorable characteristics, including low maintenance cost and high homology with mammals. Moreover, their neurotransmitter gamma-Aminobutyric acid (GABA) ergic system is highly similar to mammals despite having a less complex encephalon (Alfaro et al., 2011).
Zebrafish is a suitable model organism for studying pentylenetetrazole- (PTZ-) induced acute seizures and has been used in several studies to research the anticonvulsant activity of potential antiepileptic drugs (AEDs) (Buenafe et al., 2013; Orellana-Paucar et al., 2012). PTZ is an antagonist of the type-A γ-aminobutyric acid receptor (GABAA), acting in the same active site of picrotoxin (Ramanjaneyulu and Ticku, 1984), resulting in decreased chloride conductance and hence glutamatergic excitation (Huang et al., 2001; Sejima et al., 1997). PTZ was already employed in some studies as a seizure inducer in adult zebrafish through the immersion method (Braida et al., 2012; Menezes and Da Silva, 2017; Mussulini et al., 2013; Pineda et al., 2011; Siebel et al., 2015, 2013; Sung-joon et al., 2017; Torres-Hernández et al., 2015). However, this kind of treatment makes it difficult to replicate data in mammals further since the correlation between dose and concentration is inaccurate. Other authors also employed PTZ intraperitoneal (ip) (Banote et al., 2013; Kundap et al., 2017; Sathaye et al., 2015).
The form of AED treatment must be considered as well. Only Banote et al. (2013) and Sathaye et al. (2015) used it orally [gabapentin and diazepam (DZP)]. However, there is a need for a complete behavioral characterization or methodology considering ip PTZ administration and oral AED administration. Following the methodology described by Mussulini et al. (2013), this study aims to perform the behavioral characterization of ip PTZ treatment and oral DZP treatment in adult zebrafish.
MATERIALS AND METHODS
Ethical aspects
All procedures described in this research were approved by the Ethics Committee on the Use of Animals (CEUA) of the Federal University of Amapá under protocol number 004/2018.
Animals
Wild-type adult zebrafish (Danio rerio) were acquired from Acqua New Aquarium and Fish Ltda., ME, Igarassu-PE, Brazil. Animals (1: 1 male: female ratio) were between 4 and 6 months old and weighed 0.6 ± 0.2 g.
The animals were previously kept in quarantine for habituation under laboratory conditions. Then, they were placed in 40 l aquariums (5 animals/l) with chemical and mechanical filtration systems, controlled temperature (26°C ± 2°C), a pH between 7.0 and 8.0, controlled nitrite levels, and absence of chlorine. Room illumination was provided by fluorescent lamps on the roof (14/10 light/dark cycle), and the animals were fed twice a day with commercial ration (Alcon Basic®, Alcon, Brazil) or brine shrimps.
All animals used in this research were naïve-type, healthy, and without signs of illnesses or parasites. They were kept under the conditions described in the National Research Council of the National Academies (2011).
Treatments and seizure behavioral characterization
For DZP treatment (Brainfarma Chemical and Pharmaceutical Industry S.A., Anápolis, GO, Brazil), the animals were weighed in 500 ml containers. Three groups (n = 10/per group) were treated orally with DZP (10 mg/kg), following a method described by dos Santos Sampaio et al. (2018); the dose was chosen according to Sathaye et al. (2015). Briefly, the animals were immobilized with a damp sponge, and the product was given using a micropipette with volumes below 2 µl to avoid regurgitation. All animals were also kept fasting for 24 hours before treatment to avoid regurgitation.
The PTZ doses to induce seizures were 125, 250, 400, and 500 mg/kg, chosen according to the studies of Banote et al. (2013), Kundap et al. (2017), Choo et al. (2018), and Sathaye et al. (2015). Following the method described by Kinkel et al. (2010), the animals were previously anesthetized by cooling in cold water (~ 8°). Then, PTZ (Sigma-Aldrich St. Louis, MO) was intraperitoneally injected (via posterior pelvic girdle into the abdominal cavity, n = 10 per group), using a saline solution as a vehicle (20 µl). The observation began when the fish were recovered from the cooling (~1 minute). The methodology described here is summarized in Figure 1.
The behavioral characterization was performed during the same period of the day (from 9 am to 5 pm) to minimize the influence of the photoperiod on animals’ metabolism. The apparatus used to perform tests was a water tank (27 × 20 × 15 cm length, height, and width; 8 l). After the treatment and recovery from the anesthesia, animals were individually placed into this tank to record seizure behavior activity for over 20 minutes. The assays were performed with 10 animals per day in a silent room.
A digital camera (Sony HD) placed 55 cm away from the tank was used to ensure a good video recording of the fishs’ swimming activity. The tank was covered with a white background to avoid animals’ reflexes on the walls and floor, providing a better background to analyze the video. Two lamps were laterally placed 30 cm away from the tank to improve the contrast between the animal and the background.
Two trained observers blindly evaluated all data, and discordances were reanalyzed. All precautions were taken to avoid bias due to stress in fish and obtain representative behavior analysis results. During the experiments, animals were carefully manipulated and dislocated over tanks, beakers, and test tanks. Moreover, all animals were manipulated and tested similarly, and the behavior profile was registered all in the same room, providing uniformity of water quality and illumination.
Epileptic seizure scoring
Firstly, animals were treated with PTZ to evaluate the experimental epileptic behavior. Following the parameters that Mussulini et al. (2013) described, the behavioral manifestations were appraised in stages and replicated in ip administration.
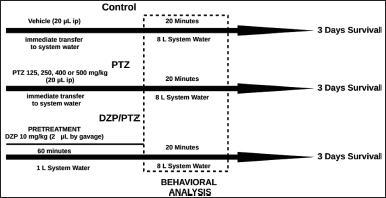 | Figure 1. Experimental protocol schematic representation. [Click here to view] |
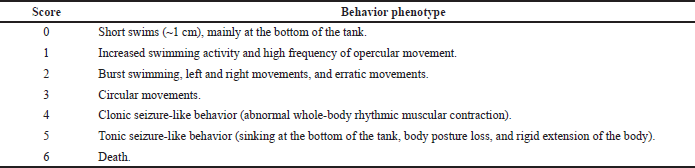 | Table 1. Acute seizure scores phenotype description. [Click here to view] |
The behavioral profile was characterized by a dose-response curve, and each observation was performed over 30 seconds. It is important to emphasize that random behavior alterations induced by PTZ, like jumping or defecating, were not considered. Each stage was attributed to a specific score described in Table 1. From the scores, we calculate the latency to seizure-related scores (4 and 5) and the severity of seizures using the area under the curve (AUC) scores.
Mortality assessment
After treatment, animals were placed in 5 l tanks without any contaminants and with water oxygenation provided by an air compressor. Half of its water was renewed daily to prevent high nitrite levels in the tank. Animals’ mortality was observed over 72 hours within fixed observation periods.
The period of mortality supervision was divided into three periods: during analysis (0–0.33 hours), immediately after analysis (1–3 hours), and after analysis (4–72 hours). During the analysis, animals were continuously observed; in the period immediately after analysis, animals were observed once every 30 minutes; in the period after analysis, animals were observed once every 12 hours. Animals were considered dead in the absence of opercular movement and the absence of response to mechanical stimulus.
Statistical analysis
The results were expressed as a mean ± SD (n = 10/group). Comparison of data among groups was performed through analysis of variance (ANOVA) and Tukey’s multiple comparison tests; p < 0.05 was considered statistically significant. The survival rate was evaluated through the logrank test.
RESULTS
The behavioral analysis shows that treatment with saline solution (vehicle) induced behavioral alterations ranging from scores 0 to 3, as shown in Table 2 and Figure 2. However, we observed that the control group did not show typical convulsion scores (equal to or higher than 4). On the other hand, animals treated with PTZ had seizures, represented by scores 4 and 5. The groups treated with the highest doses of PTZ (400 and 500 mg/kg) had higher average maximum, minimum, and starting scores compared to the group treated with the lowest PTZ dose (125 mg/kg), which had softer behavioral alterations, evidencing a dose-dependent effect (Table 3). The group treated with PTZ at 250 mg/kg had an intermediate behavior change compared to 125 mg/kg and the highest doses, and all the animals reached at least a score of 4. Animals cotreated with DZP (10 mg/kg) + PTZ (250 and 400 mg/kg) had a lower frequency of score 5 than those who received only PTZ alone at the same doses, although 100% still reached a score of 4. The average maximum and minimum and starting average also were considerably lower compared to those of PTZ alone (Tables 2 and 3; Fig. 2).
Another parameter that is generally measured in experimental seizure-like behavior is latency. Since score 4 represents a clonic-like seizure and 5 represents a tonic-like seizure, we measured the latency of these two scores (Fig. 3). It was observed that there was no statistical difference in the latency to score 4 among groups that received only PTZ; however, those who were cotreated with DZP had statistically higher latency to score 4. For score 5, it was observed that in PTZ at 125 mg/kg alone and in PTZ at 250 mg/kg + DZP 10 mg/kg, only 40% and 10% reached this score, respectively (Table 2). Hence, these groups were not counted. Unlike score 4, there were statistically significant differences in the latency for score 5 among groups treated with PTZ alone. The latency for score 5 in the group treated with PTZ at 400 mg/kg + DZP was significantly higher compared to the group that received PTZ alone at the same dose.
The analysis time was divided into three periods; then, the AUC of the scores was calculated for every animal to assess seizure severity. In the first period (0–150 seconds), animals treated with vehicle alone had the lowest AUC, as expected, and were significantly different compared to groups treated with PTZ. Furthermore, PTZ at 125 mg/kg had less seizure intensity than the other groups treated with PTZ alone, but there were no statistically significant differences among the other three doses. This pattern remained the same in the second (150–300 seconds) and third (300–1,200 seconds) periods and the entire observational period (Fig. 4). Compared to the groups that received only PTZ, the groups cotreated with DZP had less intense seizure-like behavior, but this difference was remarkably only in the group PTZ250 + DZP10.
After observing the animals’ convulsive behavior, they were monitored for survival over the next 72 hours after treatment. Deaths occurred only in the group treated with PTZ at 500 mg/kg, with two deaths totaling 20% of mortality. One death occurred in the period immediately after analysis (1 hour after treatment), and the second occurred in the period after analysis (24 hours after treatment) (Fig. 5). Since few deaths occurred, the logrank test could not identify differences.
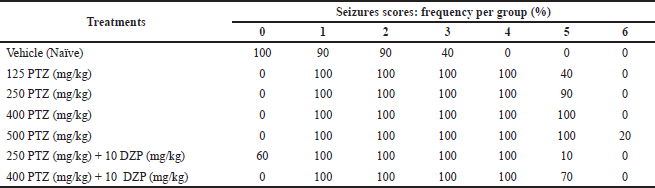 | Table 2. Seizure scores frequency (%) per group. [Click here to view] |
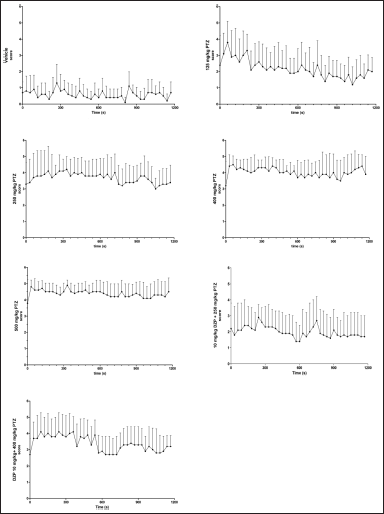 | Figure 2. Behavioral profile of PTZ-induced seizures in adult zebrafish (mean of the highest scores reached in each interval ± SD). [Click here to view] |
DISCUSSION
Several studies have shown the suitability of zebrafish in the research of epileptic seizure behavior. The first to use zebrafish as a model for this purpose was Baraban et al. (2005), who demonstrated that PTZ could induce seizures in zebrafish larvae. Subsequently, several authors used PTZ-induced seizures in zebrafish to study seizures and evaluate potential AEDs. More recently, adult zebrafish were used to test for the first time the anticonvulsive potential of perillyl alcohol, an anticancer drug (Matias Pereira et al., 2021).
Currently, researchers use this model in adult zebrafish to assess several parameters, such as the electrophysiology of immobilized animals (Banote et al., 2013; Menezes and Da Silva, 2017; Pineda et al., 2011; Sung-joon et al., 2017), c-fos expression in the central nervous system (Stewart et al., 2012), behavioral characterization (Banote et al., 2013; Choo et al., 2018; Mussulini et al., 2013; Siebel et al., 2015, 2013; Torres-Hernández et al., 2015), and AED evaluation (Braida et al., 2012; Choo et al., 2018; Matias Pereira et al., 2021; Siebel et al., 2015, 2013; Torres-Hernández et al., 2015).
Besides behavior, the cellular responses to seizures in zebrafish brains are similar to those in mammals (Duy et al., 2017). Therefore, despite having a less complex encephalon than rodents, the zebrafish brain shares numerous structural similarities with mammals, a blood-brain barrier system and a highly similar GABAergic system (Alfaro et al., 2011), corroborating its suitability as a model to investigate epileptic seizures.
A convenient method to administer PTZ to zebrafish is through immersion, where the compound is diluted in the tank’s water and absorbed through the gills, skin, and gastrointestinal system (Sukardi et al., 2011). However, as a drawback, this method makes it difficult to know the quantity of the absorbed drug, hindering the extrapolation of dose data to higher mammals. This can be bypassed by using methods of defined dose administration based on body mass. Therefore, in this study, we described the behavioral characterization of ip PTZ-induced epileptic seizures in adult zebrafish, which have a more meaningful translational value. While PTZ was administered intraperitoneally, the cotreatment with DZP was administered orally; the scores were classified based on the study by Mussulini et al. (2013).
The behavioral analysis showed that the control group treated with saline solution had spontaneous swimming activity at the tank’s bottom, indicating a score of 0. However, this control group also had scores 1–3; this can be explained by the stress induced by the intraperitoneal treatment with saline solution, which can cause bleeding in 3.9% of animals, according to Kinkel et al. (2010). This was also observed by Alfaro et al. (2011), who evaluated kainate administration in adult zebrafish and reported that phosphate-buffered saline treatment induced behavioral alterations such as immobility, hyperventilation, and whirlpool-like swimming. Overall, intraperitoneal injection induces stress in treated animals but not convulsive behavior, allowing for the differentiation between stress and seizure-like behavior.
All animals treated with PTZ reached a score of 4, and the frequency of animals that reached a score of 5 varied among the groups. This is different from the results of Jain et al. (2011), considering they reported that 0% of animals had clonic seizures when cotreated with PTZ at 225 mg/kg + DZP at 10 mg/kg. This is probably due to the different administration modes since these authors used intraperitoneal administration of DZP while we used it orally. However, it is important to notice that ip administration of DZP can be inadequate since it can induce inflammation due to the incompatibility of this drug with animals’ abdominal tissue. The administration of solid materials, such as starch glove powder, is classically known to induce inflammation and can cause granuloma formation near the injection site (Walker, 1978). Hence, oral treatment of DZP is probably the best way of administration when cotreated with PTZ.
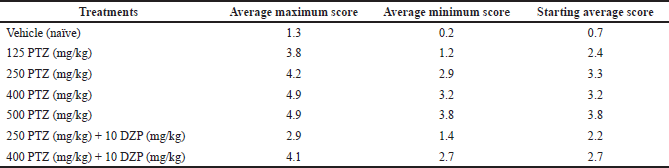 | Table 3. Average maximum score, average minimum score, and starting average score (0–30 seconds) in each group. [Click here to view] |
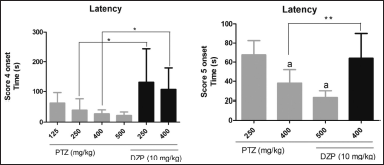 | Figure 3. Latency to score 4 onsets (clonic seizure) and latency to score 5 onsets (tonic seizure). Data are represented as mean ± SD and analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. The letter “a” indicates no statistical difference between the groups treated with PTZ (gray bars); PTZ at 250 mg/kg differed from the other groups treated only with PTZ (p < 0.05) in the latency to score 5. *p < 0.05 and **p < 0.01 indicate a significant difference between groups treated or not with DZP at the same dose of PTZ. The 125 mg/kg PTZ and 10 mg/kg DZP + 250 mg/kg PTZ groups are not represented since they did not reach a score of 5. [Click here to view] |
The sequence of epileptic seizures’ behavioral alterations was like the one induced by ip PTZ treatment, as reported by Banote et al. (2013). These authors started the analysis after 1 minute of PTZ treatment; then, they reported an initial average score of 4.2, which is similar to the initial average score of the group treated with PTZ at 250 mg/kg in our study (3.2). However, our results differ from those of Mussulini et al. (2013), who administered PTZ through immersion. For example, in our study, the treatment with PTZ at 500 mg/kg (ip) induced an initial average score of 3.8, while the group treated with PTZ at 15 mM (immersion) had an initial average score of 1. The data evidence a faster metabolization of PTZ through intraperitoneal injection compared to immersion since that prior treatment is independent of continuous absorption through gill respiration.
In rodent models, the latency for clonic and tonic seizure behavior is classically evaluated by screening novel anticonvulsant drugs. In zebrafish PTZ-induced seizures, this is possible as well. The evaluation of more than one parameter makes for a more accurate and reliable investigation; hence, the latency period was also assessed. The results show that all animals treated with PTZ had similar latency to score 4. On the other hand, the latency to score 5 tended to decrease as the dose of PTZ increased. Interestingly, DZP significantly increased the latency to scores 4 and 5.
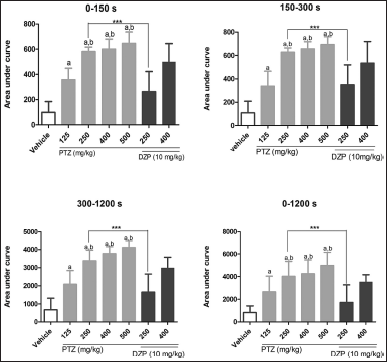 | Figure 4. Seizure intensity during different periods (0–150, 150–300, 300–1,200, 0–1,200 seconds) evaluated by the AUC observed for each treatment. Data are represented as mean ± SD and analyzed by one-way ANOVA followed by Tukey’s multiple comparison test. The letter “a” indicates a statistical difference among the groups treated with PTZ (gray bars) compared to the vehicle group (p < 0.5). The letter “b” indicates groups with no statistical difference among themselves in PTZ-treated groups (p > 0.05). DZP + PTZ is represented by black bars. ***p < 0.001 indicates a significant difference between groups treated or not with DZP with the same dose of PTZ. [Click here to view] |
Choo et al. (2018) and Kundap et al. (2017) evaluated the latency to score 4 after treatment with PTZ at 170 mg/kg. However, these authors classified circular movements as a score 4; here, this behavior is classified as a score of 3, making it impossible to compare the results. Sathaye et al. (2015) carried out treatment with PTZ at 225 mg/kg and assessed the latency to clonic-like convulsion, reporting a result of 65 seconds, which is similar to the latency to score 4 in the group treated with PTZ at 250 mg/kg.
 | Figure 5. Survival evaluation. Kaplan–Meier plot representing the animal index (%) that survived in 3 distinct periods: during exposure (0.00–0.33 hours); immediately after the treatment (0.33–3 hours); after the treatment. [Click here to view] |
The latency to score 5 (tonic seizure) was not possible to calculate in some groups, such as the group treated with PTZ at 250 mg/kg and the group cotreated with PTZ at 250 mg/kg + DZP at 10 mg/kg due to the low number of animals who showed this score. The groups treated with PTZ at 400 and 500 mg/kg had a similar latency to score 5, while that treated with PTZ at 250 mg/kg had a longer latency period. These results are similar to those of Sathaye et al. (2015) since the latency period to tonic seizures in the group treated with PTZ at 225 mg/kg was 70 seconds, and in our study, the latency to score 5 was 67 seconds in the group treated with PTZ at 250 mg/kg. It is important to notice the significant difference in the latency to score 5 between the group cotreated with DZP at 10 mg/kg + PTZ at 400 mg/kg and the group treated with PTZ alone (400 mg/kg). It is possible to infer that this PTZ dose induces higher stimuli even though the seizure intensity is like the one induced by 250 mg/kg of PTZ.
The increased latency to convulsive-like behavior caused by DZP has been reported previously. Mussulini et al. (2013) reported increased latency in fish treated with PTZ and DZP through immersion (10 + 10 mM) compared to fish receiving only PTZ. Sathaye et al. (2015) reported that 0% of animals had tonic seizures in the cotreatment with DZP + PTZ (10 + 225 mg/kg), while here 10% of animals had tonic seizures in the cotreatment with DZP + PTZ (10 + 250 mg/kg).
After assessing the latency of seizures, their intensity was appraised through the AUC. In this study, doses of PTZ higher than 150 mg/kg could not induce statistically different behavioral score alterations during all the periods. When compared with data reported by Mussulini et al. (2013), who tested PTZ through immersion at crescent concentrations (5–15 mM) in different periods (0–150, 150–300, and 300–1,200 seconds), the present study had higher seizure intensity. Overall, the seizure scores appraised through AUC indicate that PTZ at 250 mg/kg is the most appropriate dose to evaluate them. Also, at this dose, it is possible to observe a drastic decrease in convulsive-like behavior with DZP treatment. The effect of DZP in PTZ at 400 mg/kg is lower and not statistically different, evidencing that only 10 mg of DZP is insufficient to attenuate seizure intensity induced by this dose. A higher DZP dose would be necessary to do it but would compromise the survival rate of fish.
The survival rate of animals was observed in the cotreatments, and no animal died in the observation periods (Fig. 5). Mussulini et al. (2013) reported similar results in cotreatment with DZP + PTZ (10 + 10 mM). The survival rate was observed in three different periods: during analysis, immediately after analysis, and after analysis. However, animal death was observed (20%) only in the group treated with PTZ at 500 mg/kg. Other doses did not induce death over the analysis, which is advantageous in screening AEDs. Mussulini et al. (2013) reported a mortality rate of 33.3% in PTZ at 10 mM and 50% at 15 mM. The higher mortality rate reported can be explained considering the immersion method since animals absorb PTZ through gills respiration, causing damage to this organ. Gills damage in adult animals is the leading cause of mortality caused by exposure to chemical agents (Souza et al., 2016).
CONCLUSION
Here, we aimed to characterize a standardized method of intraperitoneal treatment with PTZ and oral treatment with DZP using a scoring scale based on the literature. Intraperitoneal administration of PTZ has more translational value than immersion, and intraperitoneal DZP is not the best method for this drug due to potential abdominal inflammation in the animals that can affect their behavior.
As expected, groups treated with PTZ had convulsive behavior, as evidenced by scores 4 and 5. The seizure intensity in AUC did not increase with PTZ doses higher than 250 mg/kg. The latency to score 4 (clonic seizures) was quantified, and no statistical differences were found, indicating that this parameter alone is not ideal. The latency to score 5 (tonic seizures) was statistically different among groups where it could be calculated. In the group treated with PTZ at 125 mg/kg, this parameter could not be quantified since few animals reached the score, evidencing that this dose does not induce satisfactory convulsive behavior.
The survival rate analysis shows low mortality values; only two animals died with the highest dose. Hence, this study shows the safety of intraperitoneal treatment with PTZ. Our results indicate that intraperitoneal administration of PTZ at 250 mg/kg with 10 mg/kg of DZP provides a complete model of acute seizures with the antiseizure activity of DZP (that can be used as a positive control). This replicable model can contribute to the study of convulsions and screening potential AEDs using adult zebrafish.
ACKNOWLEDGMENTS
The authors would like to acknowledge CAPES (nº 3292/2013 AUXPE) and CNPq Proc. 402332/2013-0 for the financial support.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors declare that there are no conflicts of interest.
ETHICAL APPROVALS
This study was approved by the Ethics Committee on the Use of Animals (CEUA) of the Federal University of Amapá under protocol number 004/2018.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Alfaro JM, Ripoll-Gómez J, Burgos JS. Kainate administered to adult zebrafish causes seizures similar to those in rodent models. Eur J Neurosci, 2011; 33:1252–5; https://doi.org/10.1111/j.1460-9568.2011.07622.x CrossRef
Banote RK, Koutarapu S, Chennubhotla KS, Chatti K, Kulkarni P. Oral gabapentin suppresses pentylenetetrazole-induced seizure-like behavior and cephalic field potential in adult zebrafish. Epilepsy Behav, 2013; 27:212–9; https://doi.org/10.1016/j.yebeh.2013.01.018 CrossRef
Baraban SC, Taylor MR, Castro PA, Baier H. Pentylenetetrazole induced changes in zebrafish behavior, neural activity and c-fos expression. Neuroscience, 2005; 131:759–68; https://doi.org/10.1016/j.neuroscience.2004.11.031 CrossRef
Braida D, Donzelli A, Martucci R, Ponzoni L, Pauletti A, Sala M. Neurohypophyseal hormones protect against pentylenetetrazole-induced seizures in zebrafish: role of oxytocin-like and V1a-like receptor. Peptides, 2012; 37:327–33; https://doi.org/10.1016/j.peptides.2012.07.013 CrossRef
Buenafe OE, Orellana-Paucar A, Maes J, Huang H, Ying X, De Borggraeve W, Crawford AD, Luyten W, Esguerra CV, De Witte P. Tanshinone IIA exhibits anticonvulsant activity in zebrafish and mouse seizure models. ACS Chem Neurosci, 2013; 4:1479–87; https://doi.org/10.1021/cn400140e CrossRef
Choo BKM, Kundap UP, Kumari Y, Hue SM, Othman I, Shaikh MF. Orthosiphon stamineus leaf extract affects TNF-α and seizures in a zebrafish model. Front Pharmacol, 2018; 9:1–11; https://doi.org/10.3389/fphar.2018.00139 CrossRef
dos Santos Sampaio TI, de Melo NC, de Freitas Paiva BT, da Silva Aleluia GA, da Silva Neto FLP, da Silva HR, Keita H, Cruz RAS, Sánchez-Ortiz BL, Pineda-Peña EA, Balderas JL, Navarrete A, Carvalho JCT. Leaves of Spondias mombin L. a traditional anxiolytic and antidepressant: pharmacological evaluation on zebrafish (Danio rerio ). J Ethnopharmacol, 2018; 224:563–78; https://doi.org/10.1016/j.jep.2018.05.037 CrossRef
Duy PQ, Berberoglu MA, Beattie CE, Hall CW. Cellular responses to recurrent pentylenetetrazole-induced seizures in the adult zebrafish brain. Neuroscience, 2017; 349:118–27; https://doi.org/10.1016/j.neuroscience.2017.02.032 CrossRef
Huang R, Bell-Horner C, Dibas M, Covey DF, Drewe JA, Dillon DH. Pentylenetetrazole-induced inhibition of recombinant γ - aminobutyric acid type A (GABA A) receptors : mechanism and site of action. J Pharmacol Exp Ther, 2001; 298:986–95.
Jain S, Bharal N, Khurana S, Mediratta PK, Sharma KK. Anticonvulsant and antioxidant actions of trimetazidine in pentylenetetrazole-induced kindling model in mice. Naunyn Schmiedebergs Arch Pharmacol, 2011; 383:385–92.
Kinkel MD, Eames SC, Philipson LH, Prince VE. Intraperitoneal injection into adult zebrafish. J Vis Exp, 2010; 3–6; https://doi.org/10.3791/2126 CrossRef
Kundap UP, Kumari Y, Othman I, Shaikh MF. Zebrafish as a model for epilepsy-induced cognitive dysfunction: a pharmacological, biochemical and behavioral approach. Front Pharmacol, 2017; 8:1–13; https://doi.org/10.3389/fphar.2017.00515 CrossRef
Matias Pereira AC, Sánchez-Ortíz BL, de Melo EL, da Silva Hage-Melim LI, Borges RS, Hu X, Carvalho JCT. Perillyl alcohol decreases the frequency and severity of convulsive-like behavior in the adult zebrafish model of acute seizures. Naunyn Schmiedebergs Arch Pharmacol, 2021; 394(6):1177–90; https://doi.org/10.1007/s00210-021-02050-0 CrossRef
Menezes FP, Da Silva RS. The influence of temperature on adult zebrafish sensitivity to pentylenetetrazole. Epilepsy Res, 2017; 135:14–8; https://doi.org/10.1016/j.eplepsyres.2017.05.009 CrossRef
Morimoto K, Fahnestock M, Racine RJ. Kindling and status epilepticus models of epilepsy: rewiring the brain. Prog Neurobiol, 2004; 73:1–60; https://doi.org/10.1016/J.PNEUROBIO.2004.03.009 CrossRef
Mussulini BHM, Leite CE, Zenki KC, Moro L, Baggio S, Rico EP, Rosemberg DB, Dias RD, Souza TM, Calcagnotto ME, Campos MM, Battastini AM, de Oliveira DL. Seizures induced by pentylenetetrazole in the adult zebrafish: a detailed behavioral characterization. PLoS One, 2013; 8:1–9; https://doi.org/10.1371/journal.pone.0054515 CrossRef
National Research Council of the National Academies. Guide for the care and use of laboratory animals. 8th edition, The National Academies Press, NW Washington, DC, 2011.
Orellana-Paucar AM, Serruys ASK, Afrikanova T, Maes J, De Borggraeve W, Alen J, León-Tamariz F, Wilches-Arizábala IM, Crawford AD, de Witte PAM, Esguerra CV. Anticonvulsant activity of bisabolene sesquiterpenoids of Curcuma longa in zebrafish and mouse seizure models. Epilepsy Behav, 2012; 24:14–22; https://doi.org/10.1016/j.yebeh.2012.02.020 CrossRef
Pineda R, Beattie CE, Hall CW. Recording the adult zebrafish cerebral field potential during pentylenetetrazole seizures. J Neurosci Methods, 2011; 200:20–8; https://doi.org/10.1016/j.jneumeth.2011.06.001 CrossRef
Racine R, Okujava V, Chipashvili S. Modification of seizure activity by electrical stimulation. 2. motor seizure. Electroencephalogr Clin Neurophysiol, 1972; 32:295–9; https://doi.org/Doi 10.1016/0013-4694(72)90178-2 CrossRef
Ramanjaneyulu R, Ticku MK. Interactions of pentamethylenetetrazole and tetrazole analogues with the picrotoxinin site of the benzodiazepine-gaba receptor-ionophore complex. Eur J Pharmacol, 1984; 98:337–45; https://doi.org/10.1016/0014-2999(84)90282-6 CrossRef
Sathaye S, Jain P, Tambe R, Sancheti J, Nahire M, Bhardwaj A. Screening of Pistacia integerrima extracts for their anticonvulsant activity in acute zebrafish and rodent models of epilepsy. Int J Nutr Pharmacol Neurol Dis, 2015; 5:56; https://doi.org/10.4103/2231-0738.153793 CrossRef
Sejima H, Ito M, Kishi K, Tsuda H, Shiraishi H. Regional excitatory and inhibitory amino acid concentrations in pentylenetetrazol kindling and kindled rat brain. Brain Dev, 1997; 19:171–5; https://doi.org/10.1016/S0387-7604(96)00492-5 CrossRef
Siebel AM, Menezes FP, Da Costa Schaefer I, Petersen BD, Bonan CD. Rapamycin suppresses PTZ-induced seizures at different developmental stages of zebrafish. Pharmacol Biochem Behav, 2015; 139:163–8; https://doi.org/10.1016/j.pbb.2015.05.022 CrossRef
Siebel AM, Piato AL, Schaefer IC, Nery LR, Bogo MR, Bonan CD. Antiepileptic drugs prevent changes in adenosine deamination during acute seizure episodes in adult zebrafish. Pharmacol Biochem Behav, 2013; 104:20–6; https://doi.org/10.1016/j.pbb.2012.12.021 CrossRef
Souza GC, Duarte JL, Fernandes CP, Velázquez-Moyado JA, Navarrete A, Carvalho JC. Obtainment and study of the toxicity of perillyl alcohol nanoemulsion on zebrafish (Danio rerio). J Nanomed Res, 2016; 4; https://doi.org/10.15406/jnmr.2016.04.00093 CrossRef
Stewart AM, Desmond D, Kyzar E, Gaikwad S, Roth A, Riehl R, Collins C, Monnig L, Green J, Kalueff AV. Perspectives of zebrafish models of epilepsy: what, how and where next? Brain Res Bull, 2012; 87:135–43; https://doi.org/10.1016/j.brainresbull.2011.11.020 CrossRef
Sukardi H, Chng HT, Chan ECY, Gong Z, Lam SH. Zebrafish for drug toxicity screening: bridging the in vitro cell-based models and in vivo mammalian models. Expert Opin Drug Metab Toxicol, 2011; 7:579–89; https://doi.org/10.1517/17425255.2011.562197 CrossRef
Sung-joon C, Donghak B, Na T, Seok-yong C, Lee B, Kim M, Kim S. Zebrafish as an animal model in epilepsy studies with multichannel EEG recordings. Sci Rep, 2017; 1–10; https://doi.org/10.1038/s41598-017-03482-6 CrossRef
Torres-Hernández BA, Del Valle-Mojica LM, Ortíz JG. Valerenic acid and Valeriana officinalis extracts delay onset of pentylenetetrazole (PTZ)-induced seizures in adult Danio rerio (Zebrafish). BMC Complement Altern Med, 2015; 15:1–10; https://doi.org/10.1186/s12906-015-0731-3 CrossRef
Walker EM. Effects of blood, bile and starch in the peritoneal cavity of the rat. J Anat, 1978; 126:495–507.