Development and validation of dissolution procedures
Bhavesh Vaghela, Rajan Kayastha, Nayana Bhatt, Nimish Pathak, Dashrath Rathod
Pages: 50-56

Development of validated liquid chromatographic method for estimation of levocetirizine from pharmaceutical dosage forms
Chaitanya Prasad MK, Vidyasagar G, Sambasiva Rao KRS, Madhusudhanareddy Induri, Ramanjeneyulu S
Pages: 95-97

Comparative Evaluation of Physicochemical Properties of Some Commercially Available Brands of Metformin Hcl Tablets in Lagos, Nigeria
Akinleye M. Olusola, Adelaja I. Adekoya, Odulaja J. Olanrewaju
Pages: 41-44

Generic Substitution in Malaysia: Recommendations from a Systematic Review
Mohamed Azmi Hassali, Jayabalan Thambyappa, Fahad Saleem, Noman ul Haq, Hisham Aljadhey
DOI: 10.7324/JAPS.2012.2827Pages: 159-164

Effects of substitution of pellet of Moringa oleifera to commercial feed on rabbit’s digestion, growth performance and carcass trait
Dougnon T. J., Aboh B. A., KpodékonT. M., Honvou S., Youssao I.
DOI: 10.7324/JAPS.2012.2903Pages: 015-019

Formulation and Optimization of Chronotherapeutic Drug Delivery from Carvedilol Sulphate Compression Coated Tablets by using Design of Experiment Approach
Vaishali Aggarwal, Ratendra Kumar, Rajiv Sharma, Yogendra Singh, Uday Veer Singh Teotia
DOI: 10.7324/JAPS.2013.31025Pages: 141-146

Survey on the Pharmaceutical Quality of Herbal Medicines Sold in Nigeria
Philip F. Builders, Chris A. Alalor, John A. Avbunudiogba, Isreal E. Justice
DOI: 10.7324/JAPS.2015.50616Pages: 097-103

Assessment of Medical and Pharmacy Students’ Knowledge & Perceptions about Generic Medicines’ Prices & Quality in Kabul- Afghanistan
Mohammad Bashaar, Mohamed Azmi Hassali, Fahad Saleem, Asrul Akmal Shafie
DOI: 10.7324/JAPS.2015.50816Pages: 100-104

Physicochemical quality evaluation of amoxicillin capsules produced in compounding pharmacies at Diadema, Sβo Paulo, Brazil
Fúlvio Gabriel Corazza, Blanca Elena Ortega Markman, Paulo César Pires Rosa
DOI: 10.7324/JAPS.2015.501205Pages: 029-034

Pilot Study of Quality of Diclofenac Generic Products Using Validated In-House Method: Indian Drug Regulatory Concern
Ahmed Nawaz Khan, Roop Krishen Khar, Malairaman Udayabanu
DOI: 10.7324/JAPS.2015.501226Pages: 147-153

Evaluation of the Pharmaceutical Quality of Different Brands of Ranitidine Tablets Manufactured in Bangladesh: A Pharmaceutical and Public Health Prospective
Md. Moklesur Rahman Sarker, Md. Salman Rashid, Ali Asghar Raju, Masud Rana, Mohammed Faisal Bin Karim, Rehana Akter, Abu Nayem Md. Al-Noman Howlader, Long Chiau Ming, Nahlah Elkudssiah Ismail
DOI: 10.7324/JAPS.2016.60109Pages: 055-061

Assessment of Quality of Life in Hypertensive Patients
Sattanathan Kaliyaperumal, Sagarika Binitha Hari, Prasanth Kumar Siddela, Sara Yadala
DOI: 10.7324/JAPS.2016.60522Pages: 143-147

Physalis minima Linn Methanolic Extract Reduces Blood Glucose Level without Compromising Sperm Quality in Normoglycaemic Mice
Dzulsuhaimi Daud, Siti Fatimah Elias, Fatimah Sarah Mohamad Hassan, Mohammad Noor Jalil, Alene Tawang
DOI: 10.7324/JAPS.2016.60602Pages: 008-011

Validated Ultraviolet-Spectrometric Method for Determination of Sofosbuvir in Tablets Formulation
Mohamed A. El Hamd, Ramadan Ali, Adel A. Marzouk, Osama H. Abdelmageed
DOI: 10.7324/JAPS.2017.70214Pages: 114-119

Quality Assessment and Ecotype Distinction for Panax quinquefolius L. from China and Canada by 1H NMR and Chemometrics
Caimei Gu, Zenghui Wang, Labin Wu, Linfang Huang
DOI: 10.7324/JAPS.2017.70504Pages: 018-023

Assessing the Quality of Health Economic Evaluation Research by CHEERS Instrument: A Critical Literature Review in Laos, Cambodia, and Myanmar
Hoang Nam Nguyen, Ky Nhu Ly, Quang Trung Vo
DOI: 10.7324/JAPS.2017.70633Pages: 222-228

Evaluation of Prescribing Pattern of Antiepileptic Drugs and Assessment of Quality of Life of Epileptic Patients and the Knowledge of Their Care Givers
Akhila A. Lekshmi, A. Anjana, Sajila Mary Emmanuel, V. Sreelekshmi, C. D. Shaji Selvin
DOI: 10.7324/JAPS.2017.71022Pages: 152-156

Simultaneous determination of valsartan, amlodipine besylate and hydrochlorothiazide in tablets by near infrared
Natana Becker, Gabriela R. Foresti, Willian R. R. Almeida, Karine F. Nicorena, Marco F. Ferrão, Fabiana E. B. Silva
DOI: 10.7324/JAPS.2017.71111Pages: 074-078

A Comparative Study of Psychiatric Disorders among Mothers of Children with Chronic Diseases and Mothers of Healthy Children
Zohreh Eskandari Shahraki, Mohammad Efffatpanah, Serajaddin Gray, Mitra Radfar, Mehdi Rezaei, Hamidreza Hekmat, Shokofeh Radfar
DOI: 10.7324/JAPS.2017.71216Pages: 116-120

Potential efficacy of Coenzyme Q10 against oxytetracycline-induced hepatorenal and reproductive toxicity in male rats
Samah S. Oda, Reham S. Waheeb, Zeynab Kh. El-Maddawy
DOI: 10.7324/JAPS.2018.8115Pages: 098-107

Quality of Life Assessment in Cancer Patients of Regional Centre of Hyderabad City
V. Naga Sunanda M. Priyanka, J. Architha1 , M. Shravan, A. Srinivasa Rao, Mohd. Abdul Hadi
DOI: 10.7324/JAPS.2018.8125Pages: 165-169

Investigation of Perceived Stress and Quality of Life Assessment of pharm. D. Students at Ibn Sina National College in Saudia Arabia during 2016
Lujain Turki Bin-Mallouh, Mohammed Gamal, Ahmed A H Ali, Mohamed E.A. Abdelrahim, Mohammed Safwan Ali Khan, Mohammad M. Al-Sanea, Kharrat Mohamed, Mohammed Alrashed
DOI: 10.7324/JAPS.2018.8312Pages: 082-090

A Comparative Assessment to Evaluate Enhanced External Counter Pulsation Effect on Physical Profile and Quality of Life in Diabetic and Nondiabetic Coronary Heart Disease Patients
Vikram Singh, Girija Kumari, Bimal Chhajer, Ashok Kumar Jhingan, Saurabh Dahiya
DOI: 10.7324/JAPS.2018.8615Pages: 113-123

Formulation and pharmacopoeial quality evaluation of ketorolac tromethamine IR tablet and comparison with marketed product
Aysha Akter Shetu, Suriya Sharmin, Satyajit Roy Rony, Fatema Moni, Purabi Rani Samaddar, Md. Hossain Sohrab
DOI: 10.7324/JAPS.2019.90510Pages: 082-087
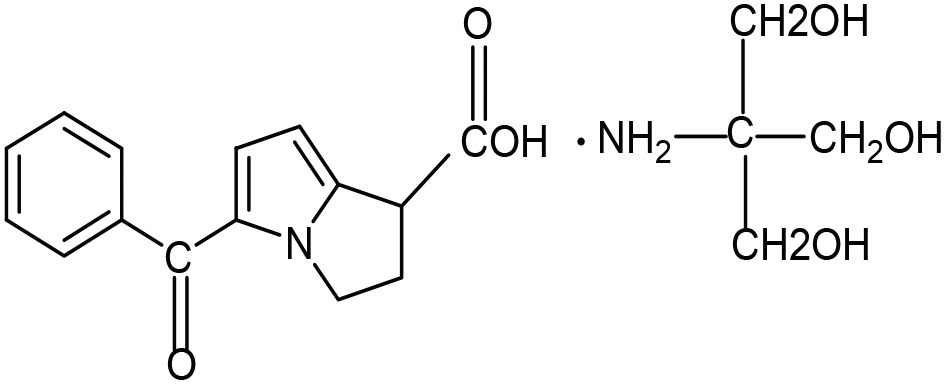
Development and validation of a stability-indicating RP-HPLC method of cholecalciferol in bulk and pharmaceutical formulations: Analytical quality by design approach
Dilipkumar Suryawanshi, Durgesh Kumar Jha, Umesh Shinde, Purnima D. Amin
DOI: 10.7324/JAPS.2019.90604Pages: 021-032

Validation of methodology for assay, pharmaceutical equivalence, and comparative dissolution profile for tablets containing amlodipine besylate
Renata Micheli Martinez, Jenifer Freitas da Silva, Larissa Regina Jorge, Rhye Lessa Ishikawa, Ana Paula Novelli, Talita Laiane Cardoso Cezar, Sandra Regina Georgetti, Marcela Maria Baracat, Rúbia Casagrande
DOI: 10.7324/JAPS.2019.91112Pages: 093-100

Quality of life of patients receiving a single or combination of calcium channel blocker-angiotensin receptor blocker: A cross-sectional study in West Java, Indonesia
Juwita Ramadhani, Mutakin Mutakin, Dyah Aryani Perwitasari, Jutti Levita
DOI: 10.7324/JAPS.2020.103011Pages: 088-092

Health-related quality of life among patients undergoing chronic disease management: A cross-sectional study
Nia Kurnia Sholihat, Vitis Vini Fera Ratna Utami
DOI: 10.7324/JAPS.2020.103009Pages: 075-079
Formulation of somatostatin analog tablets using quality by design approach
Zoya Shprakh
DOI: 10.7324/JAPS.2021.110412Pages: 096-105

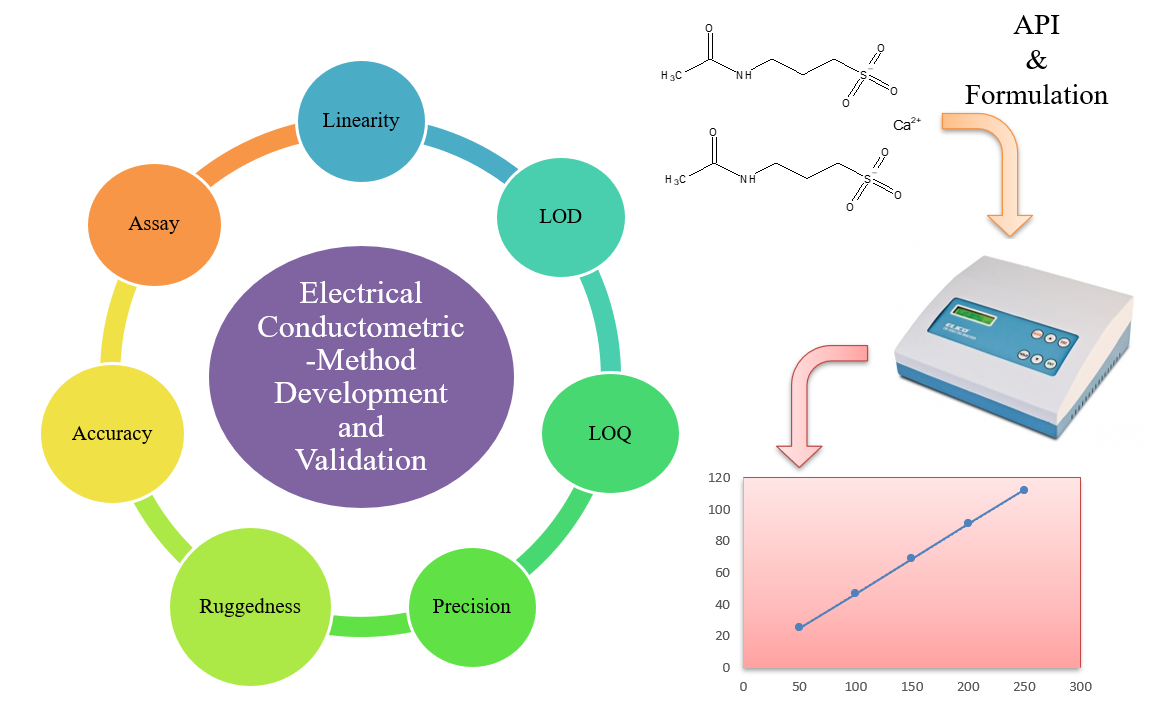
Conductometric method development and validation to estimate acamprosate calcium in API and marketed formulation
Rahul K. Yadav, Meenaxi M. Maste, Shailendra S. Surywanshi, Utkarsh Shastri
DOI: 10.7324/JAPS.2021.1101111Pages: 082–086
Meta-analysis of cost-effectiveness of three-drug therapy versus two-drug therapy in chronic obstructive pulmonary disease patients
Aamir Ali Syed, Ganesh Narayan Sharma, Birendra Shrivastav, Aleemuddin Naveed Mohd
DOI: 10.7324/JAPS.2021.110916Pages: 129-138

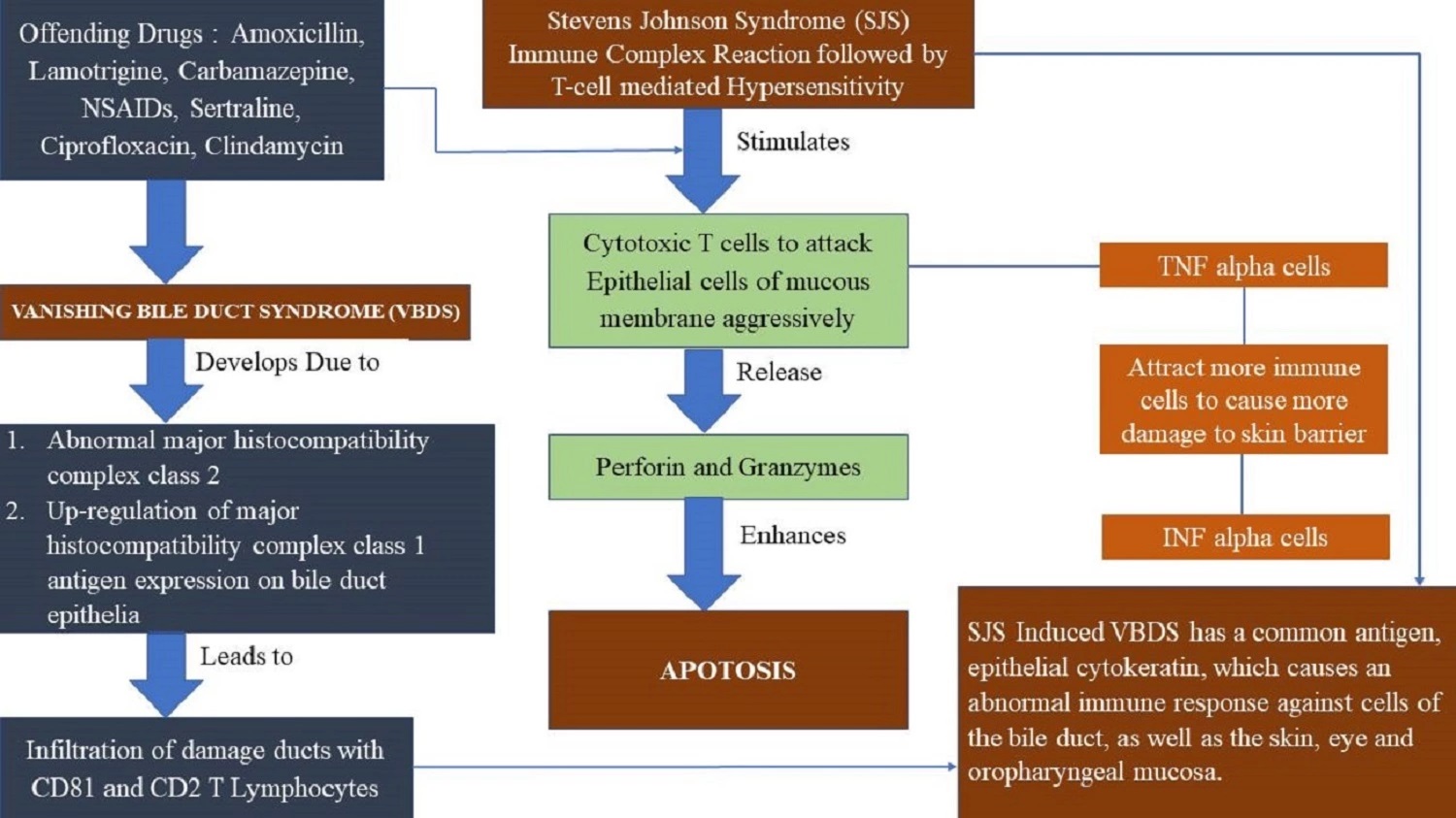
Vanishing bile duct syndrome in pediatric population: An updated case-based review
H. C. Gopika Nair, Shilpa Rachel Thomas, S. I. Prithika, P. Ajay Samraj, K. Sumathi, Keerthana Chandrasekar
DOI: 10.7324/JAPS.2021.1101018Pages: 134-139
Analytical quality by design approach for estimating rosuvastatin calcium in pharmaceutical formulation by green HPLC method: Ecologically evaluated and stability-indicating
Seetharaman Rathinam, Lakshmi Karunanidhi Santhana
DOI: 10.7324/JAPS.2021.1101119Pages: 150-160
The development of biodegradable hemostatic and absorbable sponges containing chlorhexidine digluconate and their in vitro characterization—A QbD approach
Bohdana Pavliuk, Mariana Chubka, Taras Hroshovyi, Mariana Demchuk, Iryna Stechyshyn
DOI: 10.7324/JAPS.2021.120206Pages: 056-065

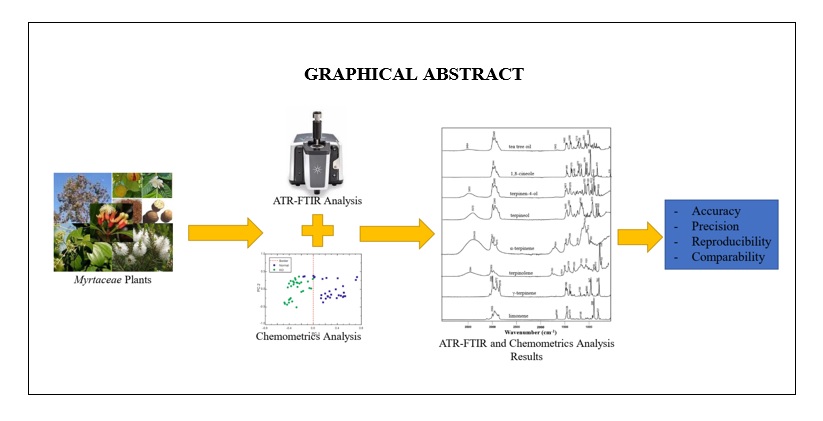
The combination of ATR-FTIR and chemometrics for rapid analysis of essential oil from Myrtaceae plants – A review
Islamudin Ahmad, Jihan Azmi Nur Fikri, Ayun Erwina Arifianti, Sarini Abdullah, Abdul Munim
DOI: 10.7324/JAPS.2022.120604Pages: 030-042
A quality by design approach for the optimization of olmesartan medoxomil-orodispersible lyophilisates: In vitro/in vivo evaluation
Shereen H. Noshi, Marwa H. S. Dawoud, Mervat S. Ibrahim
DOI: 10.7324/JAPS.2022.120617Pages: 172-185

QbD-driven HPLC method for the quantification of rivastigmine in rat plasma and brain for pharmacokinetics study
Divya Gopalan, Prajakta H. Patil, Puralae Channabasavaiah Jagadish, Suvarna G. Kini, Angel Treasa Alex, Nayanabhirama Udupa, Srinivas Mutalik
DOI: 10.7324/JAPS.2022.120606Pages: 056-067

Cost-utility analysis of olanzapine versus combination of haloperidol-diazepam in patients with acute phase schizophrenia: An Indonesian context
Hesty Utami Ramadaniati, Yusi Anggriani, Fredrick Dermawan Purba, Desweri Muhareni
DOI: 10.7324/JAPS.2022.121112Pages: 103-110

Determination of impaired quality of life of hypertensive patients and its complications
Amelia Rumi, Rudi Safarudin, Ririen Hardani, Dwi She Dewi Melinency Bokko, Nur Indah Sari, Khildayanti Saenong, Lilies Handayani, Nurulhuda Rahman, Ismail Setyopranoto, Muammar Fawwaz
DOI: 10.7324/JAPS.2022.121212Pages: 117-125

Electrical conductivity and total organic carbon analysis of water in Brazilian industrial pharmaceutical formulations
Daniela Toledo de Matos, Flávio Silva de Carvalho, Fernando Machado dos Santos
DOI: 10.7324/JAPS.2023.130118Pages: 187-192
Outcomes of different types of intermittent fasting for practitioners in terms of nutritional status and quality of life: A systematic review
Nur Asma Mohd Hafizi, Nurul Syazwani Azhari, Asma’ Ali, Noor Salihah Zakaria, Hayati Mohd Yusof
DOI: 10.7324/JAPS.2023.109827Pages: 024-031

Medication reconciliation practices in Gulf Cooperation Council countries: A review
Alaa Ahmad Farajallah, Hadzliana Zainal, Subish Palaian
DOI: 10.7324/JAPS.2024.134220Pages: 061-072

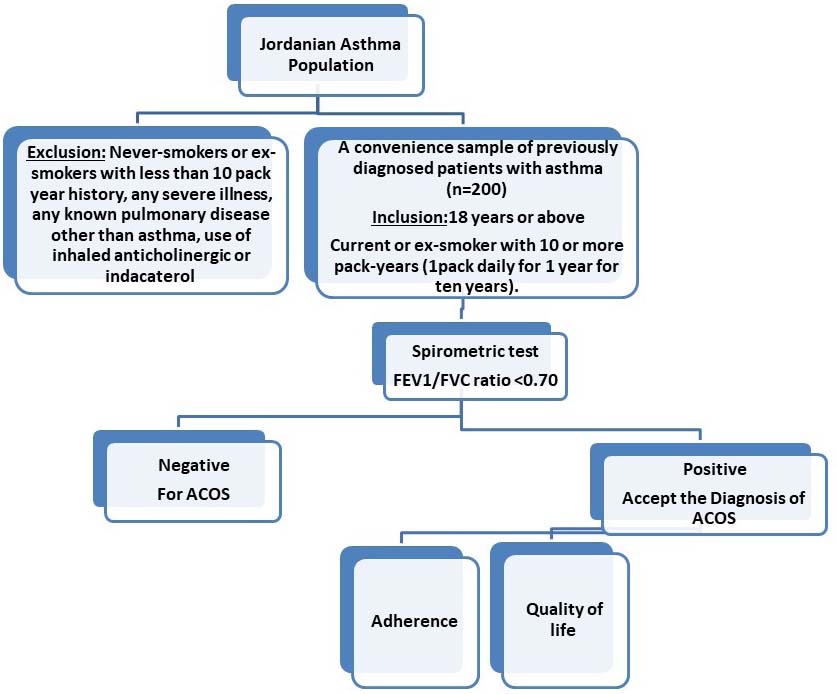
Asthma–COPD overlap syndrome among smoker subjects with asthma: A cross-sectional study
Safa Olimat, Shoroq M. Altawalbeh, Roqia S. Maabreh, Iman A. Basheti
DOI: 10.7324/JAPS.2024.143342Pages: 72-80
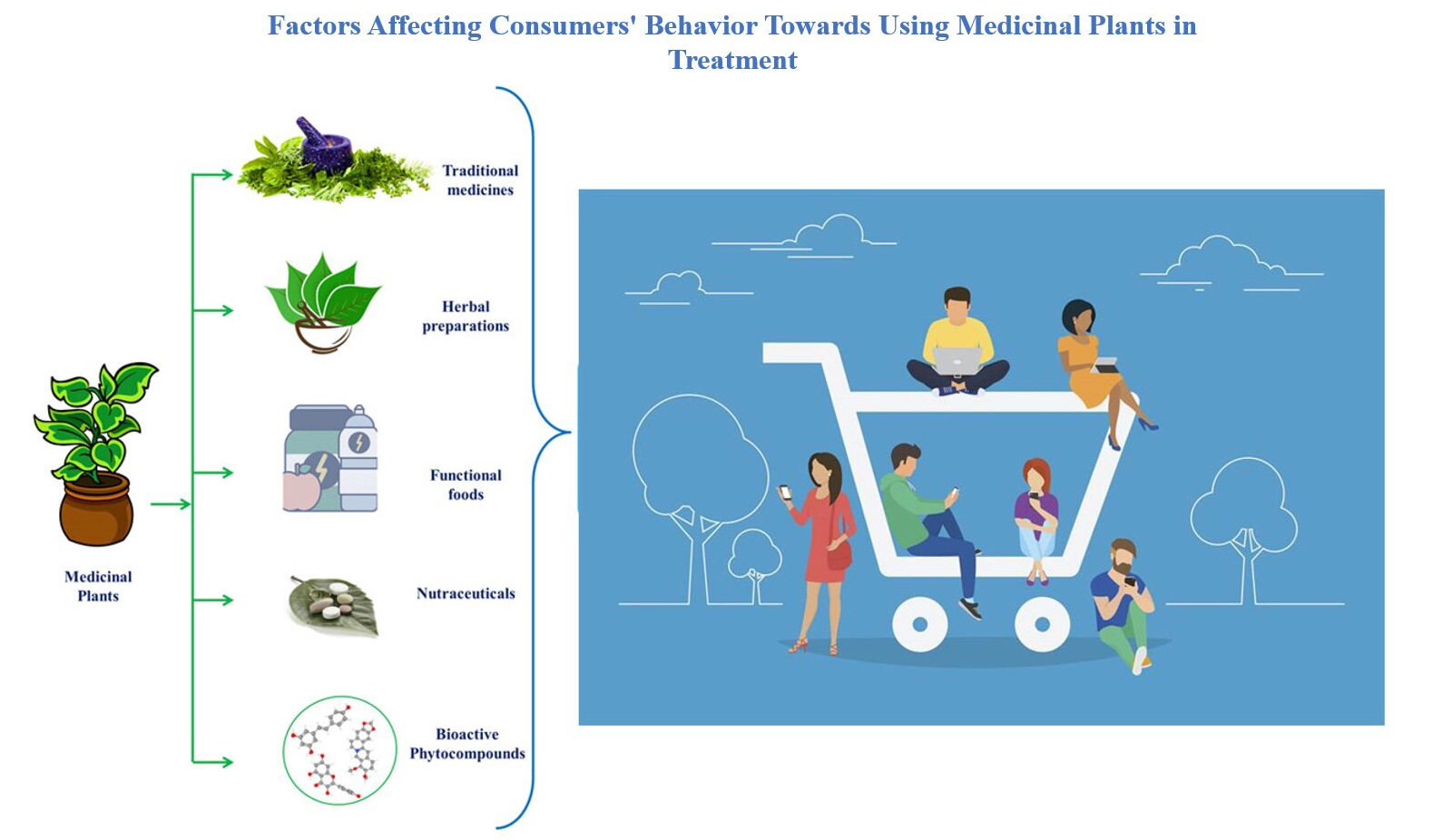
Factors affecting consumers’ behavior toward using medicinal plants
Ali Al-Saadi, Mahmood Al-Samydai, Ali AL-Samydai, Maha N. Abu Hajleh, Rudaina Othman Yousif, Dima Musa Al-Dajani, Lidia Kamal Al-Halaseh, Fatimah Akram Othman, Aburjai Ahmed
DOI: 10.7324/JAPS.2024.174011Pages: 125-130
Quality by design approach for the formulation of bilayer tablets of domperidone and itopride in gastro-esophageal reflux disease
Roshani Prajapati, Bhavna Kumar, Jagannath Sahoo, Shailendra Shakya, Diwya Kumar Lal
DOI: 10.7324/JAPS.2024.168489Pages: 169-181

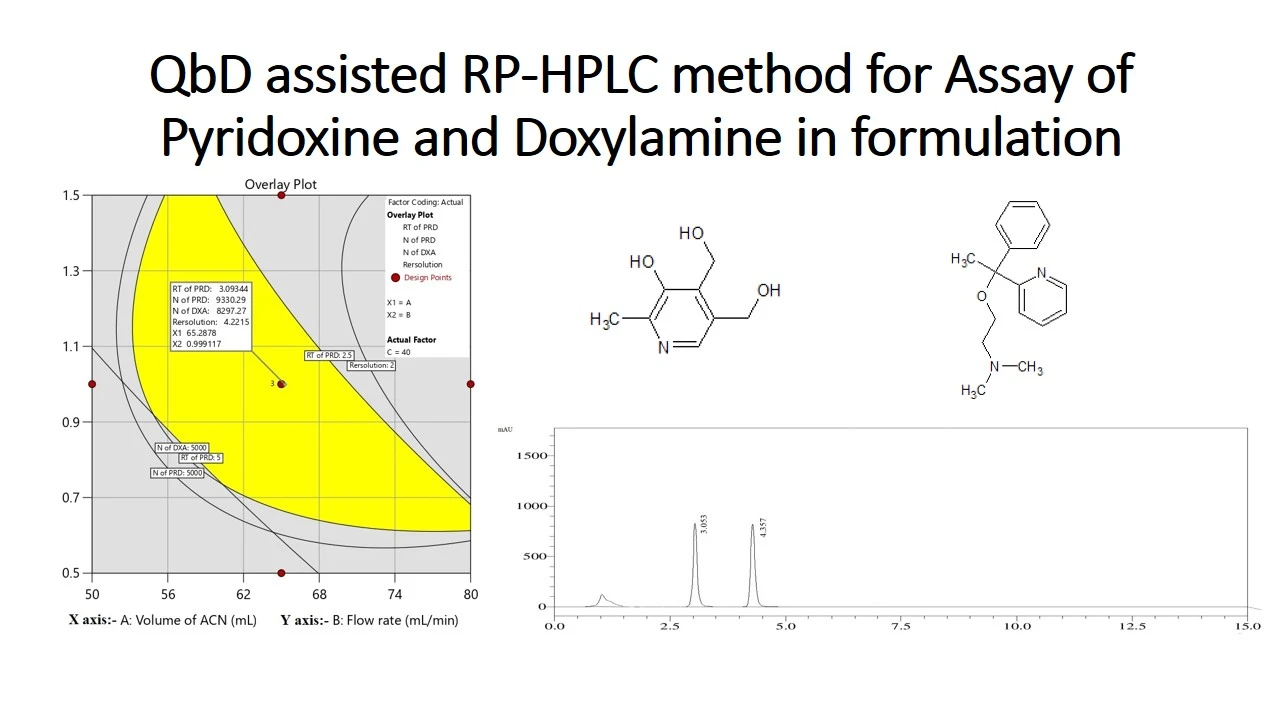
QbD assisted RP-HPLC method for determination of Pyridoxine and Doxylamine in pharmaceutical formulation using central composite design
Gangu Naidu Challa, Daniel Raju Kunda, Sheik Jakir Hussain Mustaq, Nagabharathi Marni, Srilekhya Ketha, Urmila Gorle, Shravitha Jakkula, Bhagavan Rajesh Babu Koppisetty
DOI: 10.7324/JAPS.2025.205442Pages: 072-083
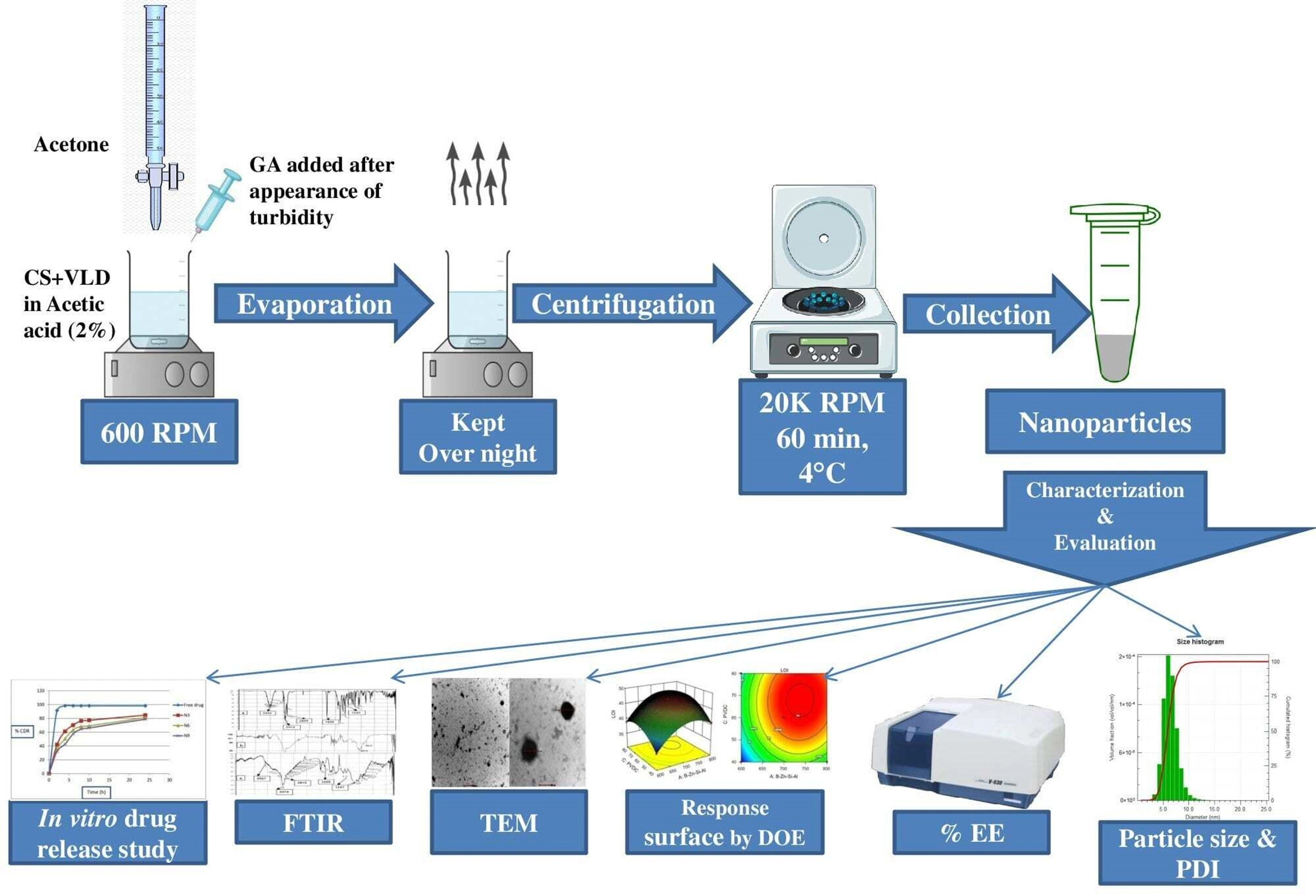
Quality by design approach assisted development and optimization of Chitosan–Vildagliptin nanoparticles using a simple desolvation technique
Anand Shripal Ammanage, Vinayak Shivamurthi Mastiholimath
DOI: 10.7324/JAPS.2025.202888Pages: 174-182
A quality by design approach with comprehensive green analytical chemistry assessment: Development, validation, and application of a high-performance liquid chromatographic method for quantifying meropenem trihydrate in nanosponges and marketed formulations
Ashwini T, Sanjay Garg, Padmaja A. Shenoy, Raghu Chandrashekhar, Yogendra Nayak, Usha Y. Nayak
DOI: 10.7324/JAPS.2025.236517Pages: 070-095
