INTRODUCTION
Schizophrenia is a psychiatric disorder characterized by symptoms of chronic or recurrent psychosis. It is commonly associated with social and occupational impairments. Unsurprisingly, this debilitating medical condition was among the major causes of disability worldwide (Institution of Health Metrics and Evaluation, 2019). The 2018 World Health Organization Report highlighted the low prevalence of schizophrenia as opposed to other mental disorders. It was cited in the report that depression is the most common mental disorder with 300 million patients globally followed by bipolar disorders (60 million), while the prevalence of schizophrenia and other psychoses constituted less than one-tenth of those with depression, i.e., 23 million cases (World Health Organization, 2018). In the context of Indonesia, the Basic Health Survey conducted by the Ministry of Health in 2013 reported the prevalence of schizophrenia was 1.7% corresponding to two households with schizophrenia in every 1,000 study households. Over the next 5 years, a similar survey signified the considerable increase of prevalence to 7% which is equivalent to 470,000 schizophrenia cases (Indonesian Ministry of Health, 2018).
Despite its low prevalence, schizophrenia is associated with a higher risk of mortality where schizophrenic patients are nearly three times more likely to die earlier than the general population mainly due to cardiovascular disease, metabolic disorders, and infections (Ringen et al., 2014). In addition, this condition has significant ramifications on social and economic aspects for patients, families, healthcare providers, and society (Chong et al., 2016). Mental health disorders including schizophrenia are part of the main causes of the global burden of disease, and around one-third of the disease burden is attributable to productivity losses (Vos et al., 2015). A systematic review to investigate the economic burden of schizophrenia in some countries revealed that annual costs for patients with schizophrenia in each country ranged from US $94 million to US $102 billion with indirect costs accounting for the largest proportion of the total costs. The aforementioned review also estimated that the economic burden of this disease was approximately 0.02%–1.65% of the gross domestic product (Chong et al., 2016). A more recent review to estimate the societal cost of schizophrenia across middle- and high-income countries signified the large economic burden of schizophrenia. It was revealed that the annual societal cost per patient varied considerably from just below US $6,000 in Thailand to almost US $100,000 in Norway. Furthermore, that review also highlighted direct medical costs and productivity losses as the major contributor (Jin and Mosweu, 2017). In reference to a report from Indonesia’s Social Security Agency for Health, total direct medical costs for mental disorders in 2016 were IDR 730 billion (US $50 million) with inpatient care-related cost dominating the proportion. Schizophrenia accounted for the highest amount of healthcare spending in comparison to other mental disorders (Badan Penyelenggara Jaminan Sosial-Kesehatan (Indonesian Social Security Agency for Health), 2017). Schizophrenia was still responsible for the highest economic burden of mental disorders in 2020 where the costs for treating inpatients and outpatients with schizophrenia were approximately IDR 282 billion (US $19.6 million) (Badan Penyelenggara Jaminan Sosial-Kesehatan (Indonesian Social Security Agency for Health), 2022).
Antipsychotic medications are the first-line medication treatment for acute psychosis, symptom reduction, and relapse prevention in patients with schizophrenia. It is recognized in numerous national and international guidelines that patients with schizophrenia should be treated with first- or second-generation antipsychotics. The choice of antipsychotics should be based on a combination of treatment efficacy, tolerability, and patient/carer preference (Kementerian Kesehatan Republik Indonesia (Indonesian Ministry of Health), 2015; National Institute for Health and Care Excellence, 2014; Remington et al., 2017; The American Psychiatric Association, 2020). The management of acute psychosis requires timely intervention using injectable antipsychotics to control severe symptoms and to minimize any harm to patients and others. Treatment with conventional (first-generation) antipsychotics such as haloperidol in conjunction with benzodiazepines has been used as the mainstay treatment for acute psychosis, yet the poor tolerability of conventional antipsychotics due to their side effects (e.g., extrapyramidal symptoms, tardive dyskinesia) was experienced by many patients compromising their benefit for long-term treatment (Uçok and Gaebel, 2008). Meanwhile, second-generation antipsychotics offer a more favorable side-effect profile despite their considerable costs (Wei Xin Chong et al., 2016). Olanzapine injection (second-generation antipsychotic) and a combination of haloperidol and diazepam injections constituted the most highly used antipsychotics in one of the major public psychiatric hospitals in Jakarta, Indonesia. Interestingly, the price of olanzapine was 44 times higher than that of the combination of haloperidol and diazepam necessitating pharmacoeconomic evaluation to justify the costs and the treatment outcomes.
In addition to epidemiological measures such as morbidity and mortality, the economic burden of mental disorders including schizophrenia should be thoroughly investigated through cost-of-illness studies within health economics. Cost-effective analysis (CEA) comparing antipsychotics in schizophrenia is widely available, yet the studies focusing on the cost-utility analysis of schizophrenic treatments are less common than their CEA counterpart. Quantifying patients’ quality of life through utility analysis is of importance in economically catastrophic and disabling medical conditions like schizophrenia. Thus, the objective of this study was to estimate the cost per quality adjusted life-year (QALY) of olanzapine injection versus the combination of haloperidol and diazepam injections in patients with acute-phase schizophrenia.
METHODS
Study design and patient selection
A prospective cohort observational three-month study was conducted in a public psychiatric hospital in Jakarta, Indonesia. A purposive sampling approach was applied in our study. Sample size was determined using Krejcie and Morgan’s table. It was estimated that over three months there were 160 patients receiving olanzapine injections and 120 patients receiving a combination of haloperidol-diazepam injections. Based on Krejcie and Morgan’s table, the sample size for the olanzapine group was 113 patients and for the haloperidol-diazepam group was 92 patients (Krejcie and Morgan, 1970). The inclusion criteria were acute schizophrenic inpatients (aged >17 years) who were treated in an acute room and received either olanzapine intramuscular injections or a combination of haloperidol and diazepam intramuscular injections during the study period. Deceased patients during hospitalization, patients referred to other hospitals, patients admitted several times during the study, and patients readministered the study medicine after being transferred to a quiet room were excluded. An acute room is an isolation room designed for schizophrenia patients with acute agitation and PANSS-EC score ≥ 20. This study had been approved by the Institutional Ethics Committee (No. Sket/02/2019/KEPK), and patient confidentiality was maintained throughout the study.
Study instrument and data collection
The principal researcher collected the patients’ sociodemographic information and clinical characteristics from medical records. The direct medical costs (medicine, medical devices, medical consumables, physician visits, laboratory testing, radiology examination, and patient accommodation) were sourced from financial records. The perspective of the study was the third-party payer. The outcome measures included Positive and Negative Syndrome Scale Excited Component (PANSS-EC) score, length of stay in the acute room, utility score, and QALY. The PANSS-EC consisted of five items: excitement, tension, hostility, uncooperativeness, and poor impulse control. The five items from the PANSS-EC were rated from 1 (not present) to 7 (extremely severe); scores ranged from 5 to 35; mean scores ≥ 20 clinically correspond to severe agitation (Montoya et al., 2011). Patients were transferred from the acute room to the quiet room if they demonstrated symptom improvement and had PANSS-EC ≤ 15 or the score for each item was ≤ 3. Meanwhile, the utility score (between 0.0 and 1.0) was measured using the Indonesian EQ-5D-5L questionnaire. The questionnaire was the most frequently used instrument to evaluate health-related quality of life. The EQ-5D-5L consisted of five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension had five levels: no problems, slight problems, moderate problems, severe problems, and unable/extreme problems. This EQ-5D-5L descriptive system was followed by rating of overall health status on a visual analog scale ranging from 0 (“worst health you can imagine”) to 100 (“best health you can imagine”) (Purba et al., 2017).
The PANSS-EC score and utility score were rated by psychiatrists and nurses, respectively. The rating of PANSS-EC score and utility score was done two times, i.e., at the initiation of study medicines in the acute room (baseline assessment) and immediately before patient transfer from the acute room to the stabilization room (after intervention). The measure for the economic evaluation was QALYs. QALYs were calculated by multiplying the utility score by the duration of the treatment effect to provide the number of QALYs gained. Utility estimates were determined by converting the results of the five dimensions obtained from the EQ-5D-5L questionnaire using the Indonesian EQ-5D-5L value set validated by Purba et al. (2017). According to Purba et al. (2017), there were approximately 3,125 combinations of five dimensions to specify health states. The resulting utility values were obtained by entering each health state combination (e.g., 12,311) into the model of the final value set. Furthermore, the duration was determined by calculating the difference between the average of life expectancy and the average age at diagnosis. The average of life expectancy was set at 69.3 years (men) and 71 years (women), whilst the average onset at diagnosis was 25 years for both genders. The cost-utility ratio was calculated by dividing the direct medical costs by the QALYs.
Data analysis
Categorical data were presented as numbers and percentages, whilst continuous data were presented as mean ± standard deviation (SD). The chi-square test was used to compare categorical data. Continuous data, i.e., PANSS-EC score, utility score, and QALY, between the two treatment groups were compared using the Mann-Whitney test. The differences in PANSS score, utility score, and QALY between the baseline and final assessment (after intervention) in each group were analyzed using the Wilcoxon test. Data were analyzed using IBM SPSS Statistics for Windows (version 23.0). A p value < 0.05 was considered to be statistically significant. For pharmacoeconomic analysis, a cost-utility analysis was conducted to calculate the cost-utility ratio (CUR) for each treatment and incremental cost-utility ratio (ICUR).
RESULTS
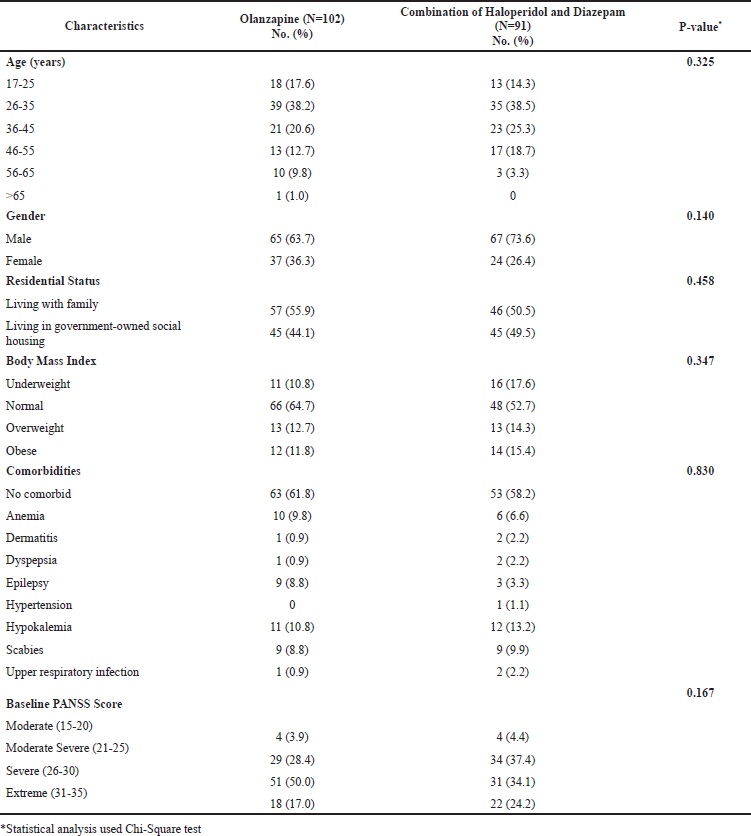
There were 193 patients in this study of whom 102 patients received olanzapine injections and 91 patients were administered a combination of haloperidol and diazepam injections for treating acute-phase schizophrenia. As depicted in Table 1, both groups were comparable in regard to sociodemographic and clinical characteristics. The aforementioned characteristics have been published elsewhere previously (Muhareni et al., 2021). It can be seen in Table 1 that patients aged 26–45 years accounted for more than half of the patients in each group. There was no discernible difference in terms of gender as more males than their counterparts were observed in either group. With respect to residential status, a similar proportion of patients either lived with their families or resided in social housing managed by the government. It is quite interesting that approximately 1 in 2 patients had normal body mass index indicating that more patients had an overall good nutritional status. A similar profile of coexisting diseases was found in both groups with the majority of the patients not having any comorbidity. Nearly all patients had a baseline PANSS-EC score > 20, and they required acute treatment to manage their schizophrenia.
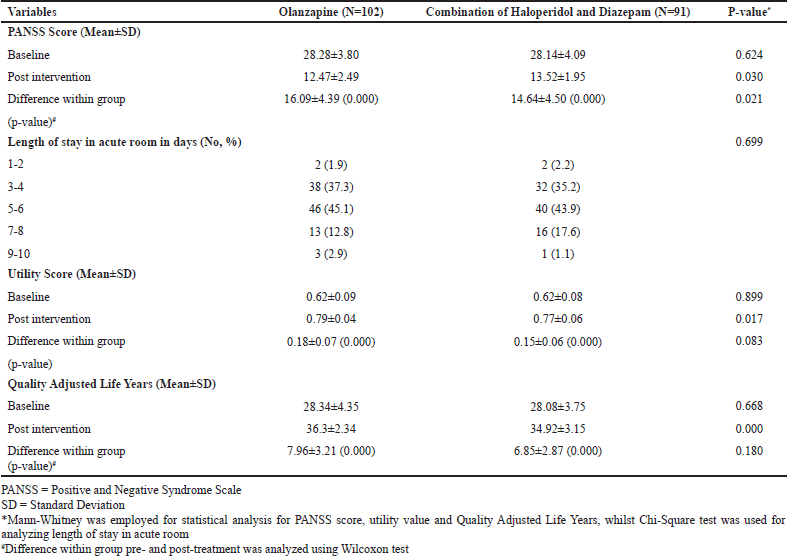
Data related to patients’ outcome measures during acute schizophrenia treatment are detailed in Table 2. At baseline, there was no significant difference in PANSS score where the patient on average had severe acute schizophrenia. After administration of study medicines, the PANSS score significantly decreased by 16.09 in the olanzapine group and 14.64 in the other group. After the intervention, there was a significant difference in change of PANSS score between both groups (p = 0.021) indicating that the administration of olanzapine injection generated greater improvement in patients’ symptoms than its counterpart. With respect to the length of stay in the acute room, there was no significant difference in duration of stay between the patients in the two groups as more than three-quarters of the patients spent around 3–6 d. Data related to the PANSS score and length of stay of this study have been published elsewhere (Muhareni et al., 2021). Similar baseline utility scores and QALYs were observed in both groups. Patients in each group had a baseline utility score of approximately 0.62 for each year of life saved, and the score increased significantly by 0.18 and 0.15 in the olanzapine group and the combination of haloperidol-diazepam group, respectively. It is worth noting that any of the study medicines was able to improve patients’ utility scores to a similar extent. In line with the postintervention utility score, there was no significant difference in additional QALYs at the final assessment of the acute phase. Patients receiving olanzapine injection could gain approximately an extra seven years versus eight years in those taking the combination of haloperidol-diazepam injections.
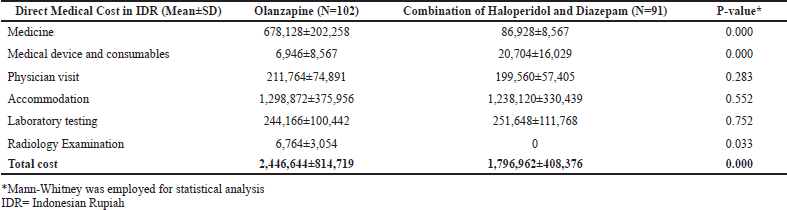
Table 3 outlines the direct medical cost for each patient during acute treatment. As detailed in Table 3, the total cost in the olanzapine group was significantly higher (p = 0.000) than its counterpart with nearly IDR 2.5 million per patient being documented in patients receiving olanzapine as opposed to approximately IDR 1,8 million in those taking haloperidol-diazepam. Furthermore, a remarkable difference was also observed in the cost of medicine in which the cost of the olanzapine injection was almost eight times higher than that of the haloperidol-diazepam injections.
The administration of either of these acute treatments was associated with increase in QALY at a relatively low increase in cost. As described in Table 4, acute treatment using olanzapine injection would cost approximately IDR 300,000 (US $20.89) per extra QALY, whilst a lower cost was observed in patients taking haloperidol-diazepam injection where this combination would cost around IDR 260,000 (US $18.10) for each additional QALY. When taking QALY into consideration, both acute treatments demonstrated the same effectiveness with a higher cost found in the olanzapine group than its counterpart indicating the combination of haloperidol-diazepam was a cost-effective option compared with olanzapine (see Table 5). In this sense, the combination of haloperidol-diazepam was the dominant choice, and ICUR calculation was not necessarily conducted.
 | Table 1. Socio-demographic and Clinical Characteristics of Acute Phase Schizophrenic Patients (Muhareni et al., 2021). [Click here to view] |
 | Table 2. Outcome Measures of the Study Patients. [Click here to view] |
 | Table 3. Direct Medical Cost Per Patient During Acute Schizophrenia [Click here to view] |
 | Table 4. Cost Utility Ratio Calculations. [Click here to view] |
 | Table 5. Alternative Position of Olanzapine Injection and Combination of Haloperidol-Diazepam Injections (Rascati, 2009). [Click here to view] |
DISCUSSION
Our study uncovered that the improvement of positive and negative symptoms of schizophrenia during exacerbation was statistically higher in patients given olanzapine than in those given haloperidol (in combination with diazepam). The PANSS score of olanzapine recipients decreased by 16.09 which was considerably lower (p = 0.02) than haloperidol recipients (PANSS score = 14.64). The superiority of olanzapine in reducing schizophrenia symptoms was also documented in some published studies (Beasley et al., 2003; Bhana et al., 2001; Leucht et al., 2009; Pinem, 2010; Tollefson et al., 1997). Consistent with our finding, a study involving schizophrenia patients with moderately severe to very severe agitation in an Indonesian hospital reported that olanzapine significantly decreased PANSS score within 2 h and 4 h following administration of the study medicines. That study also demonstrated significantly greater improvement in agitation severity (p = 0.015) at 4 hours after injection with 80% of olanzapine recipients having mild agitation compared to 50% of haloperidol recipients (Pinem, 2010). A multicenter six-week study conducted in 174 sites in Europe and North America also demonstrated better improvements in behavioral agitation during acute-phase schizophrenia in olanzapine recipients than in haloperidol counterparts (Tollefson et al., 1997). The superiority of olanzapine to haloperidol was observed not merely during acute psychosis. Olanzapine-treated patients showed a significantly lower one-year risk of psychotic relapse than those given haloperidol during long-term maintenance treatment (Beasley et al., 2003). Likewise, a meta-analysis comparing the efficacy of first-generation antipsychotics and that of second-generation comparators revealed that four second-generation antipsychotics (i.e., amisulpride, clozapine, olanzapine, and risperidone) outperformed the older antipsychotics (including haloperidol) in the improvement of positive and negative symptoms and overall symptoms (Leucht et al., 2009). Nonetheless, our results were somewhat different from those documented in a 24-week effectiveness study by Gründer et al. (2016) with no differential effects on PANSS score between typical antipsychotics (i.e., haloperidol, flupentixol) and atypical antipsychotics (i.e., aripiprazole, olanzapine, and quetiapine).
In addition to PANSS score improvement, we also assess the effectiveness of olanzapine and haloperidol on the patients’ quality of life. When comparing the findings of this study with other studies, we have found mixed findings. To some extent, the result of the present study was consistent with that of an American study where olanzapine demonstrated no significant advantages in improving compliance to treatment and overall quality of life compared with haloperidol (in combination with benztropine). Further, that study observing patients over a year found olanzapine and haloperidol had similar effectiveness in controlling positive and negative symptoms notwithstanding olanzapine’s superiority in reducing akathisia and improving cognition. Olanzapine was also associated with significantly higher costs and incidence of weight gain (Rosenheck et al., 2003). Another study conducted in the USA by Hamilton et al. (1999) also found that olanzapine was not significantly superior (p = 0.094) in improving patients’ quality of life during six-week treatment in the acute phase. It was reported in that study that 33% of olanzapine recipients and 25% of haloperidol recipients showed clinical improvement in quality of life. Contrary to our result, Hamilton et al. (1999) observed that olanzapine treatment had significantly lower inpatient total medical costs than its haloperidol counterpart (US $5,125 vs. US $5,795, p = 0.038) despite the medicine cost of olanzapine being significantly higher than haloperidol (US $5,125 vs. US $5,795, p = 0.038). These findings indicated that olanzapine would be a cost-effective option compared to haloperidol. In two six-month observational Spanish studies, patients receiving olanzapine experienced significantly greater improvement in all dimensions of the EQ-5D visual analog scale than those given haloperidol (median change 20.5 in the olanzapine group vs. 12.5 in the haloperidol group, p < 0.05) (Gómez et al., 2000; Sacristán et al., 2000). Olanzapine as the dominant strategy in improving patients’ quality of life was also reported by Tunis et al. in which olanzapine could save US $1,632.50 per unit improvement in physical health and functioning score and US $5,654.74 per additional gain in the mental health and functioning factor (Tunis et al., 1999). Similarly, better improvement in quality of life related to olanzapine use has been uncovered in a randomized double-blinded study involving schizophrenia outpatients recruited from some hospitals in Germany. This six-month study reported that patients receiving one of the second-generation antipsychotics (aripiprazole, olanzapine, and quetiapine) demonstrated significantly greater improvement in quality of life than those taking either first-generation antipsychotic (haloperidol or flupentixol). However, this German study did not report any cost calculation, so it is unlikely to assess the cost-effectiveness of the antipsychotics (Gründer et al., 2016).
The efficacy of antipsychotics is usually quantified by the reduction of psychopathological symptoms (e.g., positive and negative symptoms). Nevertheless, other effectiveness measures such as quality of life, subjective well-being, and social performance have been considered as important outcomes. Further, the importance of patients’ perspectives should be taken into account when evaluating their quality of life. In this sense, the assessment of antipsychotic effectiveness including quality of life requires a combination of subjective self-rated and observer-rated examination (Hayhurst et al., 2014). The result of our study might differ considerably if self-rated assessment by patients was applied to evaluate the quality of life. It has been suggested that the assessment of antipsychotic effectiveness solely based on objective examination may undermine the clinical profile of an individual antipsychotic (Gründer et al., 2016). Furthermore, the efficacy of antipsychotics should be balanced with their side effects. Olanzapine has a greater affinity for serotonin 5-hydroxytriptamine2A receptors than for the dopamine D2 receptor and a high affinity for all muscarinic receptor subtypes. Meanwhile, haloperidol acts as an antagonist of D2 receptors with very high affinity and has a low affinity for alpha2 receptors and all muscarinic subtypes (Zhang and Stackman, 2015). As a consequence of their mechanism of action, it was noted in a meta-analysis that olanzapine was associated with significantly less incidence of extrapyramidal side effects (relative risk = 0.39, p value < 0.0001) than haloperidol (Leucht et al., 2009). However, olanzapine and most atypical antipsychotics were associated with an increased risk of metabolic adverse effects. Different properties of the efficacy and safety of each antipsychotic require individualization strategies for treating patients with schizophrenia (Gründer et al., 2016; Leucht et al., 2009).
The results of our study should be interpreted with caution as it has some limitations. This study was conducted in one hospital thus limiting its generalizability. Furthermore, it is difficult to determine the extent to which the results of our study are generalizable to other studies. The differences in study settings, i.e., the phase of schizophrenia (acute psychosis, stabilization, or remission), type of patients (inpatients or outpatients), heterogeneity in the measured costs, and distinction in study instruments, might lead to different results between ours and other published studies. In addition, the assessment of the quality of life in our study was merely undertaken during an acute exacerbation. The nature of an acute exacerbation is likely to correlate well with more ready outcome measures, i.e., symptom reduction. Thus, the impact of antipsychotics might require long-term study (not during acute psychosis) to adequately evaluate their roles in improving quality of life. In terms of the cost component, our study focused on direct medical costs. Social care elements for patients such as employment rates and reduced work-related productivity were not considered; thus, this analysis may underestimate the benefits of treatment with olanzapine. It has been evident that the direct costs of schizophrenia treatment are substantial, yet the indirect costs including societal costs may be at least as expensive as the direct costs (Bhana et al., 2001).
CONCLUSION
When compared with olanzapine, haloperidol (combined with diazepam) appears to be a dominant strategy for treating acute psychosis in schizophrenia, resulting in cost savings and similar QALYs. However, olanzapine may represent a cost-effective treatment option when considering psychopathological symptom reduction and length of stay during the acute phase. The selection of antipsychotics should also take into account patients’ tolerability to their side effects and the outcome measures during long-term maintenance therapy.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to the psychiatrists and nurses in the study hospital for their participation and valuable insights.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study had been approved by the Institutional Ethics Committee (No.Sket/02/2019/KEPK), and patient confidentiality was maintained throughout the study.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Badan Penyelenggara Jaminan Sosial-Kesehatan [Indonesian Social Security Agency for Health]. Penderita skizofrenia bisa mendapat pelayanan Kesehatan melalui JKN-KIS [Patients with Schizophrenia can Access Healthcare Provison through National Insurance], 2017. Available via https://bpjs-kesehatan.go.id (Accessed 27 March 2020).
Badan Penyelenggara Jaminan Sosial-Kesehatan [Indonesian Social Security Agency for Health]. Gangguan Kejiwaan Dijamin BPJS Kesehatan, 2022. Available via https://bpjs-kesehatan.go.id/bpjs/post/read/2022/2161/Gangguan-Kejiwaan-Dijamin-BPJS-Kesehatan (Accessed 28 February 2022).
Beasley CM, Sutton VK, Hamilton SH, Walker DJ, Dossenbach M, Taylor CC, Alaka KJ, Bykowski D, Tollefson GD. A double-blind, randomized, placebo-controlled trial of olanzapine in the prevention of psychotic relapse. J Clin Psychopharmacol, 2003; 23:582–94. CrossRef
Bhana N, Foster RH, Olney R, Plosker GL. Olanzapine: an updated review of its use in the management of schizophrenia. Drugs, 2001; 61:111–61. CrossRef
Chong HY, Teoh SL, Wu DB, Kotirum S, Chiou CF, Chaiyakunapruk N. Global economic burden of schizophrenia: a systematic review. Neuropsychiatr Dis Treat, 2016; 12:357–73. CrossRef
Gomez JC, Sacristan JA, Hernandez J, Breier A, Ruiz-Carrasco P, Anton-Saiz C, Fontova-Carbonell E. The safety of olanzapine compared with other antipsychotic drugs: results of an observational prospective study in patients with schizophrenia (EFESO Study). Pharmacoepidemiologic Study of Olanzapine in Schizophrenia. J Clin Psychiatry, 2000; 61:335–43. CrossRef
Grunder G, Heize M, Cordes J, Muhlbauer B, Juckel G, Schulz C, Ruther E, Timm J. Effects of first-generation antipsychotics versus second-generation antipsychotics on quality of life in schizophrenia: a double-blind, randomised study. Lancet Psychiatry, 2016; 3:717–29. CrossRef
Hamilton SH, Revicki DA, Edgell ET, Genduso LA, Tollefson G. Clinical and economic outcomes of olanzapine compared with haloperidol for schizophrenia. Results from a randomised clinical trial. Pharmacoeconomics, 1999; 15:469–80. CrossRef
Hayhurst KP, Massie JA, Dunn G, Lewis SW, Drake RJ. Validity of subjective versus objective quality of life assessment in people with schizophrenia. BMC Psychiatry, 2014; 14:365. CrossRef
Indonesian Ministry of Health. Report of Basic Health Research, 2018. Available via http://labmandat.litbang.depkes.go.id/images/download/laporan/RKD/2018/Laporan_Nasional_RKD2018_FINAL.pdf (Accessed 1 April 2020).
Institution of Health Metrics and Evaluation. Global health data exchange, 2019. Available via http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/27a7644e8ad28e739382d31e77589dd7 (Accessed 25 September 2021).
Jin H, Mosweu I. The societal cost of schizophrenia: a systematic review. Pharmacoeconomics, 2017; 35:25–42. CrossRef
Kementerian Kesehatan Republik Indonesia [Indonesian Ministry of Health]. Pedoman Nasional Pelayanan Kedokteran Jiwa [National Guideline on Psychiatric Disease Management]. Kementerian Kesehatan Republik Indonesia, [Indonesian Ministry of Health], Jakarta, Indonesia, 2015.
Krejcie RV, Morgan DW. Determining sample size for research activities. Educ Psychol Measure, 1970; 30:607–10. CrossRef
Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet, 2009; 373:31–41. CrossRef
Montoya A, Valladares A, Lizan L, San L, Escobar R, Paz S. Validation of the Excited Component of the Positive and Negative Syndrome Scale (PANSS-EC) in a naturalistic sample of 278 patients with acute psychosis and agitation in a psychiatric emergency room. Health Qual Life Outcomes, 2011; 9:18. CrossRef
Muhareni D, Aanggriani Y, Purba FD. Analisis Efektivitas Biaya Terapi Olanzapin dan Kombinasi Haloperidol dengan Diazepam secara Intramuskular pada Pasien Rawat Inap Skizofrenia Fase Akut di RSKD Duren Sawit [Cost effective Analysis of Intramuscular Olanzapine and Combination of Intramuscular Haloperidol with Diazepam in Acute Phase Schizophrenia Inpatients in Duren Sawit Psychiatric Hospital]. Majalah Farmasi dan Farmakologi, 2021; 25:28–31.
National Institute for Health and Care Excellence. Psychosis and schizophrenia in adults: prevention and management, 2014. Available via https://www.nice.org.uk/guidance/cg178/resources/psychosis-and-schizophrenia-in-adults-prevention-and-management-pdf-35109758952133 (Accessed 16 July 2019).
Pinem VE. Perbandingan Olanzapin Intramuskular dan Haloperidol Intramuskular dalam penanganan Agitasi pada Pasien Skizofrenik [Comparison of Intramuscular Olanzapin and Intramuscular Haloperidol In Management of Agitation in Schizophrenia]. Postgraduate Thesis, University of North Sumatra, 2010.
Purba FD, Hunfeld JAM, Iskandarsyah A, Fitriana TS, Sadarjoen SS, Ramos-Goni JM, Passchier J, Busschbach JJV. The Indonesian EQ-5D-5L Value Set. PharmacoEconomics, 2017; 35:1153–65. CrossRef
Rascati KL. Essentials of pharmacoeconomics. Lippincott Williams & Wilkins, Philadelphia, PA, 2009.
Remington G, Addington D, Honer W, Ismail Z, Raedler T, Teehan M. Guidelines for the pharmacotherapy of schizophrenia in adults. Can J Psychiatr, 2017; 62:604–16. CrossRef
Ringen PA, Engh JA, Birkenaes AB, Dieset I, Andreassen OA. Increased mortality in schizophrenia due to cardiovascular disease—a non-systematic review of epidemiology, possible causes, and interventions. Front Psychiatr, 2014; 5:137. CrossRef
Rosenheck R, Perlick D, Bingham S, Liu-Mares W, Collins J, Warren S, Leslie D, Allan E, Campbell EC, Caroff S, Corwin J, Davis L, Douyon R, Dunn L, Evans D, Frecska E, Grabowski J, Graeber D, Herz L, Kwon K, Lawson W, Mena F, Sheikh J, Smelson D, Smith-Gamble V. Effectiveness and Cost of Olanzapine and Haloperidol in the Treatment of Schizophrenia: a randomized controlled trial. JAMA, 2003; 290:2693–702. CrossRef
Sacristan JA, Gomez JC, Montejo AL, Vieta E, Gregor KJ. Doses of olanzapine, risperidone, and haloperidol used in clinical practice: results of a prospective pharmacoepidemiologic study. EFESO Study Group. Estudio Farmacoepidemiologico en la Esquizofrenia con Olanzapina. Clin Ther, 2000; 22:583–99. CrossRef
The American Psychiatric Association. Practice guideline for the treatment of patients with schizophrenia, 2020. Available via https://psychiatryonline.org/doi/book/10.1176/appi.books.9780890424841. CrossRef
Tollefson GD, Beasley CM, Tran PV, Street JS, Krueger JA, Tamura RN, Graffeo KA, Thieme ME. Olanzapine versus haloperidol in the treatment of schizophrenia and schizoaffective and schizophreniform disorders: results of an international collaborative trial. Am J Psychiatry, 1997; 154:457–65. CrossRef
Tunis SL, Johnstone BM, Gibson PJ, Loosbrock DL, Dulisse BK. Changes in perceived health and functioning as a cost-effectiveness measure for olanzapine versus haloperidol treatment of schizophrenia. J Clin Psychiatry, 1999; 60(Suppl 19):38–45; discussion 46.
Ucok A, Gaebel W. Side effects of atypical antipsychotics: a brief overview. World Psychiatr, 7:58–62. CrossRef
Vos T, Barber R, Bell B, Bertozzi-Villa A, Biryukov S, Bolliger I, Charlson F, Davis A, Degenhards L, Dicker D, Duan L, Erskine H, Feigin V, Ferrari A, Fitzmaurice C, Fleming T, Graetz N, Guinovart C, Haagsma J, Yadav A. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet, 2015; 386:743–800. CrossRef
Wei Xin Chong J, Hsien-Jie ET, Chong CE, Ny Y, Wijesinghe R. Atypical antipsychotics: a review on the prevalence, monitoring, and management of their metabolic and cardiovascular side effects. Ment Health Clin, 2016; 6:178–84. CrossRef
World Health Organization. Mental disorders, 2018. Available via https://www.who.int/news-room/fact-sheets/detail/mental-disorders (Accessed 29 June 2020).
Zhang G, Sackman RW. The role of serotonin 5-HT2A receptors in memory and cognition. Front Pharmacol, 2015; 6:225. CrossRef