INTRODUCTION
Acamprosate calcium (calcium 3-acetamidopropane-1-sulfonate; Figure 1) is a calcium salt of acetylhomotaurine which is used for the treatment of alcohol dependence, which acts by stabilizing the chemical balance in the central nervous system, by blocking glutaminergic N-methyl-D-aspartate receptors and activation of gamma-aminobutyric acid (GABA) type A receptors (Rhee et al., 2008a; Saivin et al., 1998; Zornoza et al., 2003). This drug is available since 1989 in the tablet strength of 333 mg (Lipha Pharmaceuticals Inc., 2002). It is approved by the US Food and Drug Administration (FDA) as an antidipsotropic agent and is used to decrease alcohol hankering (Espino and Cruz, 2005). The chemical formula of acamprosate calcium is C10H20N2O8S2 Ca with molecular weight 400.48 g/mol. The physical properties of this drug are white and odorless powder, which is freely soluble in water and insoluble in organic solvents like ethanol and dimethylformamide.
 | Figure 1. Acamprosate calcium. [Click here to view] |
The literature survey reports on the capillary zone electrophoresis method (Blanchin et al., 2000; Fabre et al., 1999). The Liquid Chromatography mass spectrometry (LCMS) method for bioanalytical analysis, LC-fluorometric, and electrochemical detection was reported in human plasma, dog plasma, and urine (Chabenat et al., 1987, 1989; Ghosh et al., 2011; Girault et al., 1990; Hammarberg et al., 2010; Kanala et al., 2013; Rhee et al., 2008a; Rhee et al., 2008b; Xu et al., 2009). A few methods are developed and validated for organic acids by conductometric titration method (Umarov et al., 2020). Hence, no work has been reported on the electrical conductivity method for the quantitative estimation of acamprosate calcium. In this research, a new cost-effective, precise, and regression co-efficient on electrical conductivity method on acamprosate calcium in pure and marketed formulation is reported.
MATERIALS AND METHODS
Instruments and apparatus
An electrical conductivity meter (ELICO CM 183EC-TDS analyzer version 2.3) was used for measuring electrical conductivity of solutions. An electronic analytical weighing balance (SARTORIUS) was used for weighing the analyte.
Reagents and chemicals
The active pharmaceutical ingredient of 98% purity of acamprosate calcium was procured from BMR Pharma and Chemicals, Hyderabad; tablet formulation was purchased from a local market of Belgaum, India. Distilled water was collected from Direct-Q UV water purification system from KLE College of Pharmacy.
Method development
The method was developed using an electrical conductivity meter using distilled water as a solvent. Various concentrations of acamprosate calcium in distilled water were prepared and electrical conductivity was measured. Different concentrations of analyte were observed and showed different values of electrical conductivity. In order to test the suitability of the developed method, the commercial tablet formulation of acamprosate calcium was analyzed.
Preparation of stock solution and working standard solution
Fifty milligram of the active pharmaceutical ingredient was dissolved in 50 ml of a volumetric flask and the final volume was made with distilled water (stock solution = 1,000 μg/ml); from the above solution, 0.5–2.5 ml was pipetted and transferred in 10 ml of a volumetric flask and the final volume was made with distilled water to obtain 50–250 μg/ml of pure acamprosate calcium.
Method validation
As per the International Conference on Harmonization (ICH) Guidelines Q2 (R1), the method was validated for various parameters like specificity, linearity, limit of detection (LOD), limit of quantification (LOQ), precision, ruggedness, accuracy, and assay.
SPECIFICITY
The specificity of the method was studied by measuring the conductance of a solvent as blank and the prepared standard solution of acamprosate calcium (50 μg/ml).
LINEARITY
The linearity method was developed by measuring the electrical conductivity of different concentrations of acamprosate calcium. Dilutions containing 50–250 μg/ml acamprosate calcium were prepared and electrical conductivity was measured using electrical conductometer. The calibration curve was obtained by plotting the concentration versus electrical conductivity (μs), and the correlation coefficient (r2) was found to be 0.9999.
LIMIT OF DETECTION/LIMIT OF QUANTIFICATION
LOD is the lowest amount of analyte which can be detected but not quantified. It can be determined using following formula:
LOQ is the lowest amount of analyte which can be quantitatively determined as precision using the following formula:
where σ = standard deviation;
S = Slope of the calibration curve.
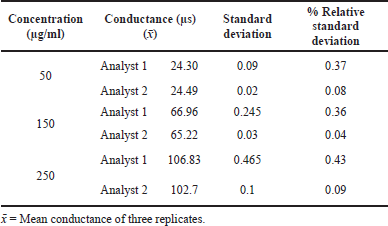
PRECISION
The precision was carried out in terms of intraday and interday. The electrical conductivity of three replicate solutions of acamprosate calcium at three different concentrations was measured, and % Relative Standard Deviation (RSD) values of each solution for its electrical conductivity were measured.
RUGGEDNESS
Ruggedness was carried out by changing the analyst to check the reproducibility of standard results and the %RSD was found to be less than 2%.
ACCURACY
Accuracy was found by means of recovery experiments, by the determination of %mean recovery of sample at three different levels: 50%, 100%, and 150%. At each level, three determinations were carried out and %mean recovery was calculated.
ASSAY
Twenty tablets of acamprosate calcium (333 mg label claim) were taken and powdered. A measure equivalent to 73.19 mg was weighed and added to a 50 ml volumetric flask, and the final volume was made up to 50 ml with diluents and shaken for 5 minutes. The solution was filtered by Whatman filter paper. From the filtered solution, 0.5–2.5 ml solution was added to 10 ml volumetric flask to make a final volume up to 10 ml using distilled water.
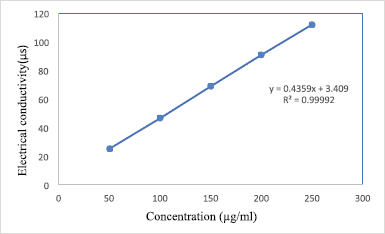 | Figure 2. Standard calibration curve of acamprosate calcium at 250 V. [Click here to view] |
RESULT
Specificity
The proposed method is specific as the conductance of water as a blank was found to be 1.75 μs and acamprosate calcium (50 μg/ml) solution showed a conductance of 25.28 μs. The results of the analysis were found to be specific and selective.
Linearity
The drug shows a linear relationship between electrical conductivity (μs) verses drug concentration level. The results are shown in Figure 2. The equation of the straight line for acamprosate calcium is y = 0.4359x + 3.409 and the regression coefficient is R2 = 0.9999.
LIMIT OF DETECTION (LOD)/LIMIT OF QUANTIFICATION (LOQ)
The LOD value obtained was 6.05 μg/ml and LOQ value was found to be 18.34 μg/ml. Both results are in the acceptance range.
PRECISION
The precision indicating the value was precise and low, and %RSD is <2%. The results are given in Tables 1 and 2.
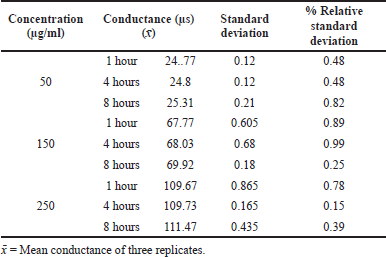 | Table 1. Intraday precision results of acamprosate calcium. [Click here to view] |
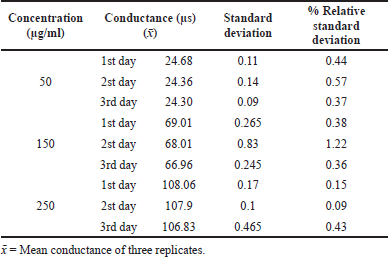 | Table 2. Interday precision results of acamprosate calcium. [Click here to view] |
RUGGEDNESS
Ruggedness was carried out at different conditions to check the expected values from one analyst to another and the %RSD shows less than 2%. The data of ruggedness are recorded in Table 3.
ACCURACY
Accuracy and recovery studies were carried out and the %mean recovery of the sample was recorded at three different levels: 50%, 100%, and 150%. Individual recovery and %mean recovery were found to be greater than 98%, which indicates that the method development is accurate and recovery values were in the range. The %mean recovery values are shown in Table 4.
ASSAY
The assay was carried out by using tablet formulation of label claim 333 mg (ACAMPROL) and the values were obtained in an acceptable range. The results were in the range of 102%–104%.
DISCUSSION
In the present research work, an attempt has been made to develop a simple conductometric method. This method was found to be economic, simple, and rugged for estimation of acamprosate calcium. The proposed method results and outcomes are compared with other research works published earlier on acamprosate calcium.
 | Table 3. Results of ruggedness for acamprosate calcium. [Click here to view] |
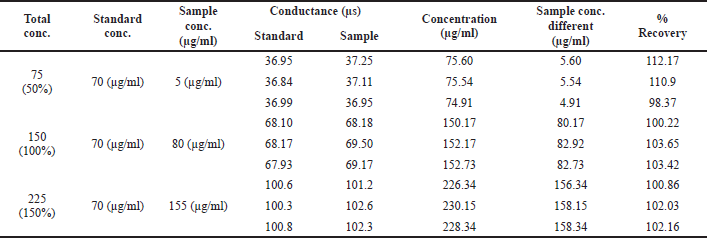 | Table 4. Results of accuracy studies for acamprosate calcium. [Click here to view] |
The literature survey reveals that acamprosate calcium can be analyzed by few chromatographic methods (Chabenat et al., 1987; Girault et al., 1990; Hammarberg et al., 2010). The published work has some limitation like use of costly solvents, hazardous chemicals, costly instruments, non-robust method, and longer time of analysis. Hence, in our proposed method, we have used water as a solvent which is universal and cheaper and the instrument cost is low. It is very simple in carrying out and is cost-effective for the method developed and analysis.
All the values obtained in the method were found to be within the acceptance range as per the ICH norms.
UV-spectrophotometric trials were carried out with different solvents such as menthol, water, etc., but they did not get the peaks at the acceptable range and it showed less than 205 nm because the acamprosate calcium drug does not contain chromophore. Furthermore, it was carried on electrical conductometric meter and the linear regression coefficient value was found to be 0.9999, Acamprosate calcium contains a cation charge and the solvent used was the distilled water. The method was developed on an electrical conductometric meter as this method is cheap, cost-effective, and no work has been reported on acamprosate calcium. The current method development and validation represent an alternative method in the laboratory which can be employed for routine analysis in the pharmaceutical industry.
CONCLUSION
The present research is a cost-effective, simple, and accurate electrical conductivity method for quantity estimation of acamprosate calcium in tablet dosage form developed as per the ICH guidelines. The method is economic, rapid, and does not need any sophisticated instruments. Hence, it may be employed for routine quality control analysis of acamprosate calcium in Active Pharmaceutical Ingrident (API) and marketed formulation.
ACKNOWLEDGMENTS
The authors are thankful to Principal Dr. S. S. Jalalpure for his support and guidance and to the Department of Pharmaceutical Chemistry, KLE College of Pharmacy. The authors are also thankful to Mr. Nikhil Gawas, Mr. Onkar S. Supe, and Ms. Sushmita Hiremath for help during the research work.
ETHICAL APPROVAL
Not applicable.
CONFLICT OF INTEREST
The authors declare that there is no conflict of interests.
FUNDING
This work did not receive any funds to declare from any national and international agency.
REFERENCES
Blanchin MD, Baalbaki B, Bosc N, Fabre H. Short-end injection technique in capillary electrophoresis for dissolution testing of tablets. Anal Chim Acta, 2000; 415(12):67–73. CrossRef
Chabenat C, Ladure P, Blanc-Continsouza D, Boismare F, Boucly P. Determination of calcium acetylhomotaurinate by liquid chromatography with fluorimetric and electrochemical detection. J Chromatogr, 1987; 414:417–22. CrossRef
Chabenat C, Ladure P, Moore N, Boucly P, Boismare F. Application of an analytical method `to calcium acetylhomotaurinate determination in urine. Arzneimittelforschung, 1989; 39(11):1413–4.
Espino D, Cruz MP. Acamprosate calcium (Campral®): an effective treatment for maintaining abstinence in alcohol-dependent patients in combination with psychosocial support. Pharm Ther, 2005; 30(1):497–505.
Fabre H, Perrin C, Bosc N. Determination of homotaurine as impurity in calcium acamprosate by capillary zone electrophoresis. J Chromatogr A, 1999; 853(1–2):421–30. CrossRef
Ghosh C, Jha V, Shinde CP, Chakraborty BS. A LC-MS analysis of acamprosate from humanplasma: pharmacokinetic application. Drug Test Anal, 2011; 3(10):735–42. CrossRef
Girault J, Gobin P, Fourtillan JB. Determination of calcium acetyl homotaurinate in human plasma and urine by combined gas chromatography-negative-ion chemical ionization mass spectrometry. J Chromatogr, 1990; 530:295–305. CrossRef
Hammarberg A, Beck O, Eksborg S, Jayaram-Lindström N, Lindefeldt A, Andersson M, Brundin L, Reid MS, Franck J. Acamprosate determinations in plasma and cerebrospinal fluid after multiple dosing measured by liquid chromatography–mass spectroscopy: a pharmacokinetic study in healthy volunteers. Ther Drug Monit, 2010; 32(4):489–96. CrossRef
ICH Guidelines. Validation of analytical procedures: text and methodology. International Conference on Harmonization (ICH) Q2 (R1), Geneva, Switzerland, p 10, vol. 11, 2005.
Kanala KM, Chandu BR, Hwisa NT, Khagga M, Katakam P, Challa BR. Quantification of acamprosate in human plasma By LC-ESI-MS/MS with solid phase extraction: application to a bioequivalence study. J Pharm Res, 2013; 7(5):389–96. CrossRef
Lipha Pharmaceuticals Inc. Briefing focument for acamprosate 333 mg tablets. Food and Drug Administration, Washington, DC, pp 1–311, 2002. CrossRef
Rhee YS, Park JH, Park S, Park CW, Ha JM, Jeong KW, Lee DS, Park ES. Analysis of acamprosate in beagle dog plasma By LC-MS-MS. Arch Pharm Res, 2008a; 31(8):1035–39. CrossRef
Rhee YS, Park S, Lee TW, Park CW, Nam TY, Oh TO, Jeon JW, Lee DS, Park ES. Investigation of the relationship between in vitro and in vivo release behaviors of acamprosate from enteric-coated tablets. Arch Pharm Res, 2008b; 31(6):798–804. CrossRef
Saivin S, Hulot T, Chabac S, Potgieter A, Durbin P, Houin G. Clinical pharmacokinetics of acamprosate. Clin Pharmacokinet, 1998; 35(5):331–45. CrossRef
Umarov UA, Maslov OY, Kolisnyk SV, Fathullaeva ?. Development and validation of the conductometric titration method of quantitative determination of free organic acids in the anise fruits. Eur J Mol Clin Med, 2020; 7(3):3874–83. CrossRef
Xu F, Qing Y, Shang B, Liang M, Zou Y, Xu G. Pharmacokinetics of acamprosate calcium in healthy Chinese subjects after oral administration of three dosage levels. Arzneimittelforschung, 2009; 59(12):631–4. CrossRef
Zornoza T, Cano MJ, Polache A, Granero L. Pharmacology of acamprosate: an overview. CNS Drug Rev, 2003; 9(4):359–74. CrossRef