Rapid high Performance liquid Chromatography- Tandem mass Spectrometry Method For Quantitation of Milnacepran in Human Plasma
Umesh Thorat
DOI: 10.7324/JAPS.2013.3427Pages: 146-151

Fast and simple multi-element determination of essential and toxic metals in whole blood with quadrupole ICP-MS
Carmen Silvia Kira, Alice Momoyo Sakuma, Nelson da Cruz Gouveia
DOI: 10.7324/JAPS.2014.40507Pages: 039-045

A Simplified Liquid Chromatography-Mass Spectrometry Method for Simultaneous Determination of Pyrimethamine, Sulphadoxine and Artesunate in Human Plasma
S M Sandhya, P S Shijikumar
DOI: 10.7324/JAPS.2015.50618Pages: 109-114

Simultaneous determination of ciprofloxacin hydrochloride and metronidazole in spiked human plasma by ultra performance liquid chromatography-tandem mass spectroscopy
Ramzia El-bagary, Asmaa Ahmed El-Zaher, Ehab Elkady, Asmaa Abdelkerim Mandour
DOI: 10.7324/JAPS.2016.60307Pages: 041-047

Bioanalytical Method Development and Validation for the Determination of Levocetirizine in Pharmaceutical Dosage Form and Human Plasma by RP-HPLC
Nilesh Jain, Deepak Kumar Jain, Ruchi Jain, Vijay Kumar Patel, Preeti Patel, Surendra Kumar Jain
DOI: 10.7324/JAPS.2016.601008Pages: 063-067

A Liquid Chromatography/Tandem Mass Spectrometric Method for Determination of Captopril in Human Plasma: Application to a Bioequivalence Study
Eman S. Elzanfaly, Hanan A. Merey
DOI: 10.7324/JAPS.2017.70202Pages: 008-015

Simultaneous quantification of ramipril, glimepiride and metformin in human plasma by ultra-performance liquid chromatography – tandem mass spectrometry
Eman S. Elzanfaly, Sherif A. Abdel-Gawad
DOI: 10.7324/JAPS.2017.70711Pages: 062-069

Antidiabetic and antihyperlipidemic activity of Euphorbia thymifolia L. extracts on streptozotocin-nicotinamide induced type 2 diabetic rats
Ghanshyam R. Parmar, Kilambi Pundarikakshudu, R. Balarama
DOI: 10.7324/JAPS.2017.70811Pages: 078-084

Estimation of Bosentan Monohydrate in Male Rabbit Plasma by using RP-HPLC Method
Revathi Mannam, Indira Muzib Yallamalli
DOI: 10.7324/JAPS.2017.71116Pages: 106-109

Validation of a simple isocratic HPLC-UV method for rifampicin and isoniazid quantification in human plasma
Laura Carolina Luciani-Giacobbe, María Laura Guzman, Rubén Hilario Manzo, María Eugenia Olivera
DOI: 10.7324/JAPS.2018.8715Pages: 093-099

Efficient validated method of UPLC-MS/MS to determine curcumin in rat plasma and ovarium
Wenny Trias Ramadanty, Wawaimuli Arozal, Melva Louisa, Vivian Soetikno, Sigit Purbadi, Priyanto Priyanto
DOI: 10.7324/JAPS.2019.90109Pages: 058-065

Papain-mediated reduction of MTT is an artifact but not an indicator of cell apoptosis in Saccharomyces cerevisiae
Shafaque Asif, Gurjeet Kaur, Usha Pendurthi, Vineet Awasthi
DOI: 10.7324/JAPS.2019.90917Pages: 119-124

Impact of sample storage conditions on gliclazide quantification in rat plasma by UHPLC/UV method: storage recommendation and pharmacokinetic application
Nesrin F. Taha, Ebtesam W. Elsayed, Ahmed A. El-Ashmawy, Aya R. Abdou, Laila H. Emara
DOI: 10.7324/JAPS.2021.110305Pages: 046-053

Morphological and immunohistochemical effect of cerium dioxide nanoparticles on reparative osteogenesis of the jaw bones
Ernest Bazikyan, Andrey Chunikhin, Grigory Volozhin, Knarik Abraamyan, Vladimir Ivanov, Mariya S. Zudina
DOI: 10.7324/JAPS.2021.120217Pages: 165-171
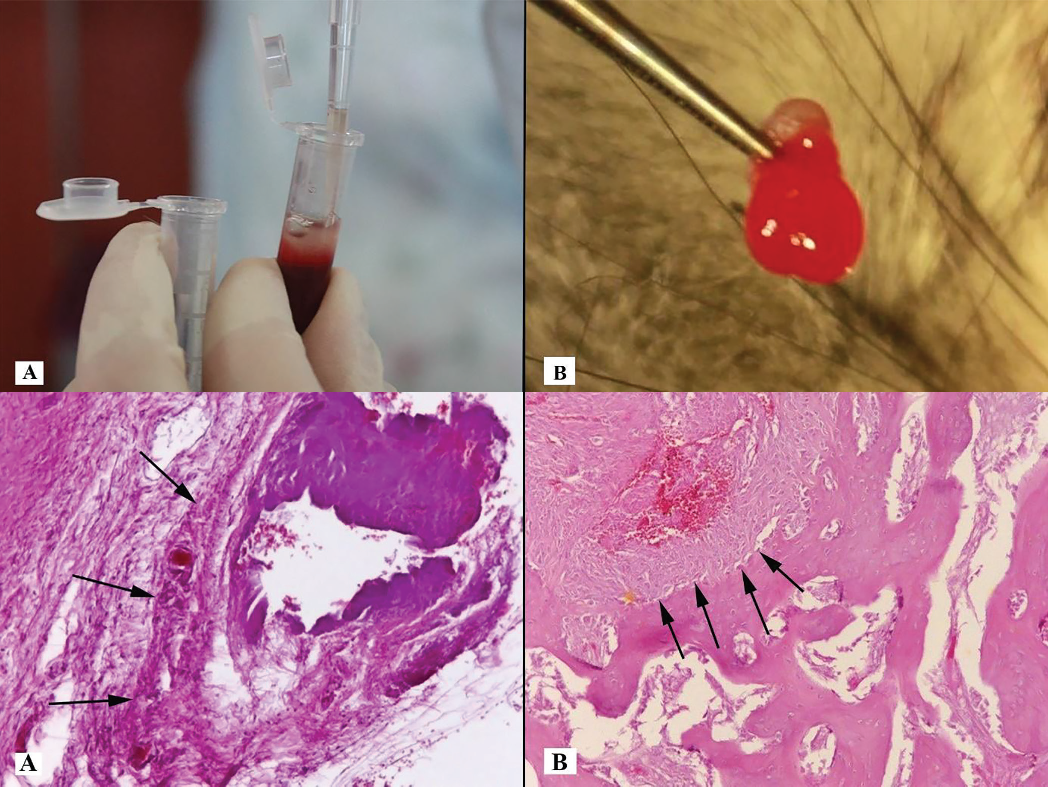
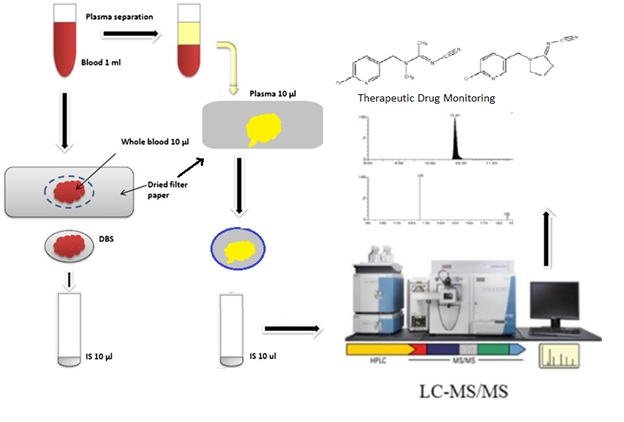
Dried spot sample and its drug detection using LC-MS/MS: Trends and advances in matrix collection and bioanalytics
Arpita Sathyanarayanan, Divyashree Mysore Somashekara
DOI: 10.7324/JAPS.2022.120602Pages: 011-022
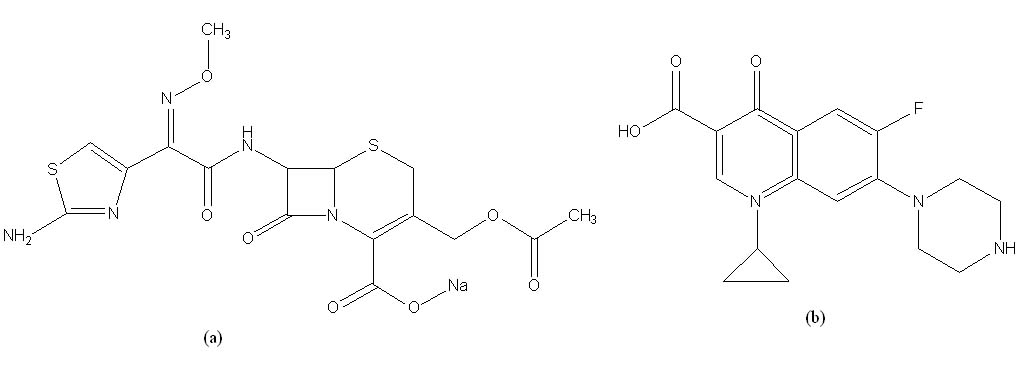
Bioanalytical and validation high-performance liquid chromatography method for simultaneous quantification cefotaxime and ciprofloxacin in human plasma
Luh Putu Mirah Kusuma Dewi, Djoko Wahyono, Ika Puspitasari, Rizka Humardewayanti, Endang Lukitaningsih
DOI: 10.7324/JAPS.2024.153492Pages: 221-229
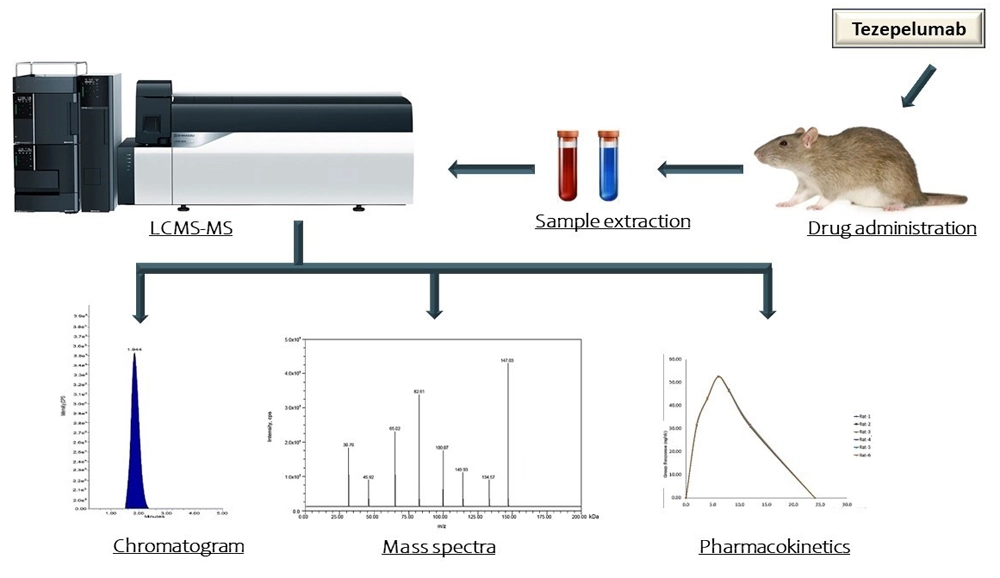
A novel LC-MS/MS method development and validation for the determination of tezepelumab in rat plasma and its application in rat pharmacokinetic studies
Madhusudhan Reddy Nimmakayala, Deepti Kolli, Murali Prakash Jatla
DOI: 10.7324/JAPS.2024.171688Pages: 154-162
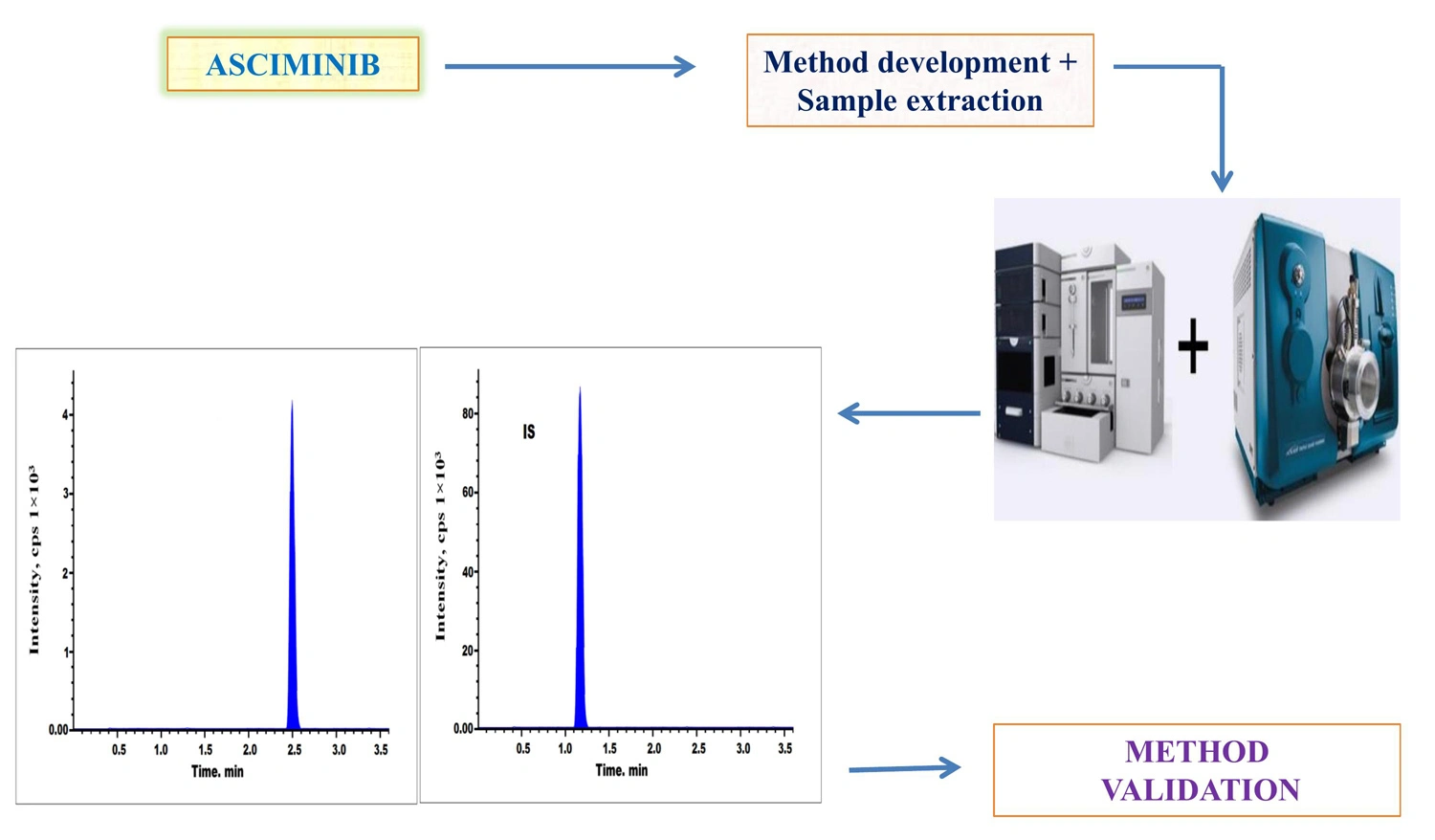
Quantification of asciminib by LC-MS/MS method in human plasma: Validation and stability studies
Hema, Naresh Panigrahi
DOI: 10.7324/JAPS.2024.163326Pages:
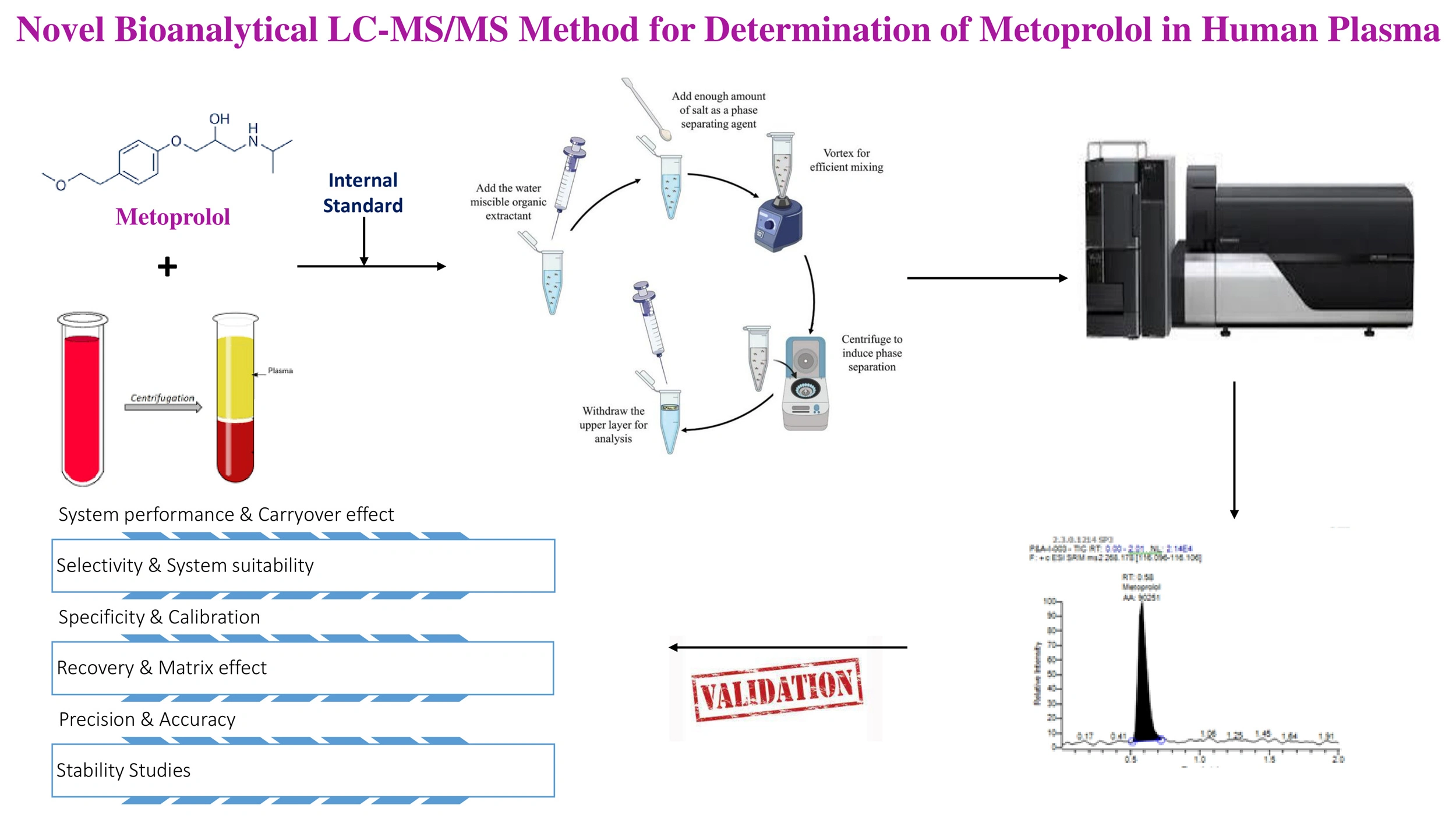
Novel bioanalytical LC-MS/MS method for determination of metoprolol in human plasma
Lakshmana Rao Atmakuri, Raveesha Peeriga, Shabana Begum, Narender Gaddamedi, Bhaskar Vallamkonda, Anupama Baratam
DOI: 10.7324/JAPS.2024.657641Pages: 131-138
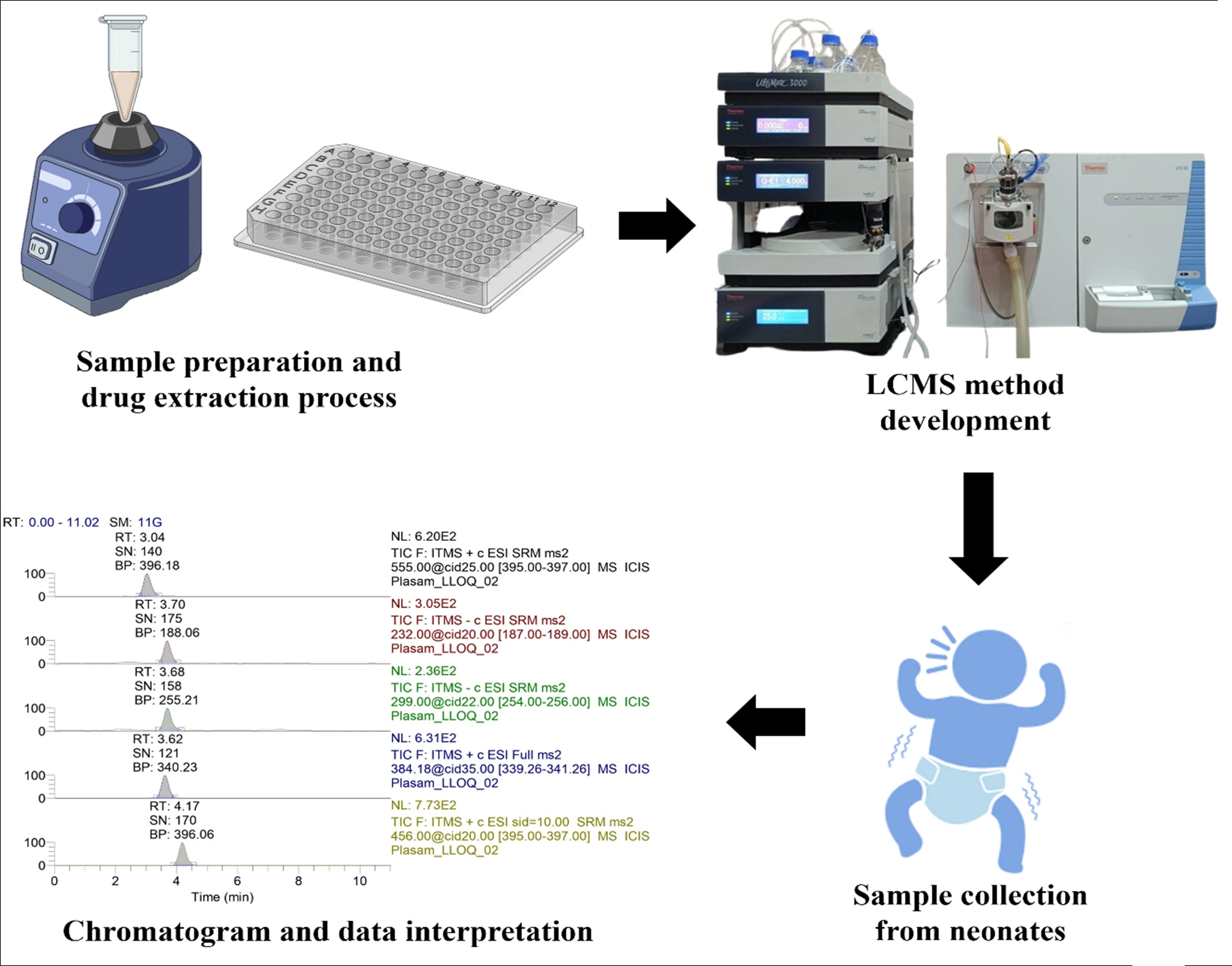
A comparative evaluation of the plasma and DBS-based LC-MS/MS methods for the simultaneous analysis of nine antibiotics for application to pharmacokinetic evaluations and precision dosing in neonates
Bhim Bahadur Chaudhari, Leslie E. Lewis, M. Surulivel Rajan, Ashutosh Gupta, Moumita Saha, Shivani Kunkalienkar, Sudheer Moorkoth
DOI: 10.7324/JAPS.2025.214611Pages: 129-142
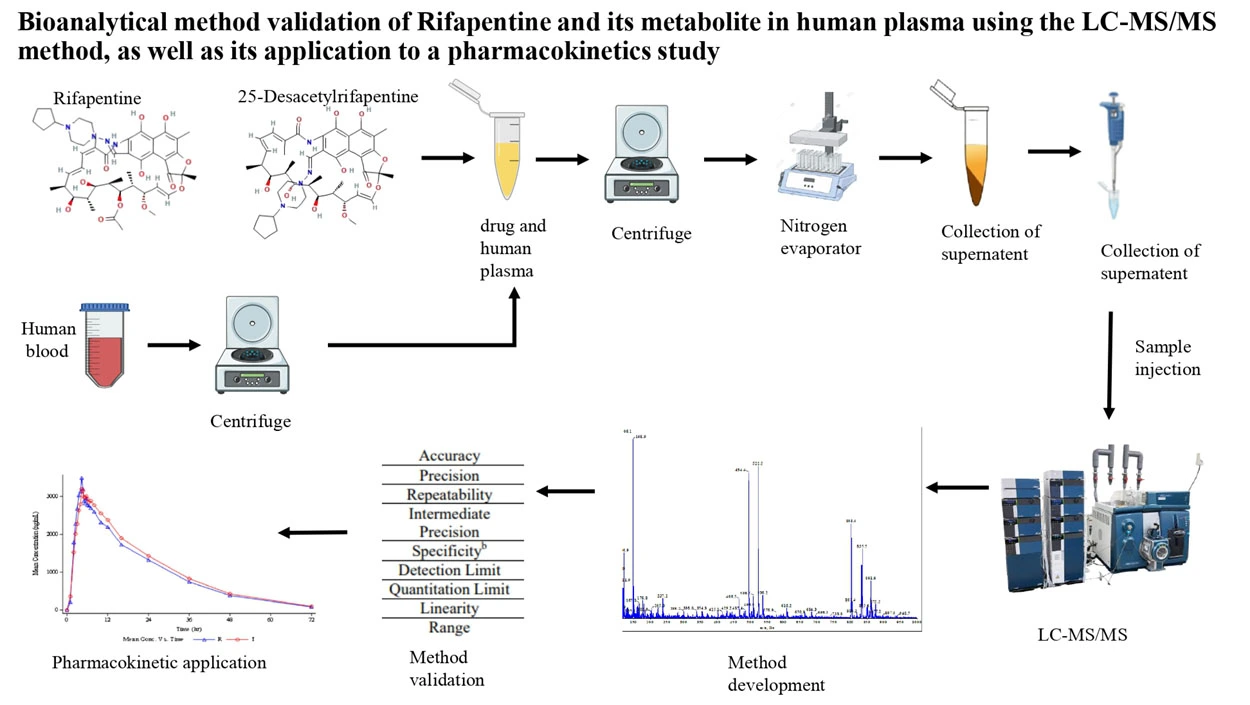
Bioanalytical method validation of rifapentine and its metabolite in human plasma using the LC-MS/MS method, as well as its application to a pharmacokinetics study
Rutuja Parghale, Radhika Inapakolla, Vijay Durga Rao Tikka, Rajesh Kumar Suvvaru, Pradnya Date, Vaishnavi Gawade, Ande Anil, Swati Changdeo Jagdale
DOI: 10.7324/JAPS.2025.210914Pages: 179-201
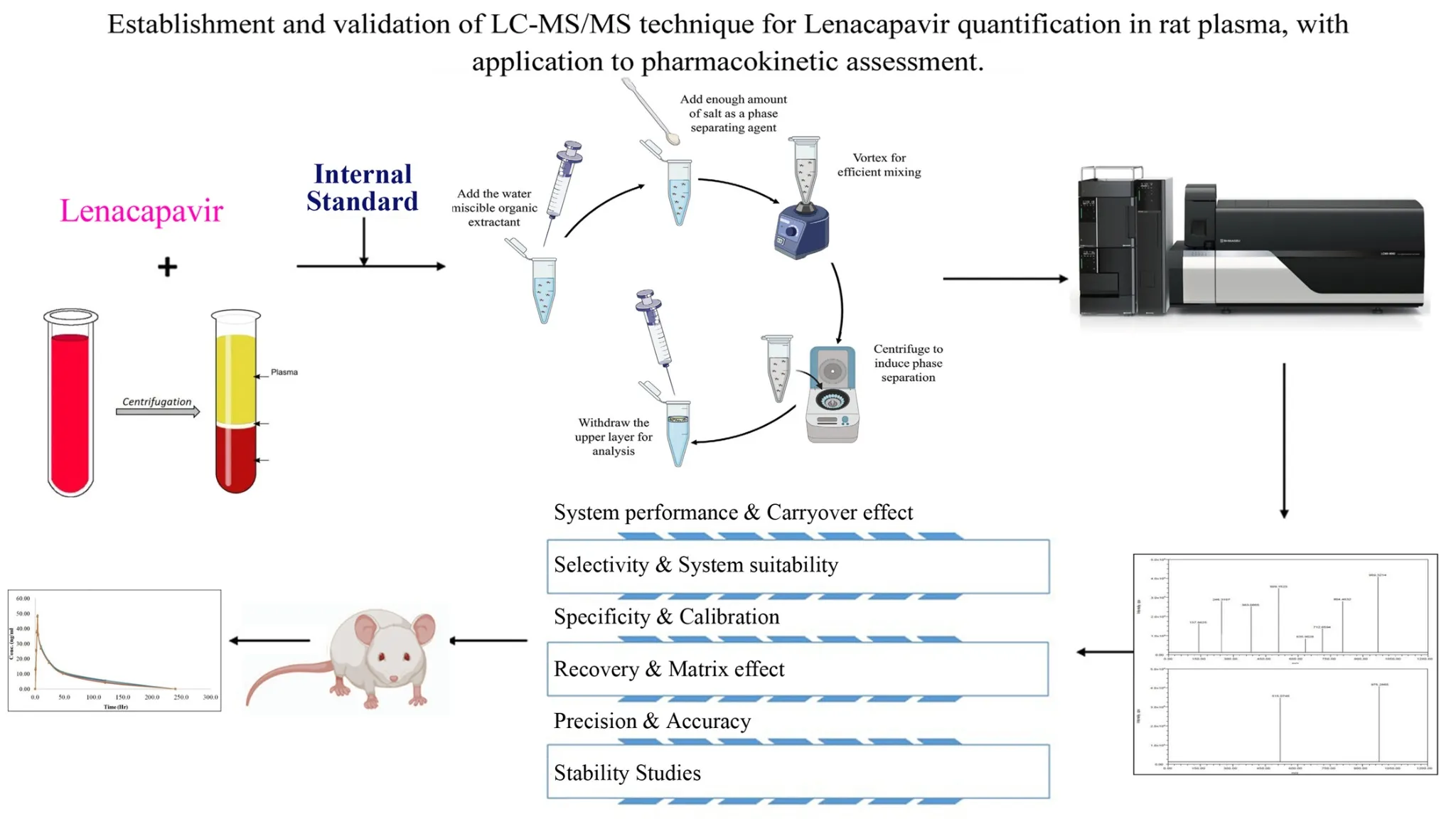
Establishment and validation of LC-MS/MS technique for Lenacapavir quantification in rat plasma, with application to pharmacokinetic assessment
Edward Raju Gope, Srikanth Pottendla, Suneetha Yaparthi
DOI: 10.7324/JAPS.2025.229006Pages: 112-120
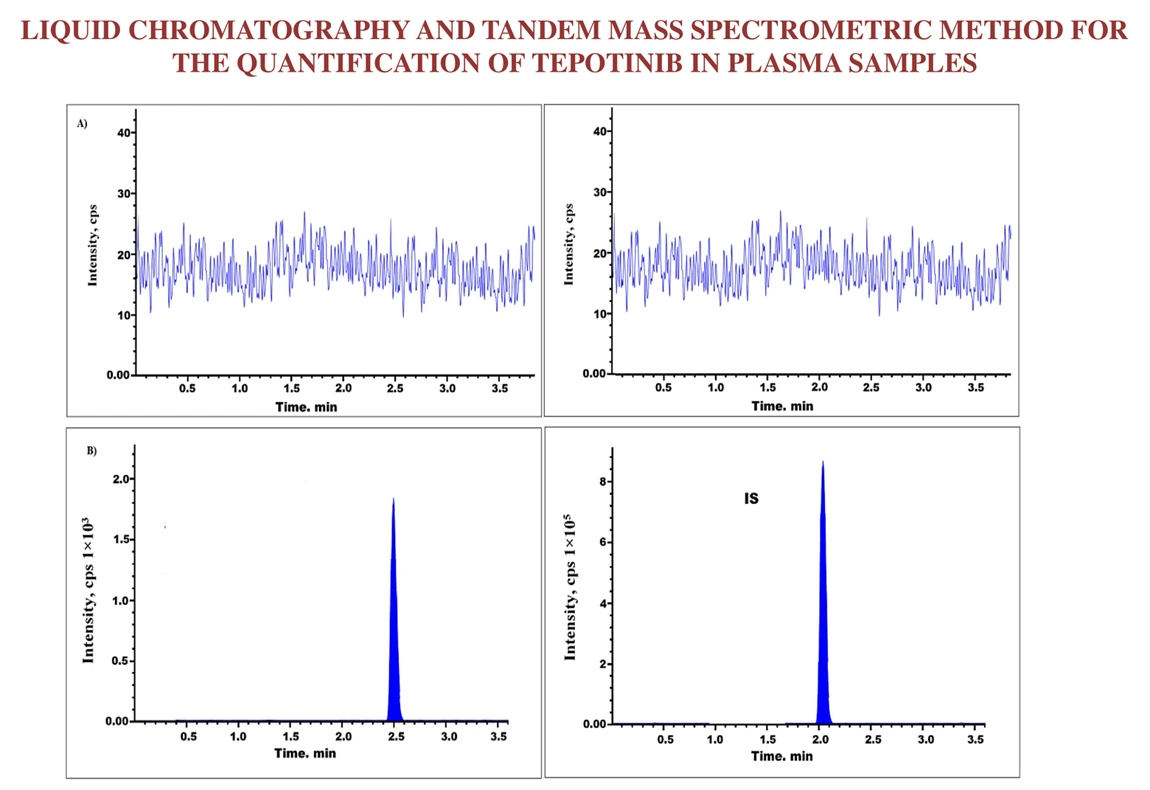
Liquid chromatography and tandem mass spectrometric method for the quantification of Tepotinib in plasma samples
Arjun Siliveri, Kavitha Pingili
DOI: 10.7324/JAPS.2025.206883Pages: