INTRODUCTION
Tezepelumab (TZP), marketed under the trade name Tezspire, is a human monoclonal antibody used to treat asthma [1–3]. This medication works by blocking thymic stromal lymphopoietin, [4,5] an epithelial mediator believed to contribute to the onset and aggravation of respiratory inflammation [6,7]. TZP has a molecular weight of 144,590.40 gmol−1 [8] and a chemical formula of C6400H9844N1732O1992S52, making it capable of targeting multiple inflammatory pathways implicated in the pathogenesis of asthma. According to clinical studies, TZP has demonstrated efficacy in reducing asthma exacerbations and improving lung function in patients with severe asthma [9,10]. The most frequent adverse effects associated with TZP therapy are pharyngitis and arthralgia, and the medication is administered via subcutaneous injection every 4 weeks [11,12]. TZP has been approved for use in the United States since 2021, and in Europe, since 2022, making it available in many countries [13,14].
Recent studies have suggested that TZP may have the potential to reduce airway smooth muscle mass and mucus production, which are significant contributors to the development of asthma symptoms [15]. However, further research is required to confirm these findings and understand the underlying mechanisms of TZP’s action in the airways. One of the major advantages of TZP is its ability to target multiple inflammatory pathways implicated in the pathogenesis of asthma, making it an attractive option for patients who have not responded well to other treatments. However, the high cost of TZP may limit its accessibility to patients, particularly in developing countries. In addition to its use in asthma, TZP is also being evaluated for its potential to treat other inflammatory diseases such as chronic obstructive pulmonary disease, atopic dermatitis, and eosinophilic esophagitis [16]. Moreover, TZP has shown a good safety profile in clinical trials, with adverse events reported being generally mild to moderate in severity [1]. Nevertheless, healthcare providers must monitor patients closely for potential side effects and take appropriate measures to manage any adverse reactions that may occur. Overall, TZP represents a significant advancement in the treatment of severe asthma, and ongoing research into its long-term safety and efficacy will continue to provide valuable insights into its potential use in other inflammatory diseases [9,10].
Bioanalysis is a critical process in drug development, where the quantitative measurement of a drug and its metabolites is necessary to evaluate its pharmacokinetic and pharmacodynamic properties [17]. TZP is a monoclonal antibody that has shown promising results in clinical trials for the treatment of asthma [1]. However, until now, no liquid chromatography-tandem mass spectrometry (LC-MS/MS) method has been available for the bioanalysis of TZP in any type of biological matrix. The development of a bioanalytical method for TZP is a significant milestone in the drug’s development. This new method will allow researchers to quantitatively measure TZP in various biological matrices, and plasma. This will facilitate the assessment of the drug’s pharmacokinetic properties, such as its absorption, distribution, metabolism, and elimination, which are crucial in determining its efficacy and safety.
Comprehensive extraction and sample preparation protocols have been meticulously established to guarantee precision and reliability in quantifying TZP levels in plasma. The LC-MS/MS instrumentation is fine tuned for both sensitivity and specificity, facilitating the detection of TZP at low concentrations, a critical factor in comprehending its pharmacological characteristics. The selection of internal standards (ISs) and calibration curves (CCs) is grounded in extensive literature reviews, ensuring alignment with best practices and industry standards (STDs). In the bioanalytical method for TZP, trastuzumab is employed as an IS, emphasizing the method’s precision and reliability. Trastuzumab, recognized for its application in breast cancer treatment, is selected strategically due to its chemical resemblance to TZP. This deliberate choice ensures that trastuzumab closely emulates the behavior of TZP during both extraction and analysis, enabling accurate compensation for variations in sample preparation, matrix effects, and instrumental conditions. The utilization of trastuzumab as an IS enhances the method’s specificity, contributing to the overall reliability of TZP quantification across diverse biological matrices. This careful selection aligns with established best practices, adding a layer of confidence to the bioanalytical method’s robustness for accurate and consistent TZP measurements.
Rigorous validation studies, adhering to regulatory guidelines, have been conducted to affirm the bioanalytical method’s robustness and reproducibility. Thorough evaluations of accuracy, precision, selectivity, and sensitivity provide a strong foundation for its widespread application. In addition, the method addresses potential interferences and matrix effects inherent in complex biological samples, underscoring a meticulous approach that enhances result reliability and contributes to a more accurate assessment of TZP’s pharmacokinetics. It is worth noting that the development of this bioanalytical method for TZP represents a considerable scientific achievement. The method will enable researchers to evaluate the drug’s pharmacokinetic properties, which will be essential in determining its safety and efficacy in clinical trials. It is hoped that the development of this bioanalytical method will accelerate the clinical development of TZP and pave the way for its approval as a new treatment option for asthma.
MATERIALS AND METHODOLOGY
Materials required
Chemicals and materials
Samples of TZP and trastuzumab from Biocon in Bangalore. All remaining ingredients, including LCMS grade formic acid (FA), methanol (MeOH), and acetonitrile (ACN), were purchased from Merck Chemical Division in Mumbai. The Merck (Manufacturer) Millipore Milli-Q water purification system’s water was utilized during the whole investigation.
Instrumentation
For analysis, a Waters, Alliance e-2695 version HPLC equipped with a column oven, auto sampler, and degasser was used. The SCIEX QTRAP 5500 mass spectrometer, which has an electrospray ionization interface, was connected to the HPLC system. The results from the chromatogram were interpreted using SCIEX software. The multiple reaction monitoring mode was adopted to record the transformation of protonated precursors to final ions at m/z 147.03 → 82.61 amu and 148.72 → 120.25 amu for sample and IS, correspondingly. The source-dependent variables that were retained for the sample and IS were as follows: GS1: 50.00 psi, GS2: 50.00 psi, IS voltage: 5,500.00 V, turbo heater temperature: 550.00°C, collision activation dissociation: 7.00 psi, and curtain gas: 20.00 psi. The compound-dependent factors such as decluttering potential were adjusted at 40.00 V, and entrance potential, collision energy, and cell exit potential were 10.00 V, 15.00 V, and 7.00 V, accordingly.
Methodology
Preparation of STD stock and plasma samples
The diluting solvent (MeOH: 0.1% FA 40:60 v/v) was used to create successive dilution series from stock solutions of TZP (6 ng/ml) and IS (5 ng/ml) to spike in plasma to generate a CC of STD. Eight nonzero values of 6.00, 15.00, 45.00, 60.00, 75.00, 90.00, and 120.00 ng/ml made up the CC STDs.
The quality control (QC) samples were made up of TZP concentrations of 6.00 ng/ml for the lower limit of quantification (LLOQ), 30.00 ng/ml for the low-QC (LQC), 60.00 ng/ml for the middle-QC (MQC), and 90.00 ng/ml for the high-QC (HQC). 300 μl of spiked samples were added to polypropylene tubes (that had already been labeled) following bulk spiking. With the exception of 30 samples every one of LQC and HQC that were shifted for preservation in a cell frost deep freezer (temp: −17°C to −27°C) to generate protracted stability at −22°C ± 5°C, the CC STD, as well as QC sample, were retained in the low-temperature freezer (temp: −55°C to −75°C). The technique validation was completed using these samples [18–25].
Extraction procedure
Plasma samples that have been centrifuged and processed should be labeled with time frames. 300 μl of diluent was added to 200 μl of plasma and thoroughly mixed. An estimated 0.5 ml of ACN was combined, followed by extensive vortexing, then swirled for 20 minutes at 4,000 rev/minute to separate all the proteins. The supernatant was filled in a vial before injecting it into the chromatogram.
Procedure for preparing mobile phase and buffer
1 ml of FA and 1,000 ml of deionized water were combined together and then the solution was passed through a 0.45 micron filter membrane. 40: 60 v/v mixture of MeOH and 0.1% FA was mixed followed by filtration using 0.45 micron filter paper.
Preparation of extracted sample
The requisite amount of plasma samples was withdrawn from the deep freezer, defrosted at ambient temperature, and the tubes were vortexed. Place the prelabeled sterile tubes in the correct order according to batches; a fraction of 200.000 μl of plasma was blended with 300.000 μl of MeOH and swirled vigorously for 15 minutes. Following this, 500.000 μl each of diluent, STD stock, and IS stock were blended together, and the solution was mixed thoroughly for 15 minutes before being vibromaxed for 5 minutes at 2,500 rpm. The solution was centrifuged for 5 minutes at 4,000 rpm and 10°C. Roughly 1.000 ml of supernatant was collected.
Preparation of unextracted sample
Put 500.000 μl of the STD stock solution in tubes that have already been labeled. 500.000 μl of the IS working solution should be added, then vortexed to blend properly. Add 1,000.000 microliters of mobile phase followed by vortexing. Move the necessary volume into auto-sampler vials that have already been labeled, then inject 10.000 μl into the column as per optimized bio-analytical conditions tabulated in Table 1.
Pharmacokinetic studies
TZP was extracted from rat plasma using the solvent extraction and partitioning technique. For this, 200 μl of plasma sample (at the appropriate concentration) were poured into polypropylene tubes and mixed thoroughly. Following the addition of 500 μl of each stock, centrifugation was performed for approximately 10 minutes at 4,000 rpm and 20°C. Every sample’s supernatant was taken in a tube, and it was then vaporized at 40°C for drying. These samples were quickly vortexed after being diluted with 500 μl of diluent and 300 μl of MeOH, and they were subsequently put into auto-sampler vials.
Ethics statement
This study was carried out in strict accordance with the recommendations and compliance of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEAs) norms. CPCSEA is a statutory body constituted by the Government of India, which regulates the experiments on animals. as part of this research work. The protocol of animal study was approved by the Institute of Animal Ethics Committee. Animals were housed in similar laboratory conditions with access to endives, carrots, and fresh corn (few amounts only), and the animals were kept at a temperature of 21°C–24°C, and humidity was 50%–55%. Before experimentation, all animals fasted overnight and had water ad libitum. Experiments are done without anesthesia and taken all such measures as may be necessary to ensure that animals are not subjected to unnecessary pain or suffering before, during, or after the performance of experiments on them.
Bioanalytical method validation
The technique was validated for sensitivity, selectivity, linearity, precision, matrix condition, accuracy, reinjection reproducibility, recovery study, and stability.
Sensitivity and selectivity
By examining the six diverse rat samples and examining interference at respective retention times (RTs), sensitivity, and selectivity were carried out.
Matrix effect
To estimate the matrix effect, the comparability of height area ratios of six diverse drug-free samples for TZP was assessed. Six diverse plasma batches were studied in repeated trials at LQC and HQC concentrations with an adequate precision below 15%.
Precision and accuracy
An LLOQ, LQC, MQC, and HQC level investigation of IS samples was used to assess it. Excluding the LLOQ, wherein 20% is required, accuracy, as well as coefficient of variation (%CV), is required to not exceed 15%.
Recovery
Through the extraction of TZP, the assessment of six samples replicates at every internal control concentration. Then, by correlating the height areas of extracted and nonextracted STDs, recovery is investigated.
Carryover
Carryover refers to the analyte recovered by chromatographic column after reconstitution of this sample using a matrix having a sample concentration upper limit of quantification (ULOQ) and beyond.
Dilution integrity
By injecting the matrix having sample over the ULOQ levels and followed by reconstitution using a blank matrix, the integrity of the dilution must be explicable.
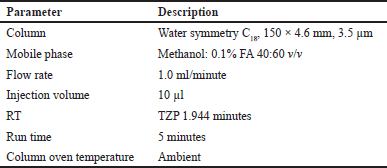 | Table 1. Optimized bio-analytical conditions. [Click here to view] |
Stability
By contrasting samples against the new stock sample, the solution stability of the sample was determined. Employing six reruns, the stability in plasma at HQC and LQC levels was ascertained. In accordance with US FDA criteria, the sample was supposed stable if the disparity remained below 15%. The accuracy and stability of spiked plasma maintained at ambient temperature were assessed after being kept in an autosampler for 24 hours, were evaluated. By studying extract plasma samples that had been pumped promptly opposite samples that had been pumped back following storage in wet extract stability at 2°C–8°C for 12 and 18 hours, the autosampler stability (LQC, MQC, and HQC) was assessed. By assessing extract plasma samples that had been pumped right away opposite samples that had been pumped back following storage in dry extract stability at −20°C ± 3°C for 12 and 18 hours, the reinjection reproducibility was assessed. Freeze-thaw stability was investigated through the assessment of freshly spiked IS samples opposite to steadiness samples that had undergone three cycles of freezing–thawing. The concentrations achieved the subsequent day were studied opposite to the original concentrations for assessing long-term stability.
High selectivity and recoveries were seen when applying the solvent extraction and partitioning technique. By the application of optimized chromatographic settings, extraction techniques, and detecting factors, TZP in rat plasma may be precisely and accurately detected in less time during the assessment. Figures 1 and 2 depict the parent and production mass spectra of TZP and IS.
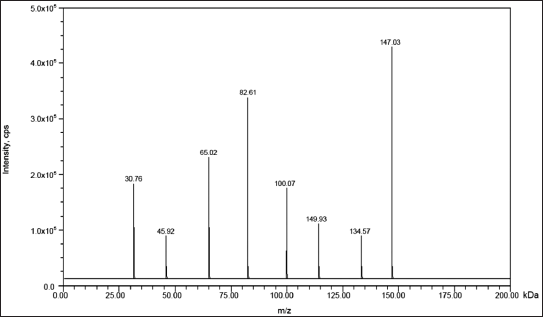 | Figure 1. Mass spectrum of the fragmentation pattern of TZP (147.03 and 82.61). [Click here to view] |
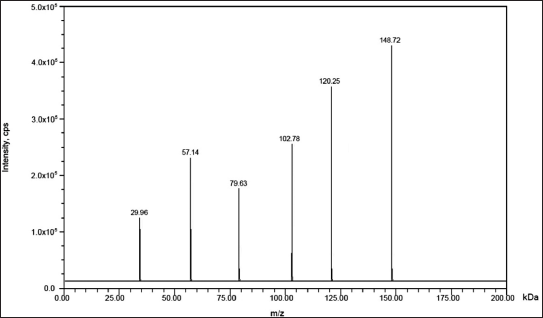 | Figure 2. Mass spectrum of the fragmentation pattern of IS (148.72 and 120.25). [Click here to view] |
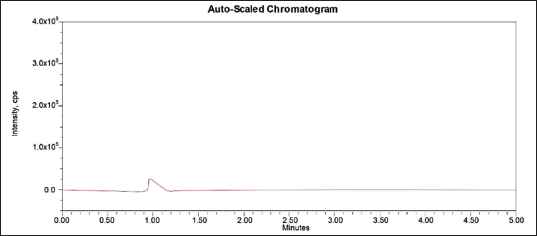 | Figure 3. Blank chromatogram TZP. [Click here to view] |
 | Figure 4. STD chromatogram of TZP. [Click here to view] |
 | Figure 5. STD chromatogram of IS. [Click here to view] |
Pharmacokinetic studies
Animals received a single 210 mg dose of TZP injection; samples were drawn 2, 4, 6, 8, 12, and 24 days later. K2 EDTA vacuum blood collection tubes were utilized for acquiring a portion of 5 ml blood at every interval. A preinjection sample was withdrawn to assess for any noticeable interferences. The plasma was extracted from the obtained samples using a centrifugation technique, and it was then kept at −70°C. At diverse concentrations, samples were spiked with IS and assessed along with QC samples. The WinNonlin (Version 5.2) software program was implemented to determine the pharmacokinetic profile of TZP.
RESULTS AND DISCUSSION
Method validation
Specificity
The chromatographs of blank plasma samples, STD, and IS, are depicted in Figures 3–5, respectively. There were no interfering peaks visible in the obtained chromatographs.
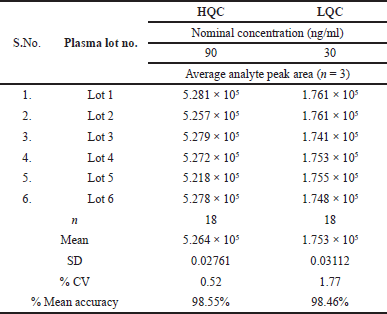 | Table 2. Matrix effect results of TZP (HQC-90 ng/ml, LQC-30 ng/ml). [Click here to view] |
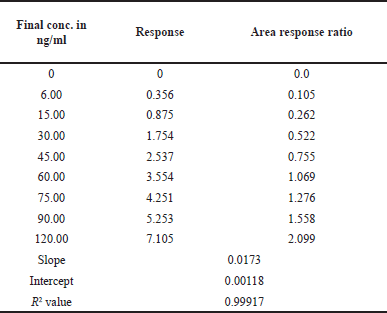 | Table 3. Linearity results of TZP. [Click here to view] |
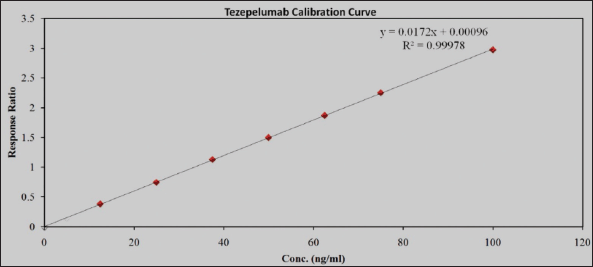 | Figure 6. Calibration plot for TZP. [Click here to view] |
Matrix effect
For TZP LC-MS/MS, the % RSD for ionic suppression/upregulation was computed to be 1.0%, signifying that with these conditions, the matrix effect on sample ionizing falls below an acceptable level. TZP’s LQC and HQC in matrix effect scored 98.46% and 98.55%, respectively, and %CV was 1.77 and 0.52, respectively. It shows that the matrix effect on sample ionizing falls below an appropriate limit of ionization. Table 2 is the matrix effect results of TZP (HQC-90 ng/ml, LQC-30 ng/ml).
Linearity
At the TZP concentrations spanning between 6 and 120 ng/ml, the CCs were linear. It was 0.999 for the average correlation coefficient. The proportion of the sample and IS peak areas was used to quantify samples. Plasma concentration was plotted across peak area ratios. Table 3 has the TZP linearity data, and Figure 6 displays their calibration graphs.
Precision and accuracy
By adding together all of the discrete test outcomes from the discrete IS samples, the accuracy and precision were computed. It was clear from the information presented that the system was precise and efficient. Table 4 presents TZP precision and accuracy findings.
Recovery
The approach has high extraction efficiency, as seen by the recovery findings for TZP at LQC, MQC, and HQC concentrations. The %CV for TZP varied between 0.44 and 2.03 and recoveries were 98.16%–105.14% at LQC, MQC, and HQC concentrations. Data implied that the proposed approach provides an effectual extraction yield.
Ruggedness
At LQC, HQC, and MQC levels, the % recoveries and CV of TZP as measured by two separate investigators on separate columns met appropriate levels. Data signified that technology is rugged. For TZP, the % recoveries spanned around 98.55% and 100.00%. The %CV figures vary around 0.4–1.83. Data implied the ruggedness of the proposed technique.
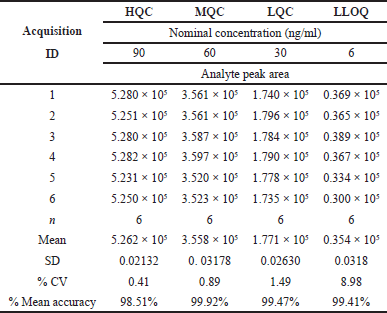 | Table 4. Precision and accuracy results of TZP. [Click here to view] |
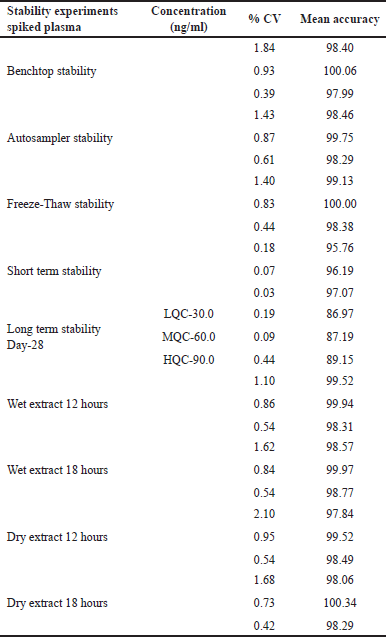 | Table 5. Stability studies of TZP. [Click here to view] |
 | Table 6. Pharmacokinetic studies of TZP. [Click here to view] |
Autosampler carryover
Following sequential loading of LLOQ, as well as ULOQ at the RTs of TZP, the peak area response of TZP, was not seen in the blank plasma samples. Consequently, autosampler carryover is not existent in this approach.
Stability
For stability investigation, TZP solutions were concocted using solvent and preserved at around 2°C and 8°C. Newly prepared stock solutions and stock solutions concocted a day before were correlated. For 24 hours at 20°C in the autosampler and 24 hours on the benchtop, the plasma stability was steady. Future stability signified that TZP remains stable for a maximum of 24 hours if maintained at a temperature of −30°C. Table 5 includes TZP outcomes for stability studies.
Pharmacokinetic studies
Six distinct rats were given injections of TZP samples at various intervals, including 2, 4, 6, 8, 12, and 24 days. Samples are then concocted in accordance with the test methodology, loaded into the chromatographic device, and the findings are noted and tabulated in Table 6. By incurred sample reanalysis (ISR), the stability of the research samples was determined. Close to Cmax as well as the elimination phases in the pharmacokinetics, two samples from every subject were chosen for ISR. The percentage difference should not be beyond 20%, and the samples were thought to be stable. Figure 7 represents the recovery graph for TZP in rat plasma.
SUMMARY AND CONCLUSION
In conclusion, an accurate, precise, sensitive, and rapid HPLC-MS/MS method was developed and validated to quantify TZP in rat plasma. The method was successfully applied to quantify TZP in pharmacokinetics studies using Sprague–Dawley rats. For TZP the CCs were found to be consistently accurate and precise over the linear dynamic range of 6–120 ng/ml. The coefficient of determination (R2) is greater than or equal to 0.99. The extraction method gave consistent and reproducible recoveries for TZP from plasma with ?90% recovery. Intra and inter-day accuracy and precision were found to be acceptable as per the guidelines. The method was run through a battery of stability studies and the results were found to be within the assay variability limits during the entire process. The pharmacokinetic study of TZP in rat plasma was effective under this optimized method. In addition, a simple liquid–liquid extraction was developed for the biological sample pretreatment, which was simpler, more efficient, and inexpensive. The plasma concentrations of TZP were quantified and the main pharmacokinetic parameters including the absolute oral bioavailability were determined. Since the oral bioavailability of TZP is low, efforts on absorption/metabolism are needed to develop TZP as a drug administered through the oral route. This study demonstrates that the method may be suitable for various exploratory and other preclinical studies.
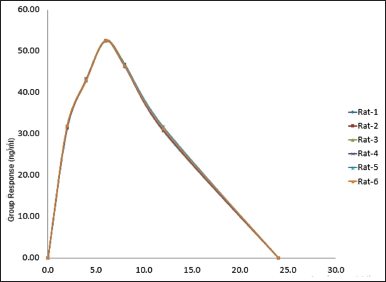 | Figure 7. Recovery graph for TZP in plasma. [Click here to view] |
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The Animal House Facility of Manisha Laboratories (City: Mumbai, State: Maharashtra, Country: India) licensing with CPCSEA has been revised for experimental studies on small animals for educational reasons under registration number 1074/PO/Re/S/05/CPCSEA, and this has authorized the pharmacokinetic performance methods. All experimentations on rats were performed at the Animal House Facility of Manisha Laboratories with protocol No: CPCSEA/MS Lab/PK/58796.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
DISCLOSURE
This manuscript is accessible as a preprint with the identifier 10.1101/2022.12.14.520442 on bioRxiv: https://biorxiv.org/cgi/content/short/2022.12.14.520442v1, and its corresponding DOI is https://doi.org/10.1101/2022.12.14.520442. The copyright for this preprint is held by the corresponding author, who has granted bioRxiv the license to present the preprint. It is released under the terms of a CC-BY 4.0 International license.
REFERENCES
1. Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med. 2017 Sep 7;377(10):936–46. CrossRef
2. Marone G, Spadaro G, Braile M, Poto R, Criscuolo G, Pahima H, et al. Tezepelumab: a novel biological therapy for the treatment of severe uncontrolled asthma. Expert Opin Investig Drugs. 2019 Nov 2;28(11):931–40. CrossRef
3. Ragnoli B, Morjaria J, Pignatti P, Montuschi P, Barbieri M, Mondini L, et al. Dupilumab and tezepelumab in severe refractory asthma: new opportunities. Ther Adv Chronic Dis. 2022 May;13:20406223221097327. CrossRef
4. Pelaia C, Pelaia G, Crimi C, Maglio A, Gallelli L, Terracciano R, et al. Tezepelumab: a potential new biological therapy for severe refractory asthma. Int J Mol Sci. 2021 Apr 22;22(9):4369. CrossRef
5. Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol. 2012 Jan 1;129(1):104–11. CrossRef
6. Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol A J Pathol Soc Great Britain Ireland. 2004 Feb;202(2):145–56. CrossRef
7. Adatia A, Wahab M, Satia I. Is tezepelumab more than just an anti-eosinophil drug?. Eur Respir J. 2021;59(1):2101700. CrossRef
8. Menzies-Gow A, Corren J, Bourdin A, Chupp G, Israel E, Wechsler ME, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021 May 13;384(19):1800–9. CrossRef
9. Papi A, Brightling C, Pedersen SE, Reddel HK. Pathogenesis of asthma. Lancet. 2018;391:783–800. CrossRef
10. Verstraete K, Peelman F, Braun H, Lopez J, Van Rompaey D, Dansercoer A, et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat Commun. 2017 Apr 3;8(1):14937. CrossRef
11. Matera MG, Rogliani P, Calzetta L, Cazzola M. TSLP inhibitors for asthma: current status and future prospects. Drugs. 2020 Apr;80:449–58. CrossRef
12. Nakajima S, Kabata H, Kabashima K, Asano K. Anti-TSLP antibodies: targeting a master regulator of type 2 immune responses. Allergol Int. 2020;69(2):197–203. CrossRef
13. Ho Hoy SM. Tezepelumab: first approval. Drugs. 2022 Mar;82(4):461–8. CrossRef
14. Cloutier MM, Dixon AE, Krishnan JA, Lemanske RF, Pace W, Schatz M. Managing asthma in adolescents and adults: 2020 asthma guideline update from the National Asthma Education and Prevention Program. JAMA. 2020 Dec 8;324(22):2301–17. CrossRef
15. Ferrazzoni S, Scarpa M, Agostini S, Calabrese F. Novel therapeutic approaches to asthma treatment. Front Pharmacol. 2020;11:616.
16. Mackus M, Loo AJ, Garssen J, Kraneveld AD, Scholey A, Verster JC. The role of alcohol metabolism in the pathology of alcohol hangover. J Clin Med. 2020 Oct 25;9(11):3421. CrossRef
17. Li W, Wu S. Role of bioanalysis in drug development. Bioanalysis. 2019;11(7):547–50. CrossRef
18. Guidance for Industry: bioanalytical method validation. Rockville, MD: U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Biologics Evaluation and Research (CBER); 2001.
19. Sathyamoorthy N, Rajendran V, Naveena VSH, Dhanaraju MD. An approach for validated Rp-Hplc method for the analysis of paclitaxel in rat plasma. J App Pharm Sci. 2014;4(09):073–6.
20. Ramadanty WT, Arozal W, Louisa M, Soetikno V, Purbadi S, Priyanto P. Efficient validated method of UPLC-MS/MS to determine curcumin in rat plasma and ovarium. J Appl Pharm Sci. 2019;9(01):058–65. CrossRef
21. Ravi Y, Rajkamal BB. A validated LC-MS/MS method for the pharmacokinetic study of alogliptin in healthy rabbits. J Appl Pharm Sci. 2019;9(2):29–37. CrossRef
22. Sharath Kumar LMK, Azeemuddin MM, Routhu KC, Priya K, Babu UV, Pai SR. A validated LC-MS/MS method for simultaneous quantification of antitubercular drugs in rat plasma and its application for a pharmacokinetic interaction study with Immusante®. J Appl Pharm Sci. 2023;13(07):151–8.
23. Amar Babu NL, Koganti K, Palakeeti B, Srinivas KS, Rao KP. Bioanalytical LC-MS/MS method for determination and comparison of selexipag assay in various biological materials and its application to pharmacokinetics studies in rat plasma. Int J Res Pharm Sci. 2020;11(2):2210–20. CrossRef
24. Rao KP, Babu NL, Koganti K, Palakeeti B, Srinivas KSV. Related substances method development and validation of an LCMS/MS method for quantification of selexipag and its related impurities in rat plasma and its application to pharmacokinetic studies. SN Appl Sci. 2021;3:321. CrossRef
25. Thulaseedhar A, Karunasree M, Namburi LAA, Aravind K. Peramivir and related impurities in rat plasma and its applications in pharmacokinetic studies (Bioanalytical method development and validation by LC-MS/MS). Int J App Pharm Sci. 2022;5(14):53–61. CrossRef