Development and Validation of RP-HPLC Method for the Simultaneous Estimation of Domperidone and Naproxen in Tablet Dosage Form
Md. Shozan Mondal, Md. Ahsanul Haque, Mohammad Safiqul Islam, S.M. Ashraful Islam
Pages: 145-148

RP HPLC Method for the determination of Tamsulosin in bulk and Pharmaceutical Formulations
Manish Kumar Thimmaraju, Venkat Rao, Hemanth .K, P. Siddartha Kumar
Pages: 177-180

Simultaneous Estimation of Finasteride andTamsulosin Hydrochloride in Combined DosageForms by RP-HPLC-PDA Method
M. Sindhura, K. Raghavi, R. Prashanthi, Buchi N. Nalluri
DOI: 10.7324/JAPS.2012.2626Pages: 203-209

Development and Validation of RP-HPLC Method for Simultaneous Estimation of Enalapril Maleate and Amlodipine Besylate in Combined Dosage form
Bharat G. Chaudhari
DOI: 10.7324/JAPS.2012.2911Pages: 054-057

A RP-HPLC Method Development and Validation for the Estimation of Gliclazide in bulk and Pharmaceutical Dosage Forms
B.V.V Ravi kumar, A.K. Patnaik, Saroj Kumar Raul, Nagireddy Neelakanta Rao
DOI: 10.7324/JAPS.2013.3410Pages: 059-062

RP-HPLC Method Development and Validation of Gallic acid in Polyherbal Tablet Formulation
Kamal Kardani, Nilesh Gurav, Bhavna Solanki, Prateek Patel, Bhavna Patel
DOI: 10.7324/JAPS.2013.3508Pages: 037-042

Bioavailability of karanjin from Pongamia pinnata L. in Sprague dawley rats using validated RP-HPLC method
Naresh Shejawal, Sasikumar Menon, Sunita Shailajan
DOI: 10.7324/JAPS.2014.40303Pages: 010-014

Simultaneous estimation of Cefpodoxime proxetil and Ofloxacin In tablet dosage form using RP-HPLC
Annadi Chiranjeevi and Medidi Srinivas
DOI: 10.7324/JAPS.2014.40508Pages: 046-050

A comparative estimation of quercetin content from Cuscuta reflexa Roxb.using validated HPTLC and HPLC techniques
Sunita Shailajan, Harshvardhan Joshi, Bhavesh Tiwari
DOI: 10.7324/JAPS.2014.40721Pages: 123-128

An approach for validated RP-HPLC method for the analysis of paclitaxel in rat plasma
Nandhakumar Sathyamoorthy, Vijayalakshmi Rajendran, Naveena V.S.H, Magharla Dasaratha Dhanaraju
DOI: 10.7324/JAPS.2014.40913Pages: 073-076

Development and validation of a new RP-HPLC method for the estimation of dutasteride in bulk and pharmaceutical formulations
Poonguzhali Subramanian, P. S. Rajinikanth
DOI: 10.7324/JAPS.2016.601207Pages: 047-055

Application of modern RP-HPLC technique for the quantitation of betulinic acid from traditional drug Symplocos racemosa Roxb.
Sunita Shailajan, Sasikumar Menon, Dipti Singh, Gauri Swar, Suhina Bhosale
DOI: 10.7324/JAPS.2017.70321Pages: 129-134

A new quantitative reverse phase high-performance liquid chromatographic method for the quantification of Rilpivirine hydrochloride in bulk and dosage form
Sonam Patel, Krishnaveni Nagappan, Gouru Santhosh Reddy
DOI: 10.7324/JAPS.2018.81122Pages: 157-162

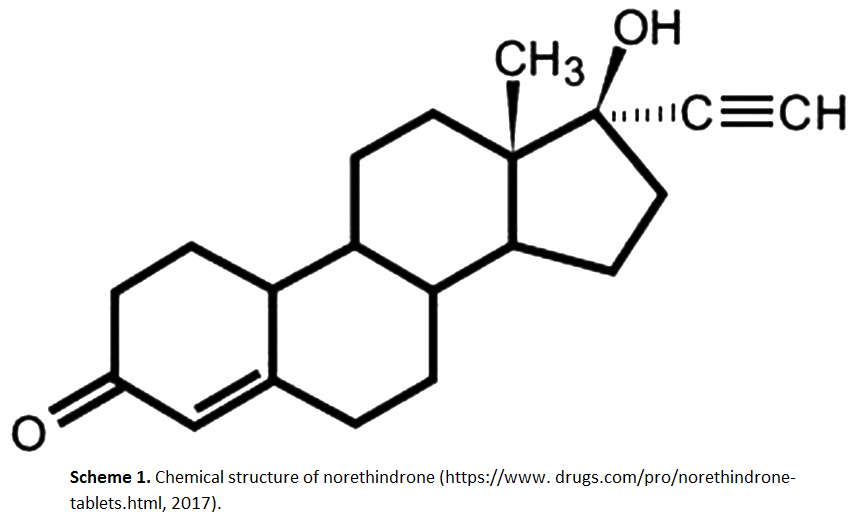
RP-HPLC method for determination of norethindrone in dissolution media and application to study release from a controlled release nanoparticulate liquid medicated formulation
Suhair S. Al-Nimry, Bashar M. Altaani, Razan H. Haddad
DOI: 10.7324/JAPS.2019.90211Pages: 079-086
Validation and application of reversed-phase high-performance liquid chromatography for quantitative analysis of acid orange 7 and Sudan II in blusher products
Novalina B. R. Purba, Abdul Rohman, Sudibyo Martono
DOI: 10.7324/JAPS.2019.90714Pages: 100-105

Development and validation of RP-HPLC method for pitavastatin calcium in bulk and formulation using experimental design
Vinodkumar D. Ramani, Girish K. Jani, Ashim Kumar Sen, Girish U. Sailor, Vijaykumar B. Sutariya
DOI: 10.7324/JAPS.2019.91010Pages: 075-083

Stability indicating RP-HPLC method for simultaneous determination of pyrimethamine and sulfamethoxypyrazine in pharmaceutical formulation: Application to method validation
Shankaranahalli Gurusiddappa Keshava, Gurupadayya Bannimath, Prachi Raikar, Maruthi Reddy
DOI: 10.7324/JAPS.2020.102008Pages: 049-055
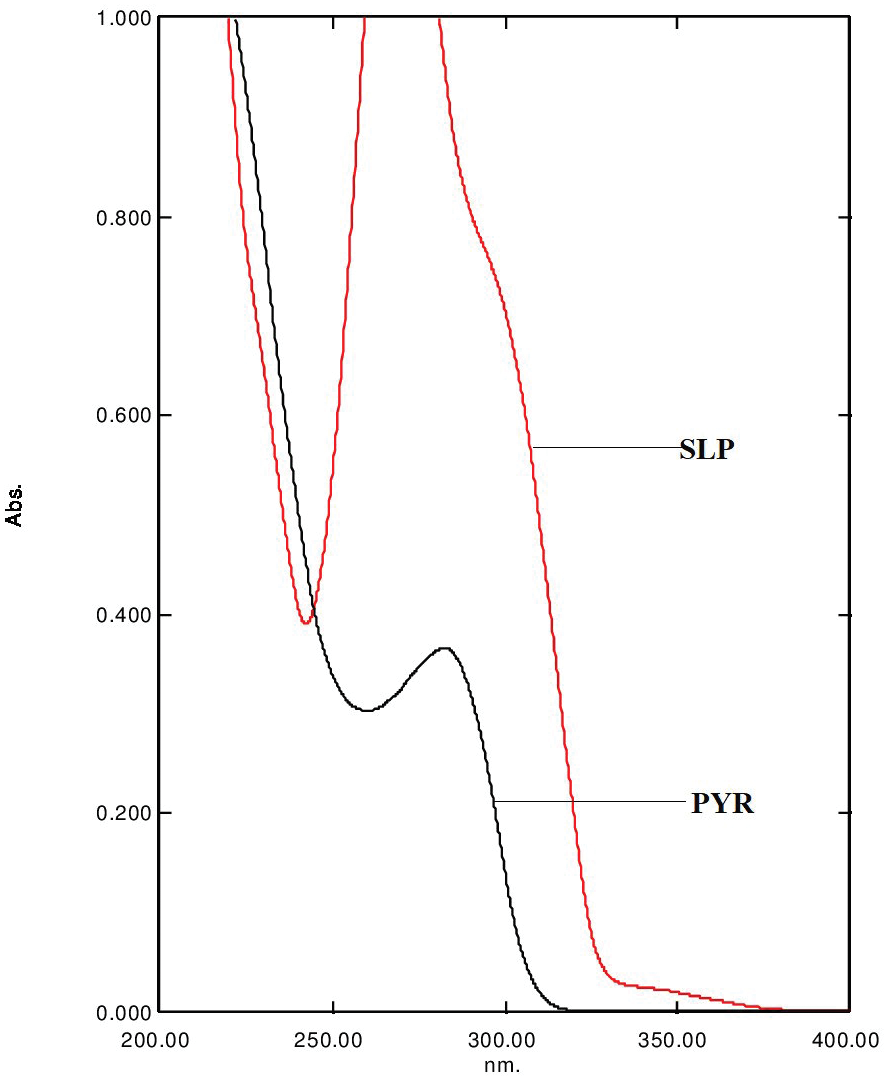
Pharmaceutical equivalence study of marketed ibuprofen tablets of UAE using a validated RP-HPLC method
Fazilatun Nessa, Ruqaiya Salim, Susan George, Saeed Ahmed Khan
DOI: 10.7324/JAPS.2021.1101118Pages: 141-149
A new approach for evolution and quantification of Triamcinolone acetonide in medication shots by using RP-HPLC
Manikantha Naveen Vuddagiri, Veeraswami Boddu
DOI: 10.7324/JAPS.2021.1101216Pages: 169–174

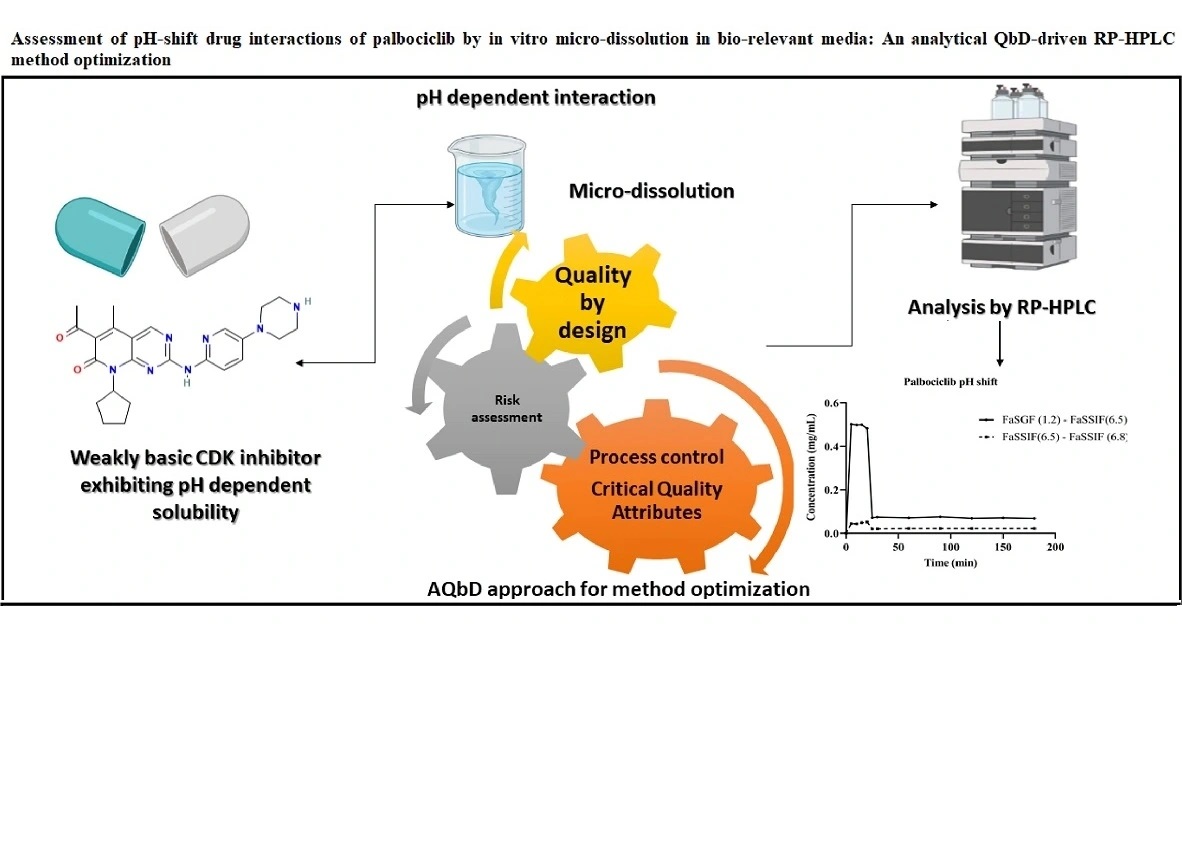
Assessment of pH-shift drug interactions of palbociclib by in vitro micro-dissolution in bio relevant media: An analytical QbD-driven RP-HPLC method optimization
Prajakta Harish Patil, Mrunal Desai, Rajat Radhakrishna Rao, Srinivas Mutalik, Gurupur Gautham Shenoy, Mahadev Rao, Puralae Channabasavaiah Jagadish
DOI: 10.7324/JAPS.2022.120505Pages: 078-087
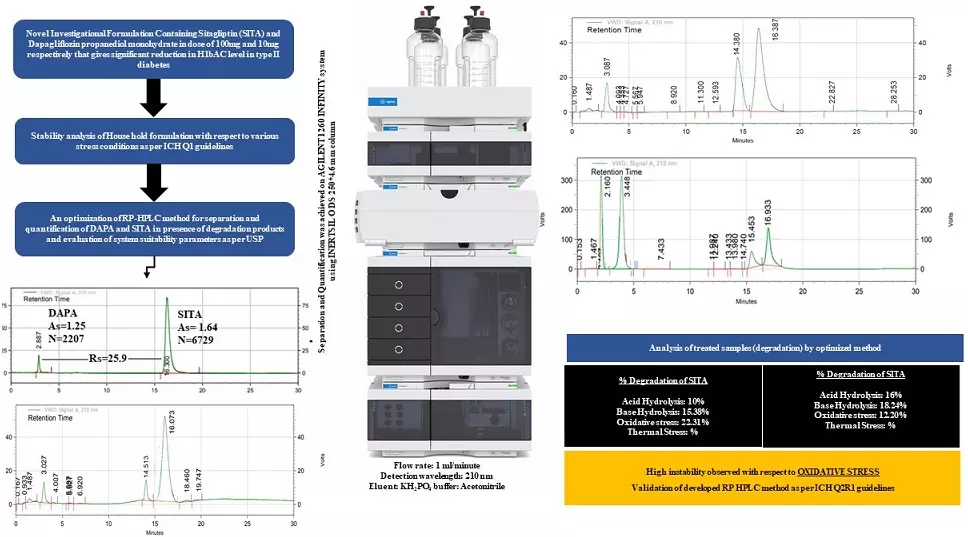
Quantitative computation and stability evaluation of phase III composition comprising sitagliptin and dapagliflozin propanediol monohydrate by RP-HPLC
Yesha Darshak Patel, Pinak Rameshbhai Patel, Jigna Bhatt, Binny Mehta, Krunal Detholia
DOI: 10.7324/JAPS.2022.120614Pages: 148-155
QbD-based RP-HPLC method development for quantitative computation of phase III composition comprising apixaban and clopidogrel
Rashmi Shukla, Ankit Chaudhari, Pinak Patel, Krunal Detholia
DOI: 10.7324/JAPS.2024.181311Pages: 085-093

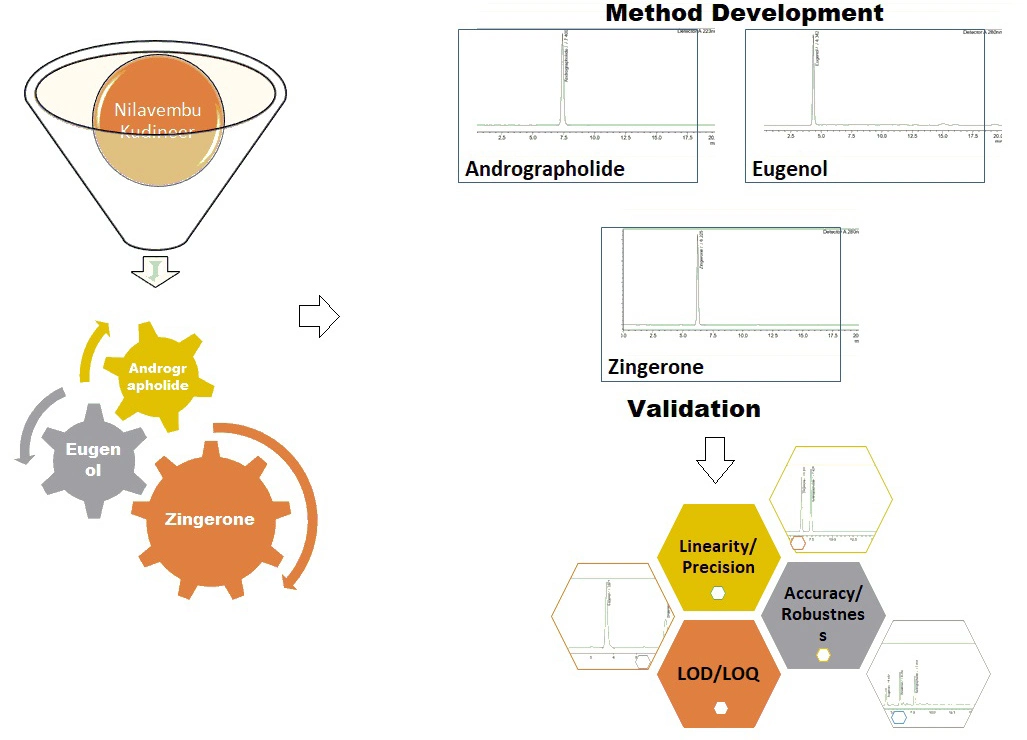
Determination of phytochemical markers andrographolide, eugenol and zingerone in nilavembu kudineer by RP-HPLC method
B. Sivagami, B. Sailaja
DOI: 10.7324/JAPS.2024.180359Pages: 128-134
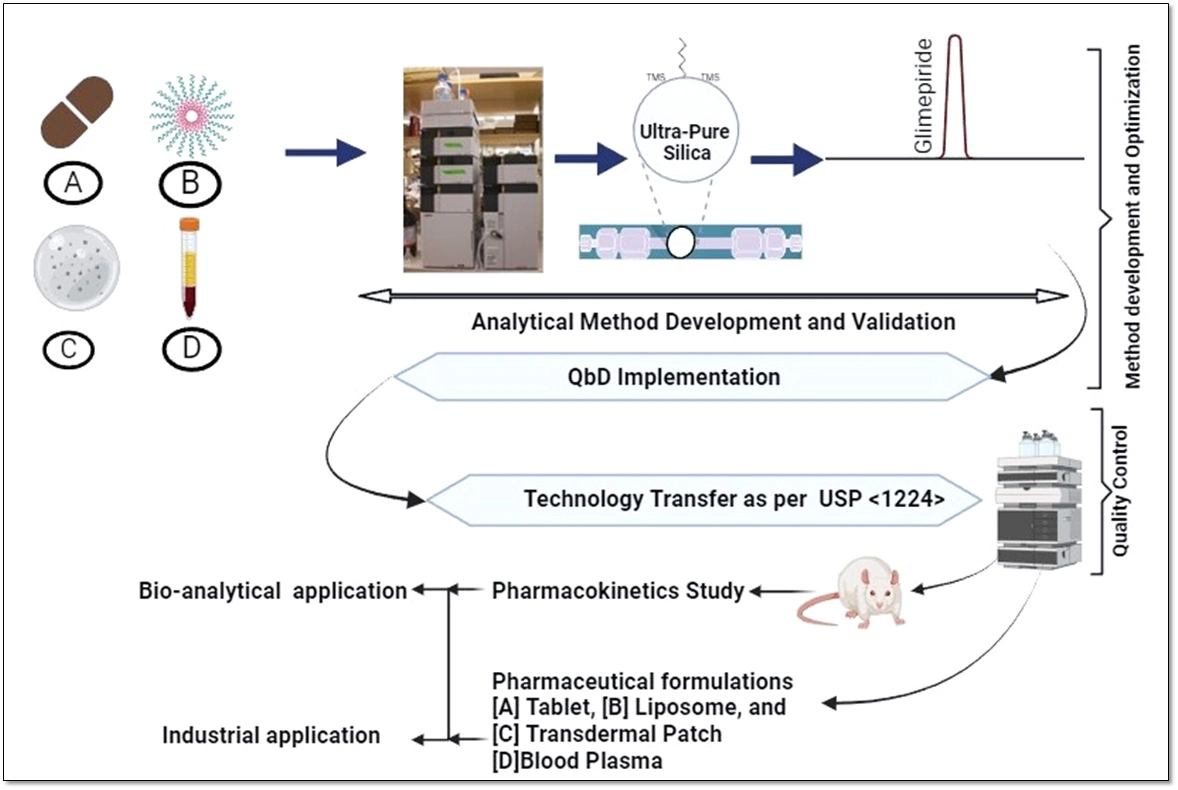
Development of a quality by design based hybrid RP-HPLC method for Glimepiride: Bioanalytical and industrial applications
Abhiram Kumar, Chhavi Dhiman, Madhaw Kumar, N. Kannappan, Deepak Kumar, Manish Kumar Chourasia, Kumar Pranav Narayan
DOI: 10.7324/JAPS.2025.214654Pages: 102-115
Supercritical fluid extraction, LC-MS profiling, and QbD-guided green HPLC method for standardization of Careya arborea Roxb. nanoemulsion
Abhijit S. Salokhe, Archana S. Patil, Yadishma Gaude, Pooja Rayanade, Rahul Koli, Namdeo S. Jadhav
DOI: 10.7324/JAPS.2025.266427Pages:
_.jpg)