INTRODUCTION
An alternative system of medicine known as a traditional system of medicine is an embodiment containing more than 45,000 species of medicinal plants [1]. Siddha medicine called the ancient Indian medicine, which is prominently followed for maintaining health. In addition to treatment of various complications, mainly relies on therapeutic efficacy using medicinal plants [2]. Siddha medicine focuses on diagnosing diseased conditions (imbalance) and healthy states (balanced condition) to maintain a holistic approach to treatment in human beings and provides a healthy lifestyle in completely eliminating the root cause of the disease. Quality control and standardization of phytochemicals are important criteria to ensure the quality and purity of herbal materials [3].
Separation and standardization of phytoconstituents present in the polyherbal formulation is a great challenge to researchers. Sophisticated methods such as high performance liquid chromatography (HPLC), high performance thin layer chromatography, gas chromatography (GC), liquid chromatography mass spectrometry, and GC mass spectrometry are available to estimate phytochemicals in medicinal plants and herbal formulations. Herbal formulations are available as solid, semisolid, and liquid dosage forms and choornam like nilaveembu kudineer (NK). NK is available as choornam (mixture of powdered herbs) and syrup form. Compared to choornam, syrup is more acceptable as there are no prepreparatory requirements like choornam. The polyherbal formulation contains many herbal plant materials prepared by powdering herbal raw materials and prepared as a decoction for the treatment of viral disorders such as dengue fever and many complications such as anti-inflammatory activity, antipyretic, digestive disorders, hepatoprotective, and so on [4].
NK is a Siddha polyherbal medicine available in liquid and powdered form called as NK choornam. NK comprises 8 to 9 herbs namely Andrographis paniculata Burm.f. (Nees) (whole plant) Kalmegh containing the major constituent (Andrographolide), which is a labdane derivative, Vetiveria zizanioides (L) Roberty (root), Plectranthus vettiveroides, Cyperus rotundus L. (rhizome), Santalum album L. (heart wood), Zingiber officinale Roscoe (rhizome), Piper nigrum L. (fruit), Trichosanthes cucumerina L. (whole plant), and Mullugo cerviana (L.) Ser. (whole plant) in equal proportions. NK consists predominantly andrographolide, eugenol and zingerone, diterpenoids, glycosides, flavonoids, and lactones [5].
Eugenol has a polar hydroxy group and polar ether group in the structure. The molecular weight of eugenol is 164 and the molecular formula is C10H12O2 [6]. Zingerone which has a polar hydroxy group with a mol. Wt. of 194.22 g/mol and the molecular formula of zingerone is C11H14O3. Andrographolide is a polar compound because of hydroxy and carbonyl groups attached to the rings. When carbon count increases the non-polarity increases, hence andrographolide is less polar when compared to eugenol and zingerone [7]. Zingerone is mid-polar compared with eugenol and andrographolide, whereas eugenol is more polar because of its less carbon atom and more polar hydroxy group [8]. The chemical structures of eugenol, andrographolide, and zingerone are depicted in Figure 1.
The shim-Pack C18 column has superior inertness, which increases column stability. It is a hybrid silica ODs column. Shim-Pack C18 column with 150 mm gives better Rt when compared to the published articles. Andrographolide Rt is less than 7 in reported studies due to an increase in the organic phase concentration. For zingerone, very few studies were reported; it shows a very good symmetrical peak with a retention time of 6.2 minutes. Eugenol Rt is 4.3 minutes, within 7 minutes all three compounds can be eluted with good resolution. It shows that the shim pack C18 column is better when compared to previous methods.
There are numerous reports available in the literature for determining andrographolide [9], eugenol [10], and zingerone [11] individually and in combination with other drugs. On the other hand, there is no reverse phase (RP)-HPLC method described for the simultaneous quantification of these marker standard compounds as well as these markers in herbal formulations, combined marker compounds in any formulation. Consecutively, the present research was proposed to develop and validate the RP-HPLC method for simultaneous determination of standard samples of andrographolide [12], eugenol [13], and zingerone in further the optimized method was planned to apply for the estimation of andrographolide, eugenol, and zingerone NK a Siddha formulation [14]. HPLC is an advanced sophisticated instrument that can detect multicomponent analysis of mixtures present in phytoconstituents in medicinal plants and polyherbal formulations. The suggested approach is an excellent quality control tool for the concurrent quantitative assessment and detection of phytochemical markers present in polyherbal formulations.
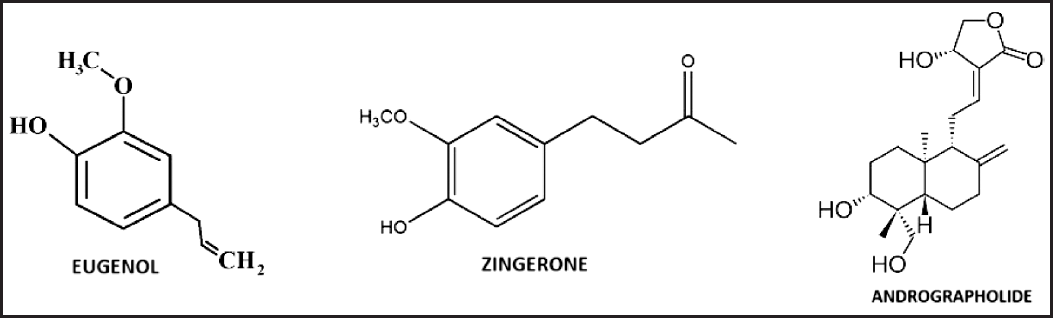 | Figure 1. Structures of eugenol, zingerone, and andrographolide. [Click here to view] |
MATERIALS AND METHODS
Chemicals and reagents
Andrographolide, eugenol, and zingerone were procured from Yucca Enterprises Mumbai, India. Acetonitrile—HPLC, Methanol-HPLC, Water—HPLC, (Merck, India).
Chromatographic system and conditions
The chromatographic analysis was performed on the Shimadzu HPLC-ultravoilet (UV) system. The instrument consisted of a quaternary pump gradient LC 20 AD, the injector used was an autosampler SIL 20 AC, the column oven consisted of CTO 10 AS, the column is a C18, 100 A°, (5 μm, 4.6 × 150 mm) and the detector was a UV detector SPD M 20 A (Shimadzu, Japan).
Preparation of standard solution of andrographolide (100 µg/ml)
About 1 mg of andrographolide was accurately weighed and transferred into a 10 ml volumetric flask containing 1 ml of HPLC grade methanol and the volume was adjusted with methanol up to 10 ml. The solution was sonicated for 5 minutes to remove air bubbles [15].
Preparation of standard solution of eugenol (100 µl/ml)
About 1 ml of eugenol (1.06 g/cm³) was exactly measured and transferred to a 10 ml volumetric flask containing 1 ml of HPLC grade methanol and made up the volume up to 10 ml with HPLC methanol [16].
Preparation of standard solution of zingerone (100 µg/ml)
Exactly weighed 1 mg of Zingerone was transferred into a 10 ml volumetric flask containing 1 ml of HPLC grade water and the volume was adjusted to 10 ml with water and sonicated for 5 minutes [17].
Fractionation of phytoconstituents from NK
NK 10 ml was taken and transferred into a separating funnel, to this 30 ml of hexane was added and gently shaken for 5 minutes and left for 30 minutes until two clear layers were formed. The hexane layer was separated and collected separately in a beaker, and labeled as hexane fraction, and kept aside. To the remaining layer, chloroform was added and extracted in the same way and the organic layer was collected separately. To the remaining layer, 10 ml of acetonitrile was added and extracted leaving behind the residual fraction. The residual fraction was evaporated on a rotary evaporator and subjected to sample preparation.
Preparation of sample solution
The sample solution was established by exactly weighing 1 mg fraction residue in a 10 ml volumetric flask and dissolved in 1 ml of HPLC grade water and the solution was adjusted with HPLC grade water up to 10 ml, sonicated for 5 minutes to get the required concentration of 100 µg/ml.
Analytical method development
The best optimal conditions were chosen for selecting the mobile phase, establishing the column, and determining the detection wavelength, a number of factors were taken into consideration. Following multiple trials, the most successful chromatographic trial that achieved ideal peak resolution and high symmetry for the simultaneous estimation of three phytomarkers is reported in Table 1.
Validation of developed method
Validation of the developed method was established as per International Council for Harmonization (ICH) guidelines which include accuracy, limit of detection (LOD), limit of quantification (LOQ), linearity, precision, robustness, and system suitability.
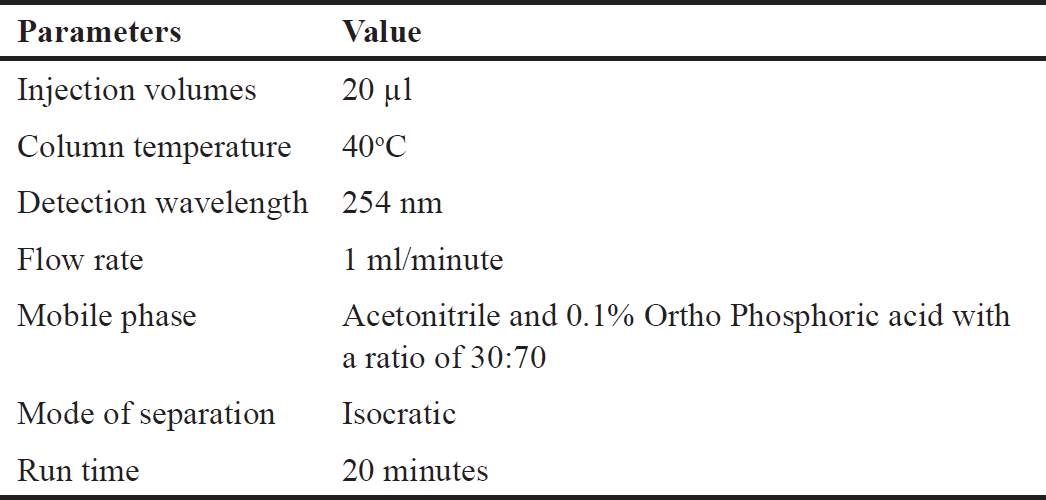 | Table 1. Chromatographic conditions of the developed method. [Click here to view] |
 | Figure 2. Chromatogram of blank. [Click here to view] |
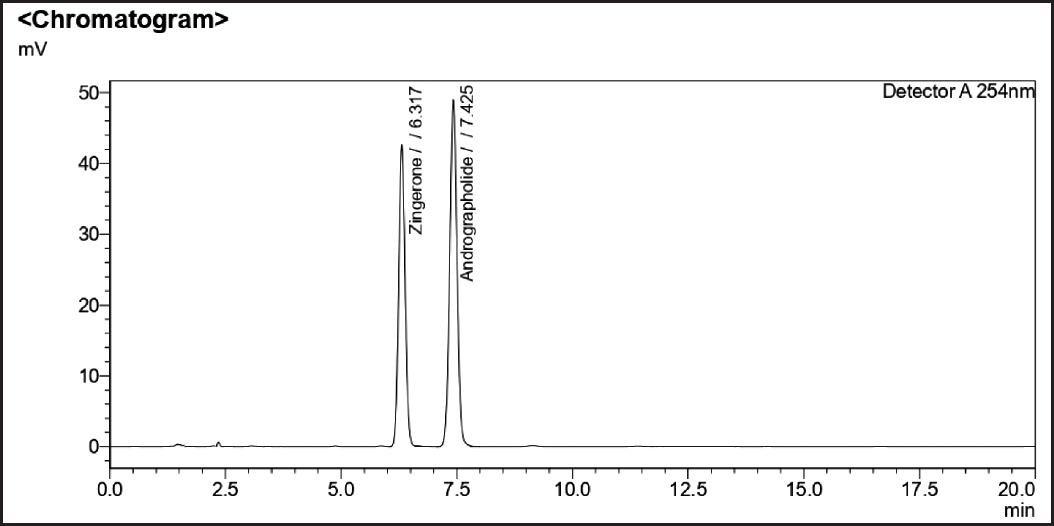 | Figure 3. Standard chromatograms of andrographolide and zingerone. [Click here to view] |
Specificity
The specificity of the method was determined by injecting blank samples to demonstrate the absence of interference with the elution of phytomarkers andrographolide, zingerone, and eugenol in the polyherbal formulation.
Linearity
The linearity was tested by preparing a stock solution of three phytomarkers andrographolide, zingerone, and eugenol. Dilution was carried out to achieve a final concentration of 25–200 µg/ml for all three marker compounds. The linearity was assessed and calibration curves were established by plotting average peak area vs standard concentrations.
LOD and LOQ
The LOD and LOQ of the study were achieved by assuming the linearity of the standards and repeating the procedure three times to acquire the standard deviation (SD) of the intercept and slope of the regression equation (S) values. The following formulas were used to establish and determine the SD technique for LOD and LOQ:
LOD = 3.3 × SD/S and LOQ = 10 × SD/S
Precision
The precision study was demonstrated by accurately weighing and preparing a stock solution (100 µg/ml) of three phytomarkers. The method precision was shown by determining the sample response six times a day. The procedure outlined in the “Preparation of sample solution” section was used to prepare each sample in turn. Relative standard deviation (RSD) was established to document the findings of all precision-related data.
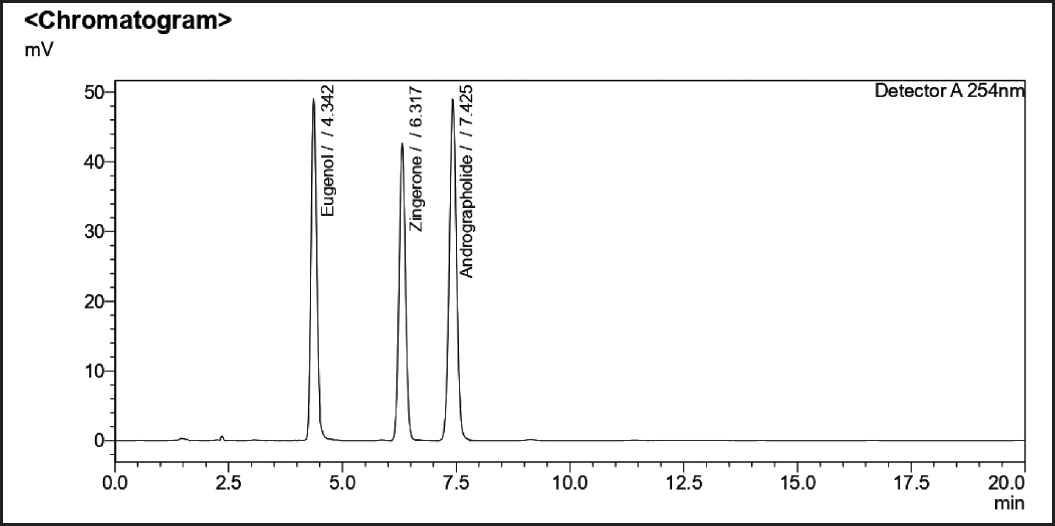 | Figure 4. Standard chromatogram of andrographolide, eugenol, and zingerone. [Click here to view] |
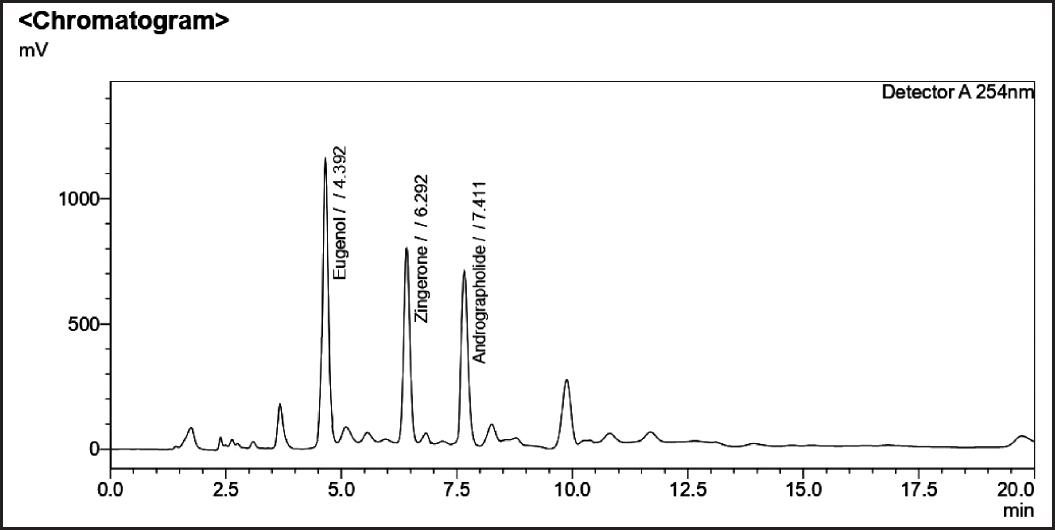 | Figure 5. Sample chromatogram of andrographolide, eugenol, and zingerone. [Click here to view] |
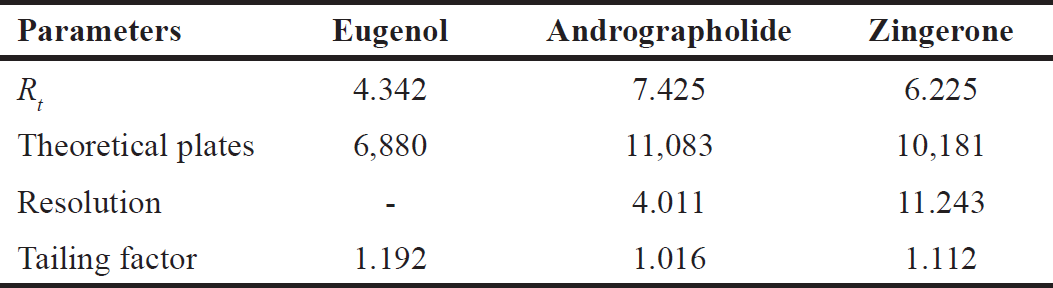 | Table 2. System suitability parameters for marker compounds. [Click here to view] |
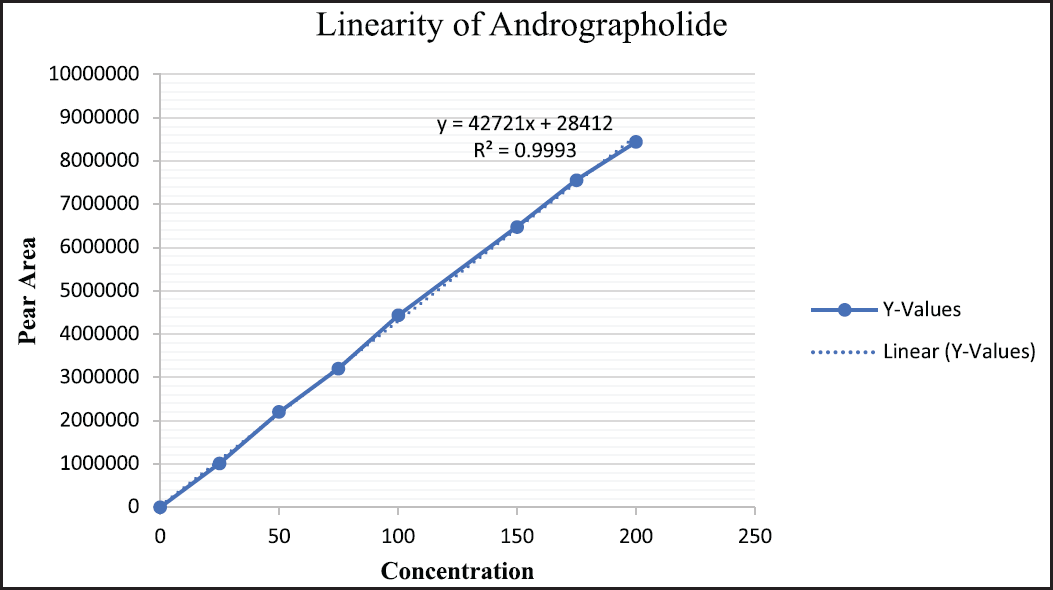 | Figure 6. Linearity of andrographolide. [Click here to view] |
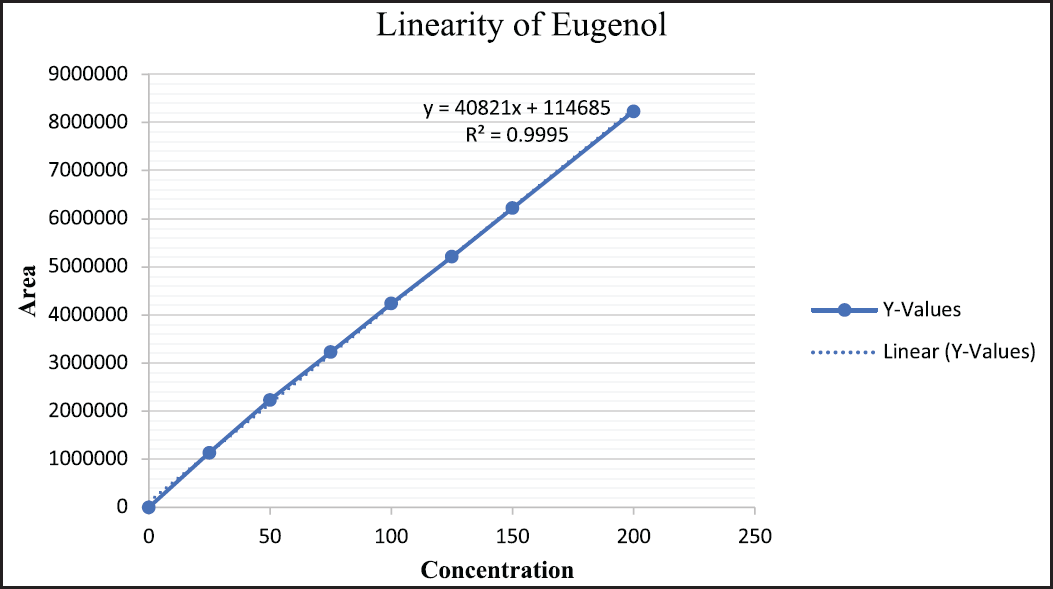 | Figure 7. Linearity of eugenol. [Click here to view] |
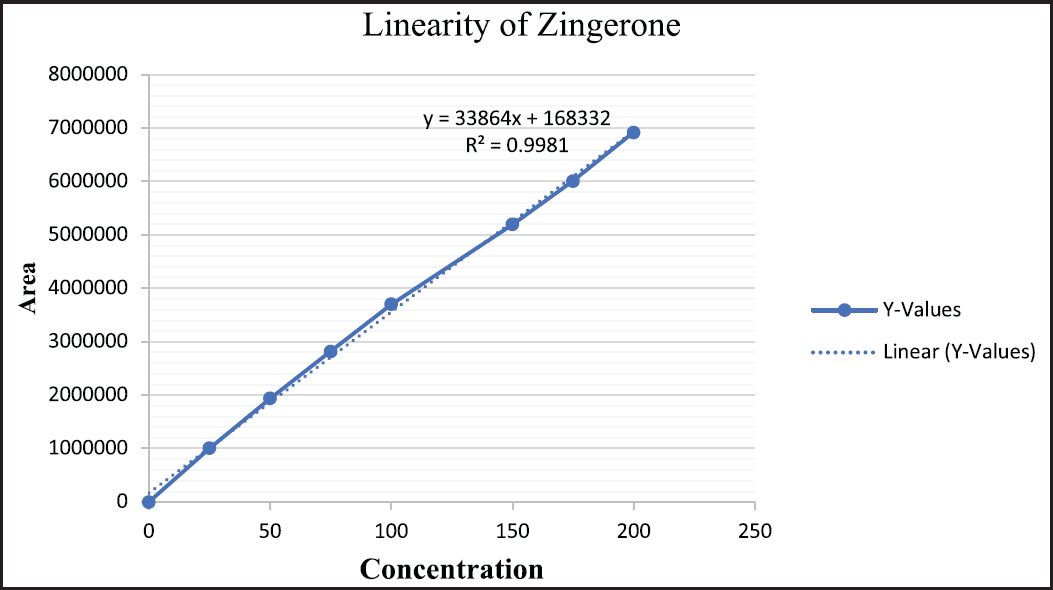 | Figure 8. Linearity of zingerone. [Click here to view] |
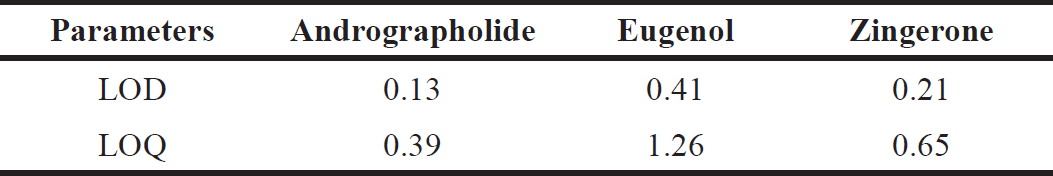 | Table 3. LOD and LOQ data for phytochemical markers. [Click here to view] |
Accuracy
The relative recovery, which was calculated at three different concentrations at three levels in the sample (50%, 100%, and 150%) was used to determine the accuracy of the method.
Robustness
Robustness is altering the parameters such as temperature and flow rate following deliberate conditions. By purposefully varying experimental parameters including column temperature and flow rate, the method’s durability was tested. To test the method’s robustness, flow rates of ± 1 ml/minutes and column temperatures of ± 40°C were used.
RESULTS
Optimization of chromatographic conditions
Mobile phase combinations containing methanol: water and acetonitrile: water in varying ratios were used in the trials but the Rt value was found to be high. These solvent combinations were used in a number of studies but they resulted in unsatisfactory peak shapes and unsuccessful separations. Finally, several ratios of ortho phosphoric acid and acetonitrile were explored for improved resolution of phytomarkers. Blank chromatogram is shown in Figure 1.
Quantification of phytomarkers
Optimized chromatographic parameters were established to estimate the amount of sample solutions to determine the phytomarkers present along with a mixture of individual standards. The blank chromatogram is shown in (Fig. 2). The mixture of standards andrographolide and zingerone (Fig. 3) standard chromatograms andrographolide, eugenol, and zingerone (Fig. 4). The sample chromatogram of NK is depicted in (Fig. 5). Based on the calibration curve, the results for each individual marker were determined.
System suitability
By extrapolating chromatographic conditions from the chromatogram of standard solutions, such as the number of theoretical plates (N), resolution (Rs), and tailing factor (Tf), the method’s system suitability was established. The results are displayed in Table 2. The specified chromatographic conditions were determined by system suitability parameters to be suitable for the development and validation of the method.
Linearity data for marker compounds
The linearity of calibration curves for andrographolide, eugenol, and zingerone was established in a range of 25–200 µg/ml. Representation of results obtained and the calibration curves are depicted in Figures 6–8.
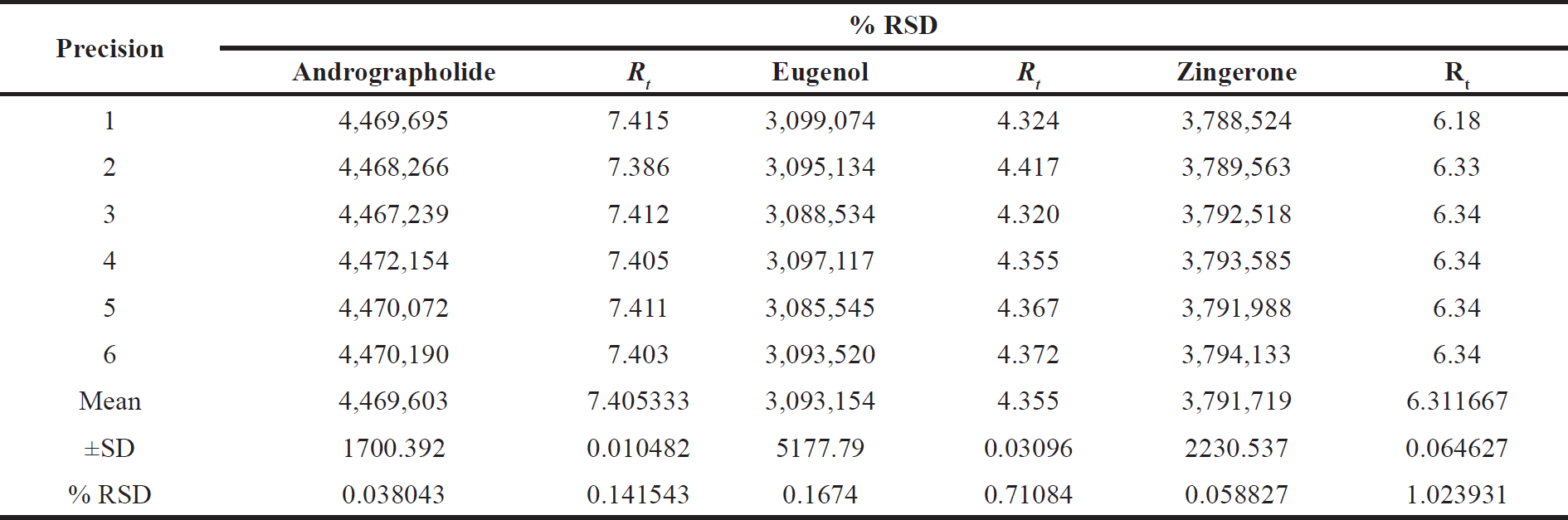 | Table 4. Precision study for Marker compounds. [Click here to view] |
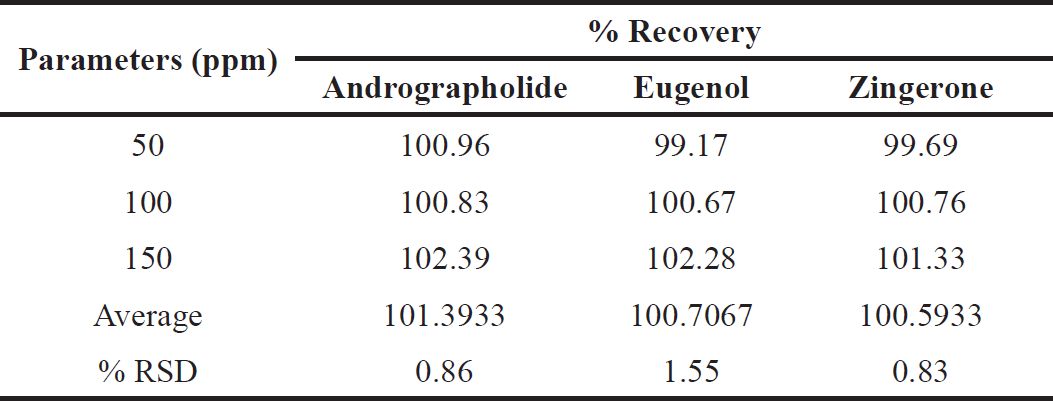 | Table 5. Accuracy data of marker compounds. [Click here to view] |
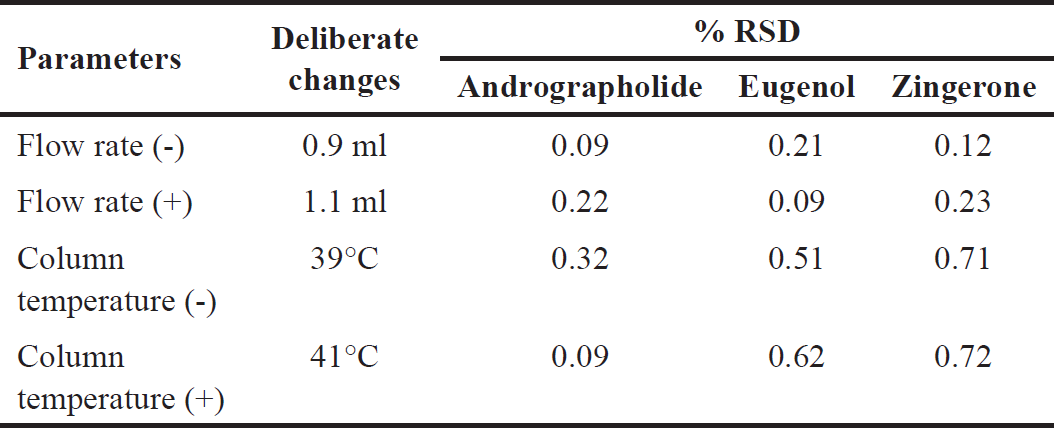 | Table 6. Results of robustness study. [Click here to view |
Detection limit and LOQ
The LOD and LOQ for andrographolide are 0.13 and 0.39 µg/ml, when compared to other methods andrographolide LOD and LOQ is superior because the published article shows the average range of 0.40 to 1.82. Very few studies were reported on zingerone which shows the LOD and LOQ level from 0.2 to 0.95. Hence, the reported LOD and LOQ for zingerone are superior then other methods. Eugenol shows slightly higher LOD and LOQ values when compared to other methods. Eugenol shows the average LOD and LOQ values from 0.09 to 1.34 µg/ml. The LOD and LOQ values were determined and results obtained are shown in Table 3.
Precision
The precision was measured six times a day and the % relative standard deviation (RSD%) ranged from 0.1% to 1.0%. The results obtained from precision study are shown in Table 4.
Accuracy
The accuracy of the assay method, measured as relative recovery at three concentration levels, was 100.5%–101.39%, with all RSD% values ≤ 2%. Recovery study results are shown in Table 5.
Robustness
The robustness of the developed method was established by intentionally adjusting the experimental conditions such as flow rate from ± 1 ml/min and the column temperature was varied from ±40°C. Results obtained from the robustness study are shown in Table 6.
DISCUSSION
The research involves the development of a new, simple, rapid, and sensitive RP-HPLC approach for the synchronous determination of andrographolide [18] eugenol [19] and zingerone [20] marker compounds in NK, a Siddha polyherbal formulation. Previous research has revealed that there are numerous methods for determining andrographolide [21] eugenol and zingerone separately. However, no RP-HPLC methods were reported for the simultaneous determination of these marker compounds in any herbal formulation. The research is primarily aimed at developing a rapid, accurate, and cost-effective method involving minimal sample preparation.
Several mobile phase combinations were tried during method development and validation. The isocratic mobile phase consisting of 30:70 acetonitrile and 0.1% ortho phosphoric acid was finalized to precisely estimate all three marker chemicals. This developed method was validated through factors including system suitability, accuracy, precision, linearity, LOD, quantification, and robustness.
Utilizing variables such as the theoretical plate count (N), resolution (Rs), and tailing factor (Tf), the system suitability research was established. Each parameter was perceived to be within the recommended range. The developed method was found to be appropriate for analyzing andrographolide, eugenol, and zingerone in the system. The proposed approach is linear for all three phytomarkers in the specified range, according to the obtained R2 > 0.999 for all standards in the linearity experiment. The least concentration required to quantify and identify the markers in the sample solution was estimated using LOD and LOQ as well. The intra-day precision study was used to determine the precision study. The study results, i.e., % RSD (2%), were within acceptable range in accordance with ICH recommendations.
Three different concentration levels of the phytomarkers were used to test their accuracy. For the compounds of interest, complete recovery of marker compounds was obtained, demonstrating the method’s ability to recover markers completely. The recovery study sample preparation process was simple and rapid mainly due to the short variation time. The method was determined to be resilient for simultaneous analysis by altering flow rate and temperature, according to the robustness study findings [22].
This optimized and developed method can be used in the estimation of andrographolide, eugenol, and zingerone siddha polyherbal formulations such as NK Ayurvedic and homeopathic formulations containing these three phytomarkers. These compounds can be used as a quality control tool for the standardization and evaluation of formulations. This study can be an imperative tool in developing new methods and validating these novel methods for utilization in Herbal industries.
CONCLUSION
With the increased demand for herbal medicine and enhanced usage of herbal medicines, the development of an authentic standardization method will help sustain the quality of such predominant polyherbal preparations. The developed RP-HPLC method is suitable for the simultaneous quantification of standard phytochemical markers andrographolide, eugenol, and zingerone and also for their estimation in NK liquid siddha formulation.
LIST OF ABBREVIATIONS
NK: nilavembu kudineer; HPLC: high performance liquid chromatography; Rt: retention time; N: number of theoretical plates; Tf: Tailing factor; RS: resolution factor; R2: regression coefficient; RSD: relative standard deviation; SD: standard deviation; LOD: limit of detection; LOQ: limit of quantification; ICH: International Council for Harmonization.
ACKNOWLEDGMENTS
The authors express their sincere gratitude to Seven Hills College of Pharmacy, Tirupati, for providing all required facilities to accomplish the entitled work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Pandey MM, Rastogi S, Rawat AK. Indian traditional ayurvedic system of medicine and nutritional supplementation. Evid Based Complement Alternat Med. 2013;2013:376327.
2. Chauhan A, Semwal DK, Mishra SP, Semwal RB. Ayurvedic research and methodology: present status and future strategies. Ayu. 2015;36(4):364–9.
3. Parasuraman S, Thing GS, Dhanaraj SA. Polyherbal formulation: concept of ayurveda. Pharmacogn Rev. 2014;8(16):73–80.
4. Srivastava A, Rengaraju M, Srivastava S, et al. Efficacy of two siddha polyherbal decoctions, nilavembu kudineer and kaba sura kudineer, along with standard allopathy treatment in the management of mild to moderate symptomatic COVID-19 patients-a double-blind, placebo-controlled, clinical trial. Trials. 2021;22(1):570.
5. Jain J, Kumar A, Narayanan V, et al. Antiviral activity of ethanolic extract of nilavembu kudineer against dengue and chikungunya virus through in vitro evaluation. J Ayurveda Integr Med. 2020;11(3):329–35.
6. Jayakumar T, Hsieh CY, Lee JJ, Sheu JR. Experimental and clinical pharmacology of andrographis paniculata and its major bioactive phytoconstituent andrographolide. Evid Based Complement Alternat Med. 2013;2013:846740.
7. Haro-González JN, Castillo-Herrera GA, Martínez-Velázquez M, Espinosa-Andrews H. Clove essential oil (Syzygium aromaticum L. Myrtaceae): extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules. 2021;26(21):6387.
8. Ahmad B, Rehman MU, Amin I, Arif A, Rasool S, Bhat SA, et al. A review on pharmacological properties of zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). Sci World J. 2015;2015:816364.
9. Song YX, Liu SP, Jin Z, Qin JF, Jiang ZY. Qualitative and quantitative analysis of Andrographis paniculata by rapid resolution liquid chromatography/time-of-flight mass spectrometry. Molecules. 2013;18(10):12192–207.
10. Pramod K, Ilyas UK, Kamal YT, Ahmad S, Ansari SH, Ali J. Development and validation of RP-HPLC-PDA method for the quantification of eugenol in developed nanoemulsion gel and nanoparticles. J Anal Sci Technol. 2013;4:1–16.
11. You H. et al. Determination of bioactive nonvolatile ginger constituents in dietary supplements by a rapid and economic HPLC method: analytical method development and single-laboratory validation. Talanta. 2019;194:795–802.
12. Karioti A, Timoteo P, Bergonzi MC, Bilia AR. A Validated method for the quality control of andrographis paniculata preparations. Planta Med. 2017;83(14–15):1207–13.
13. Gursale A, Dighe V, Parekh G. Simultaneous quantitative determination of cinnamaldehyde and methyl eugenol from stem bark of Cinnamomum zeylanicum Blume using RP-HPLC. J Chromatogr Sci. 2010:48(1):59–62.
14. Zick SM, Ruffin MT, Djuric Z, Normolle D, Brenner DE. Quantitation of 6-, 8- and 10-gingerols and 6-shogaol in human plasma by high-performance liquid chromatography with electrochemical detection. Int J Biomed Sci. 2010;6(3):233–40.
15. Patel MB, Kadakia VM, Mishra SH. Simultaneous estimation of andrographolide and wedelolactone in herbal formulations. Indian J Pharm Sci. 2008;70(5):689–93.
16. Yun SM, Lee MH, Lee KJ, Ku HO, Son SW, Joo YS. Quantitative analysis of eugenol in clove extract by a validated HPLC method. J AOAC Int. 2010;93(6):1806–10.
17. Kamal YKTK, Singh M, Ahmad S, Alam P, Salam S. Stability-indicating RP-HPLC method for the determination of 6-gingerol in polyherbal formulations. J Anal Sci Technol. 2015;6:1–23.
18. Villedieu-Percheron E, Ferreira V, Campos JF, Destandau E, Pichon C, Berteina-Raboin S. Quantitative determination of andrographolide and related compounds in Andrographis paniculata extracts and biological evaluation of their anti-inflammatory activity. Foods. 2019;8(12):683.
19. Xu T, Pan J, Zhao L. Simultaneous determination of four andrographolides in Andrographis paniculata Nees by silver ion reversed-phase high-performance liquid chromatography. J Chromatogr Sci. 2008;46(8):747–50.
20. Zhao Y, Kao CP, Wu KC, Liao CR, Ho YL, Chang YS. Chemical compositions, chromatographic fingerprints and antioxidant activities of Andrographis Herba. Molecules. 2014;19(11):18332–50.
21. Foudah AI, Shakeel F, Alqarni MH, Ross SA, Salkini MA, Alam P. Simultaneous estimation of cinnamaldehyde and eugenol in essential oils and traditional and ultrasound-assisted extracts of different species of cinnamon using a sustainable/green HPTLC technique. Molecules. 2021;26:2054.
22. Xiong H, Yu LX, Qu H. Batch-to-batch quality consistency evaluation of botanical drug products using multivariate statistical analysis of the chromatographic fingerprint. AAPS Pharmscitech. 2013;14(2):802–10.