An unlimited scope for novel formulations as orally disintegrating systems: Present and future prospects
Reeta Rani Thakur, Mridul Kashi
Pages: 13-19

Design and development of Orally Disintegrating Tablets of Famotidine Prepared by Direct Compression Method Using Different Superdisintegrants
Mahaveer Pr. Khinchi, M.K.Gupta, Anil Bhandari, Natasha Sharma, Dilip Agarwal
Pages: 50-58

Isolation of Mucilage from Cydonia vulgaris Pers. Seeds and its Evaluation as Superdisintegrant
Nisarg C Patel, Vaishali N Shah, Ashok N Mahajan, Dushyant A Shah
Pages: 110-114

Formulation and evaluation of orally disintegrating tablet containing tramadol hydrochloride by mass extrusion technique
Mansing G. Patil, Suhas M. Kakade, Sandhya G. Pathade
Pages: 178-181

Development of validated liquid chromatographic method for estimation of levocetirizine from pharmaceutical dosage forms
Chaitanya Prasad MK, Vidyasagar G, Sambasiva Rao KRS, Madhusudhanareddy Induri, Ramanjeneyulu S
Pages: 95-97

Formulation and Evaluation of Gastroretentive Floating Tablets of Domperidone Maleate
D. Saritha, D. Sathish, Y. Madhusudan Rao
DOI: 10.7324/JAPS.2012.2311Pages: 68-73

Formulation and Evaluation of Bilayer Tablets of Diclofrenac Sodium with Ranitidine HCL for Sustained and Immediate Release
Prabhakar Shirse
DOI: 10.7324/JAPS.2012.2523Pages: 136-141

Formulation and Evaluation of Sustained ReleaseMatrix Tablets of Glimepiride Based on Combinationof Hydrophilic and Hydrophobic Polymers
Mohd Abdul Hadi, V. Lokeswara Babu, Narottam Pal
DOI: 10.7324/JAPS.2012.2613Pages: 101-107

Effect of Excipients on Oxcarbazepine Release from Modified Release Matrix Tablets
Buchi N. Nalluri, S. Vidyasagar, K. M. Maheswari
DOI: 10.7324/JAPS.2012.2826Pages: 150-158

In-vitro bioequivalence study of 8 brands of metformin tablets in Iran market
Parvin Zakeri-Milani, Peyman Nayyeri-Maleki, Saeed Ghanbarzadeh, Mahboob Nemati, Hadi Valizadeh
DOI: 10.7324/JAPS.2012.2834Pages: 194-197

Orally Disintegrating Tablets: An Overview
Evren ALÄžIN YAPAR
DOI: 10.7324/JAPS.2014.40219Pages: 118-125

Formulation of Desloratadine Oral Disintegrating Tablets
Mohamed A. Etman, Mona Gamal, Aly H. Nada, Mohamed A. Shams-Eldeen
DOI: 10.7324/JAPS.2014.41110Pages: 054-061

Comparative Evaluation of the Disintegrant Properties of Rice Husk Cellulose, Corn Starch and Avicel in Metronidazole Tablet Formulation
Onyinye Jennifer Uwaezuoke, Oluyemisi A. Bamiro, Ndidi C. Ngwuluka, Omolola Tolulope Ajalla, Aderonke O. Okinbaloye
DOI: 10.7324/JAPS.2014.41219Pages: 112-117

The Compaction, Mechanical and Disintegration Properties of Modified Pennisetum glaucum (Poaceae) Starch in Directly Compressed Chloroquine Tablet Formulations
Mbang N. Femi-Oyewo, Tolulope O. Ajala, Damilola Babs-Awolowo
DOI: 10.7324/JAPS.2015.50207Pages: 043-050

Fabrication of Bucco-matrix tablets of Amoxicillin trihydrate on the basis of release and permeation kinetics
Gopa Roy Biswas, Subhasis Chakraborty, Nabarun Ghosh, Sutapa Biswas Majee
DOI: 10.7324/JAPS.2015.50408Pages: 048-052

Development of Enteric Coated Sustained Release Matrix Tablets of Sertraline Hydrochloride
Pravallika Uppala, Salma shaik, Saisri Anusha Valluru, Buchi N Nalluri
DOI: 10.7324/JAPS.2015.50410Pages: 058-064

A Five-Year Stability Study of Controlled-Release Diltiazem Hydrochloride Tablets Based on Poly(Ethylene Oxide)
Laila H. Emara, Ahmed A. El-Ashmawy, Nesrin F. Taha
DOI: 10.7324/JAPS.2015.50703Pages: 012-022

Formulation, in vitro Characterization and Stability Studies of Fast Dispersing Tablets of Diclofenac Sodium
Ranjit Prasad Swain, R. Nagamani, Satyajit Panda
DOI: 10.7324/JAPS.2015.50715Pages: 094-102

In-vitro bioequivalence, physicochemical and economic benefits study for marketed innovator and generic ciprofloxacin hydrochloride tablets in Saudi Arabia
Ahmed F. Hanafy
DOI: 10.7324/JAPS.2016.60909Pages: 063-068

Fast disintegrating tablets of amiodarone for intra-oral administration
Ebtessam Essa, Marwa Negm, Esmat Zin Eldin, Gamal El Maghraby
DOI: 10.7324/JAPS.2017.70109Pages: 064-072

Validated Ultraviolet-Spectrometric Method for Determination of Sofosbuvir in Tablets Formulation
Mohamed A. El Hamd, Ramadan Ali, Adel A. Marzouk, Osama H. Abdelmageed
DOI: 10.7324/JAPS.2017.70214Pages: 114-119

In vitro and In vivo Evaluation of Tablets Containing Meloxicam- PEG 6000 Ball-Milled Co-Ground Mixture
Mohamed Etman, Mustafa Shekedef, Aly Nada, Assem Ismail
DOI: 10.7324/JAPS.2017.70306Pages: 031-039

Design of buccal mucoadhesive tablets: understanding and development
Laisa Lis Fontinele de Sá, Naiane Carvalho Nogueira, Edson Cavalcanti Da Silva Filho, Ana Figueiras, Francisco Veiga, Lívio César Cunha Nunes, José Lamartine Soares-Sobrinho
DOI: 10.7324/JAPS.2018.8223Pages: 150-163

Design and Evaluation of Zolpidem Tartrate Matrix Tablets for Extended Release Using Natural Gums and HPMC K100M
Siramsetti Dhanalakshmi, Srinivasa Rao Baratam
DOI: 10.7324/JAPS.2018.8712Pages: 072-077

Design and characterization of intra-oral fast dissolving tablets containing diacerein-solid dispersion
Azza Hasan, Abd El hakim Ramadan, Mahmoud Abd Elghany, Shereen Sabry
DOI: 10.7324/JAPS.2020.10607Pages: 044-053

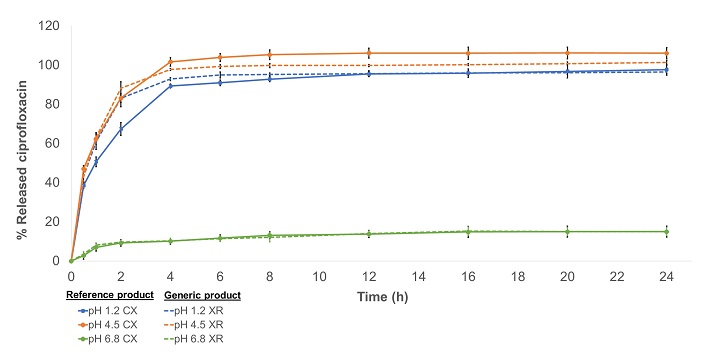
Comparison between the dissolution profiles of prolonged-release ciprofloxacin tablets available in the Colombian market
Andrés Vicente De la Cruz Gómez, Raynni Marcela Ramos Iglesias, Tatiana Sugey Ruiz Afanador, Indira Beatriz Pájaro Bolívar, Gina Paola Domínguez Moré
DOI: 10.7324/JAPS.2022.120322Pages: 209–217
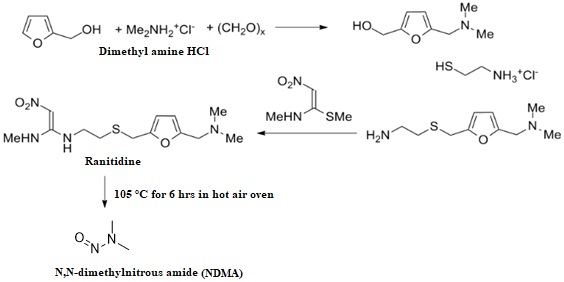
Novel stability indicating LC-MS method for N-Nitroso dimethyl amine genotoxic impurity quantification in ranitidine drug substance and drug product
Ganpisetti Srinivasa Rao, Dharamasoth Ramadevi, B. M. Rao, Nagaraju Rajana, K. Basavaiah
DOI: 10.7324/JAPS.2022.120711Pages: 106-114
Quality by design approach for the formulation of bilayer tablets of domperidone and itopride in gastro-esophageal reflux disease
Roshani Prajapati, Bhavna Kumar, Jagannath Sahoo, Shailendra Shakya, Diwya Kumar Lal
DOI: 10.7324/JAPS.2024.168489Pages: 169-181

Development and in-vitro evaluation of multilayer mucoadhesive buccal tablets of metoprolol tartrate with chitosan extracted from crustacean shells
V. V. Siva Krishna Pushadapu, Srinivasa Babu Puttagunta, Javed Ali
DOI: 10.7324/JAPS.2024.197009Pages: 224-233
.jpg)