Quantitative analysis of valsartan in tablets formulations by High Performance Thin-Layer Chromatography
Della Grace Thomas Parambi, Molly Mathew, V.Ganesan
Pages: 76-78

Comparative in vitro dissolution study of Aceclofenac Marketed Tablets in Two Different Dissolution Media by Validated Analytical Method
S.M. Ashraful Islam, Sharmi Islam, Mohammad Shahriar, Irin Dewan
Pages: 87-92

Selective High Performance Liquid Chromatographic Determination of Amodiaquine and Artesunate in bulk and pharmaceutical formulation
P. S. Jain, A. J. Chaudhari and S. J. Surana
DOI: 10.7324/JAPS.2013.30313Pages: 066-070

Simple Spectrophotometric Method for Estimation of Raltegravir Potassium in Bulk and Pharmaceutical Formulations
Girija B. Bhavar, Sanjay S. Pekamwar, Kiran B. Aher, Sanjay R. Chaudhari
DOI: 10.7324/JAPS.2013.31026Pages: 147-150

RP-HPLC-PDA method development and validation for the analysis of Tadalafil in bulk, pharmaceutical dosage forms and in-vitro dissolution samples
Aziz Unnisa, Yogesh Babu, Santosh kumar suggu, Siva Chaitanya
DOI: 10.7324/JAPS.2014.41213Pages: 072-076

Method Development and Validation using UV Spectrophotometry for Nigella sativa Oil Microparticles Quantification
Ahmad Fahmi Harun Ismail, Abd Almonem Doolaanea, Farahidah Mohamed, NurIzzati Mansor, Mohd Affendi Mohd Shafri, Fathin Athirah Yusof
DOI: 10.7324/JAPS.2015.50915Pages: 082-088

A new rapid Stability indicating RP-PDA-UPLC method for the estimation of Assay of Pemetrexed disodium-An anti-Lung cancer drug from lyophilized parenteral formulation
Vamsi Krishna Galla, V. Archana, Rajeswari Jinka
DOI: 10.7324/JAPS.2017.71019Pages: 131-137

Development and validation of high-performance liquid chromatography method for simultaneous determination of acyclovir and curcumin in polymeric microparticles
Jéssica Brandão Reolon, Maicon Brustolin, Sandra Elisa Haas, Eduardo André Bender, Marcelo Donadel Malesuik, Letícia Marques Colomé
DOI: 10.7324/JAPS.2018.8120Pages: 136-141

A new quantitative reverse phase high-performance liquid chromatographic method for the quantification of Rilpivirine hydrochloride in bulk and dosage form
Sonam Patel, Krishnaveni Nagappan, Gouru Santhosh Reddy
DOI: 10.7324/JAPS.2018.81122Pages: 157-162

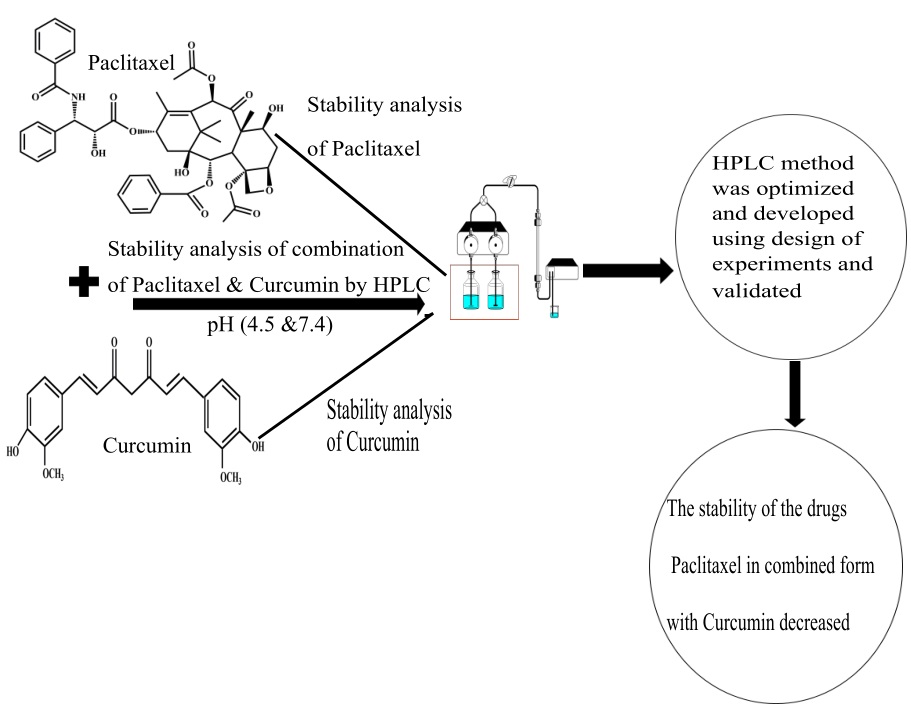
Simultaneous estimation of paclitaxel and curcumin in nano-formulation: Stability analysis of drugs, optimization and validation of HPLC method
Joyceline Praveena, Bharath Raja Guru
DOI: 10.7324/JAPS.2021.110308Pages: 071-083
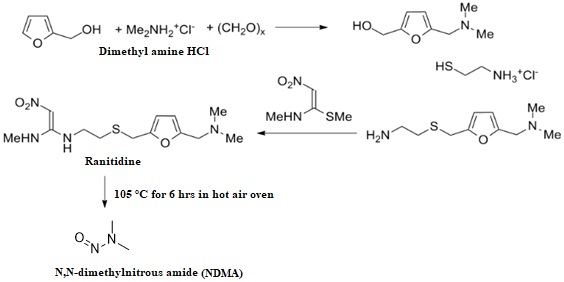
Novel stability indicating LC-MS method for N-Nitroso dimethyl amine genotoxic impurity quantification in ranitidine drug substance and drug product
Ganpisetti Srinivasa Rao, Dharamasoth Ramadevi, B. M. Rao, Nagaraju Rajana, K. Basavaiah
DOI: 10.7324/JAPS.2022.120711Pages: 106-114
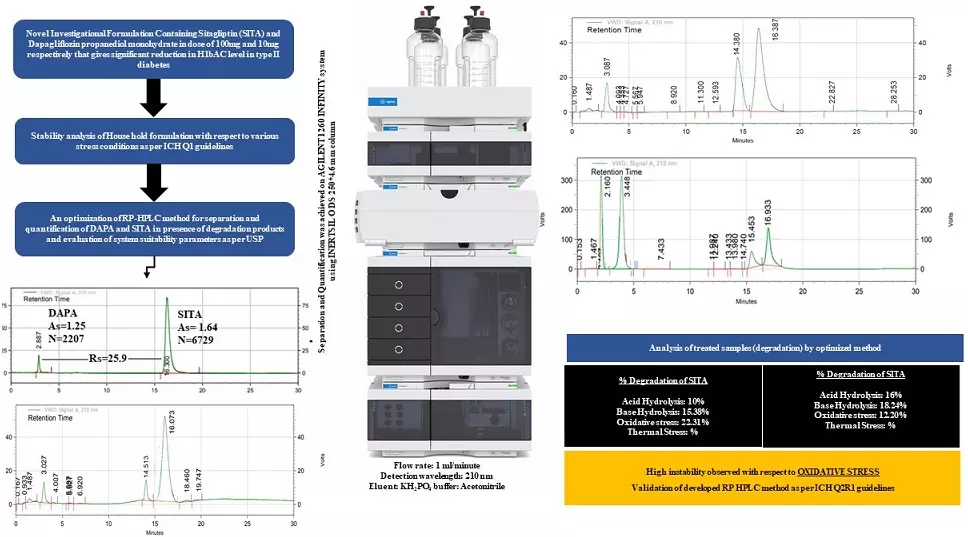
Quantitative computation and stability evaluation of phase III composition comprising sitagliptin and dapagliflozin propanediol monohydrate by RP-HPLC
Yesha Darshak Patel, Pinak Rameshbhai Patel, Jigna Bhatt, Binny Mehta, Krunal Detholia
DOI: 10.7324/JAPS.2022.120614Pages: 148-155
QbD-based RP-HPLC method development for quantitative computation of phase III composition comprising apixaban and clopidogrel
Rashmi Shukla, Ankit Chaudhari, Pinak Patel, Krunal Detholia
DOI: 10.7324/JAPS.2024.181311Pages: 085-093

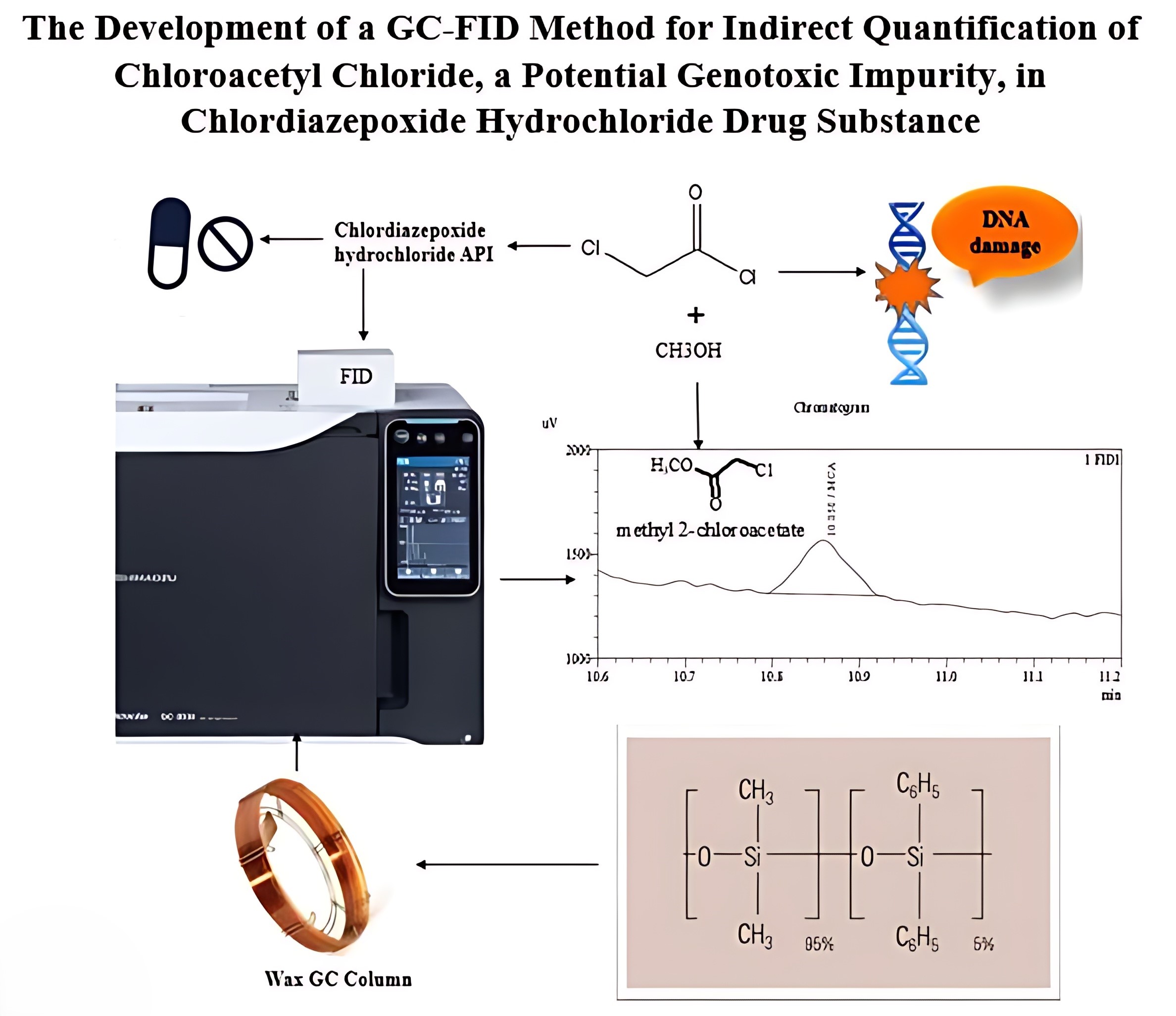
The development of a GC-FID method for indirect quantification of chloroacetyl chloride, a potential genotoxic impurity, in chlordiazepoxide hydrochloride drug substance
Srinivas Birudukota, Bhaskar Mangalapu, Ramesha Andagar Ramakrishna, Swagata Halder, Venkata Narayana Palakollu
DOI: 10.7324/JAPS.2024.182017Pages: 196-207
A sensitive liquid chromatography tandem mass spectrometric method development and validation for ribociclib and its formulation
J. Ramesh, B. Babu, R. Sangamithra, D. Anandha Jothi, S.N. Meyyanathan, B. Gowramma
DOI: 10.7324/JAPS.2024.178562Pages: 227-232
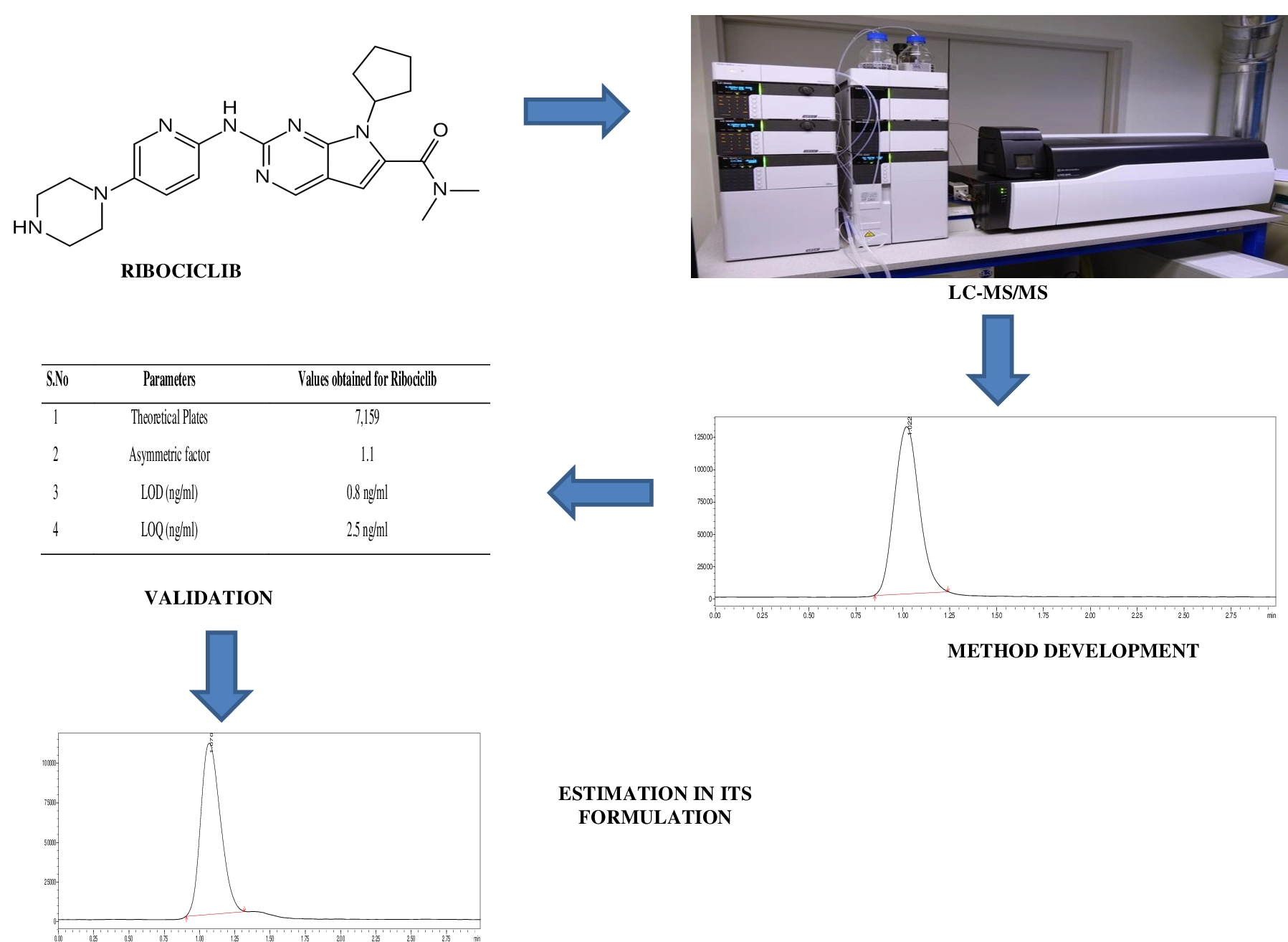
Novel bioanalytical LC-MS/MS method for determination of metoprolol in human plasma
Lakshmana Rao Atmakuri, Raveesha Peeriga, Shabana Begum, Narender Gaddamedi, Bhaskar Vallamkonda, Anupama Baratam
DOI: 10.7324/JAPS.2024.657641Pages: 131-138
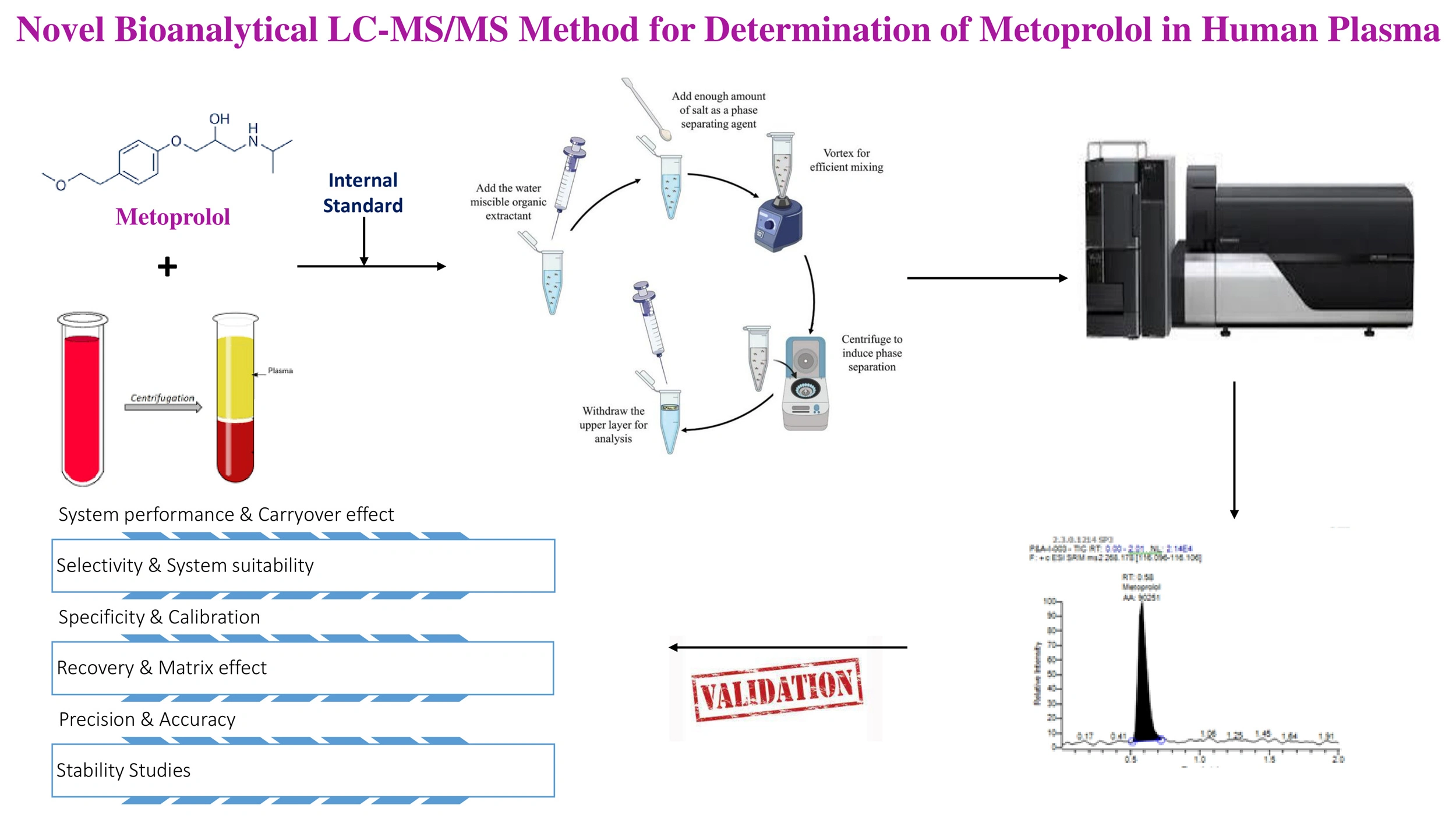
QbD assisted RP-HPLC method for determination of Pyridoxine and Doxylamine in pharmaceutical formulation using central composite design
Gangu Naidu Challa, Daniel Raju Kunda, Sheik Jakir Hussain Mustaq, Nagabharathi Marni, Srilekhya Ketha, Urmila Gorle, Shravitha Jakkula, Bhagavan Rajesh Babu Koppisetty
DOI: 10.7324/JAPS.2025.205442Pages: 072-083
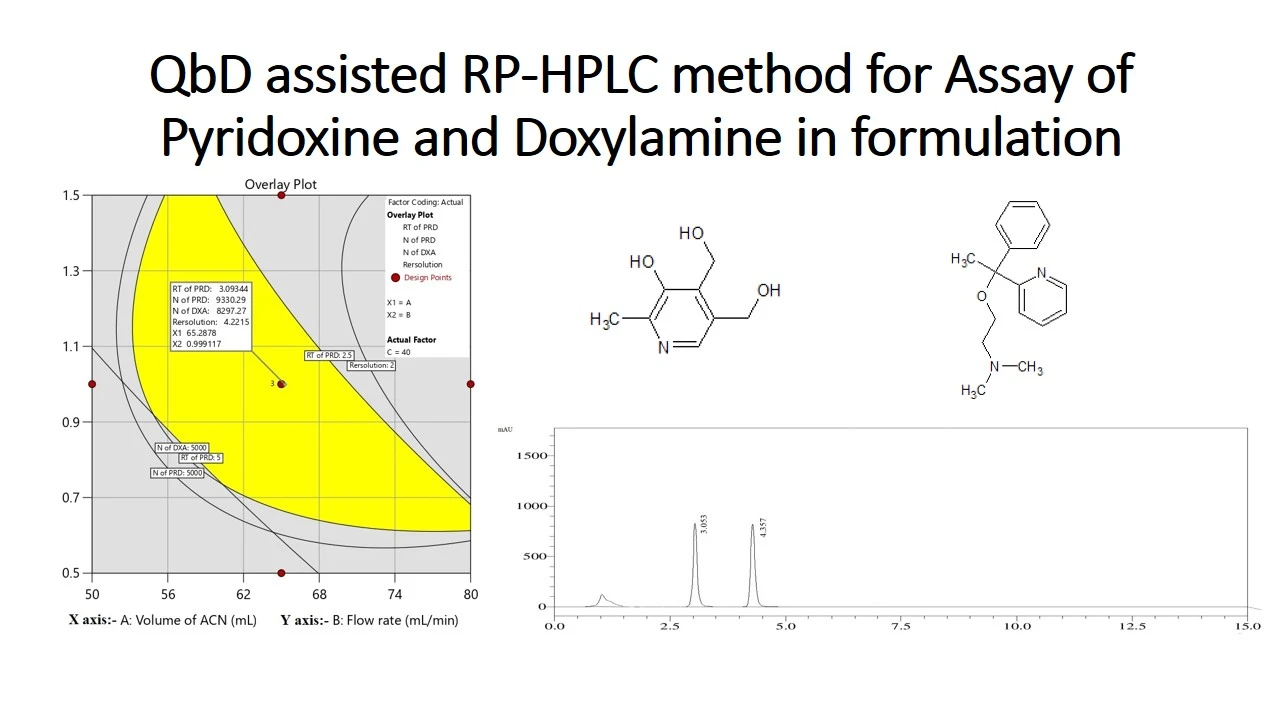
Development and application of an LC-MS/MS method for the detection of N-nitrosochlordiazepoxide as a potential genotoxic impurity
Srinivas Birudukota, Bhaskar Mangalapu, Ramesha Andagar Ramakrishna, Swagata Halder
DOI: 10.7324/JAPS.2025.199616Pages: 242-253
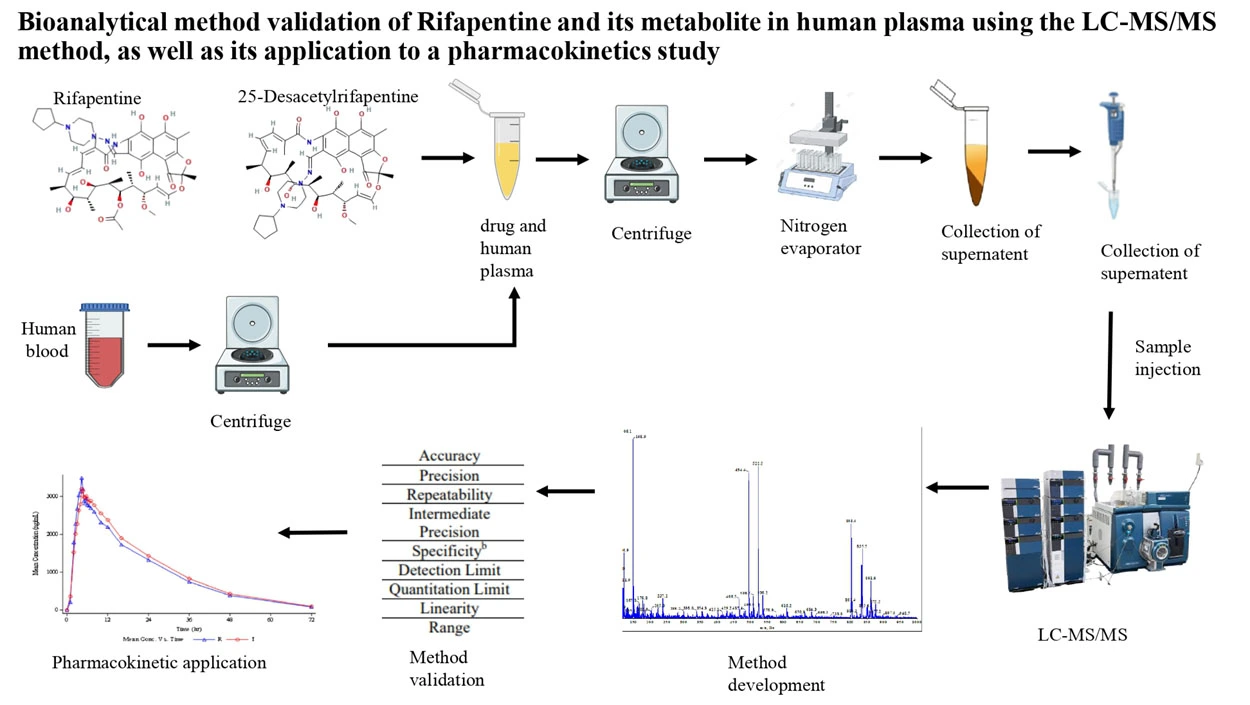
Bioanalytical method validation of rifapentine and its metabolite in human plasma using the LC-MS/MS method, as well as its application to a pharmacokinetics study
Rutuja Parghale, Radhika Inapakolla, Vijay Durga Rao Tikka, Rajesh Kumar Suvvaru, Pradnya Date, Vaishnavi Gawade, Ande Anil, Swati Changdeo Jagdale
DOI: 10.7324/JAPS.2025.210914Pages: 179-201
Development and validation of an LC-MS/MS method for pharmacokinetic assessment of tucatinib in rat plasma
Bandaru Venkata Ramarao, Anand Solomon Kamalakaran
DOI: 10.7324/JAPS.2025.202389Pages: 225-233
_.jpg)