Adherence to the Standard Guidelines for Prescription of Antidiabetic Agents in Patients with Type 2 DM
Qasim M. Alhadidi, Ahmed S. Sahib, Ali M. Jaffer, Maha H. Ismael, Ekhlas K. Hassan, Saja M. Shareef, Asmaa M. Shoesh, Asia S. Dawood
DOI: 10.7324/JAPS.2012.2719Pages: 138-143

Simple Spectrophotometric Method for Estimation of Raltegravir Potassium in Bulk and Pharmaceutical Formulations
Girija B. Bhavar, Sanjay S. Pekamwar, Kiran B. Aher, Sanjay R. Chaudhari
DOI: 10.7324/JAPS.2013.31026Pages: 147-150

Drug promotional literatures (DPLs) evaluation as per World Health Organization (WHO) criteria
Jadav SS, Dumatar CB and Dikshit RK
DOI: 10.7324/JAPS.2014.40613Pages: 084-088

Survey on the Pharmaceutical Quality of Herbal Medicines Sold in Nigeria
Philip F. Builders, Chris A. Alalor, John A. Avbunudiogba, Isreal E. Justice
DOI: 10.7324/JAPS.2015.50616Pages: 097-103

Stability Indicating RP-HPLC Method for the Simultaneous Estimation of Pyrimethamine and Sulphadoxine in Bulk and Tablet Dosage Form
Veeragoni Anil Kumar, Vasudeva Murthy Sindgi, Shoba Rani Satla, Manish Kumar Thimmaraju
DOI: 10.7324/JAPS.2016.60312Pages: 071-076

Estimation of Bosentan Monohydrate in Male Rabbit Plasma by using RP-HPLC Method
Revathi Mannam, Indira Muzib Yallamalli
DOI: 10.7324/JAPS.2017.71116Pages: 106-109

Evaluation of Proton Pump Inhibitors Prescribing among Non-Critically Ill Hospitalized Patients in a Malaysian Tertiary Hospital
Mohamed Hassan Elnaem, Mohamad Haniki Nik Mohamed, Amirul Hazim bin Nazar, Rabiatul Nur Khaliesa binti Ibrahim
DOI: 10.7324/JAPS.2017.71210Pages: 077-083

Simultaneous estimation of lidocaine and prilocaine in topical cream by green gas chromatography
Hashim Chekku Marakkarakath, Gurupadayya Bannimath, Prachi Pramesh Raikar
DOI: 10.7324/JAPS.2019.90310Pages: 066-072
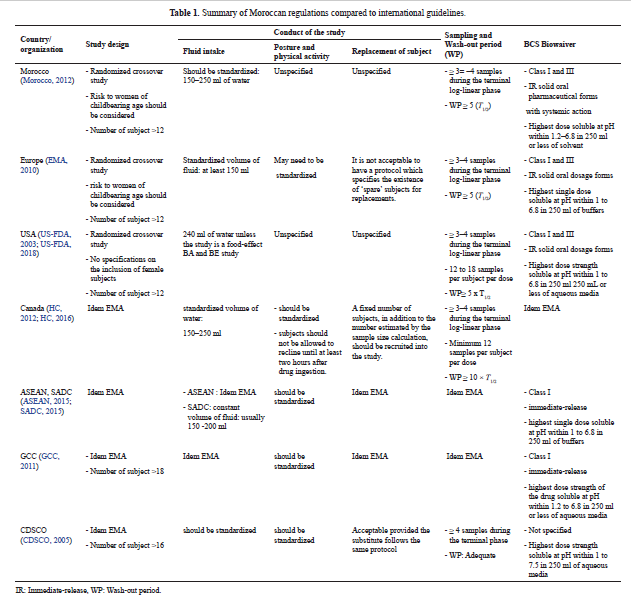
Bioequivalence regulation in emerging countries: Example of Moroccan regulations on immediate release formulations and comparison with international guidelines
Casimir Adade Adade, Amine Cheikh, Yahia Cherrah, Mustapha Bouatia, Jean Michel Cardot
DOI: 10.7324/JAPS.2019.91104Pages: 028-035
Stability indicating RP-HPLC method for simultaneous determination of pyrimethamine and sulfamethoxypyrazine in pharmaceutical formulation: Application to method validation
Shankaranahalli Gurusiddappa Keshava, Gurupadayya Bannimath, Prachi Raikar, Maruthi Reddy
DOI: 10.7324/JAPS.2020.102008Pages: 049-055
LC-MS method development for the quantitation of potential genotoxic impurity 2-Methyl-6-nitro aniline in Telmisartan API
Duvvuri Suryakala, Sivakumar Susarla, Bandlamudi Mallikarjuna Rao
DOI: 10.7324/JAPS.2020.10512Pages: 092-096
Simultaneous detection and quantification of bronchodilators in pure form and from in-vitro drug release of a novel combinational formulation
Sheena M. Raj, Vilas G. Jamakandi, Sunil S. Jalalpure, Pradeepkumar M. Ronad
DOI: 10.7324/JAPS.2020.10815Pages: 131-138

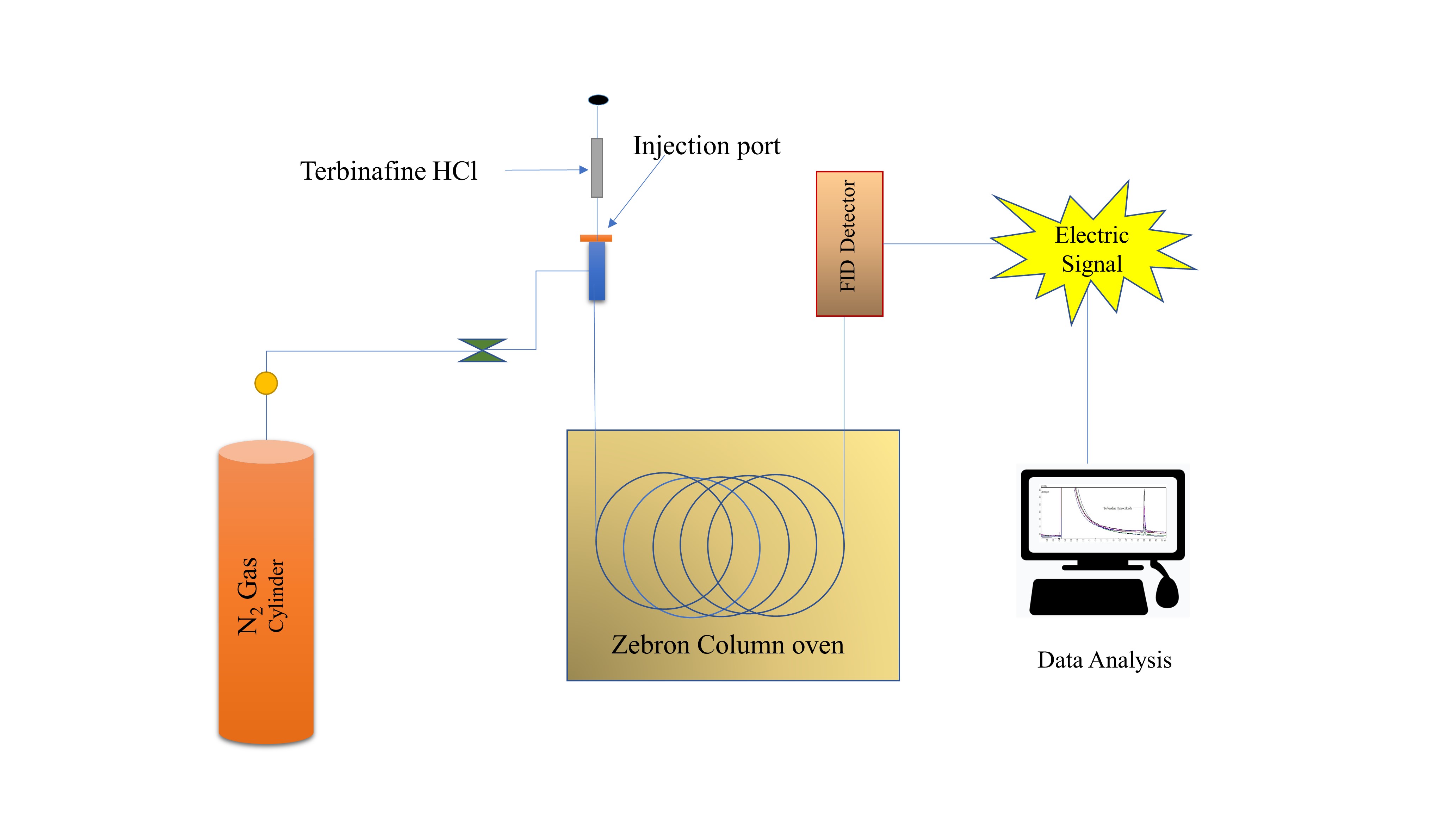
Estimation of terbinafine HCl in tablet dosage form by green gas chromatography
Kalyani Reddy, Gurupadayya Bannimath, Maruthi Reddy, Akshay Nanjundappa
DOI: 10.7324/JAPS.2021.110610Pages: 087-093
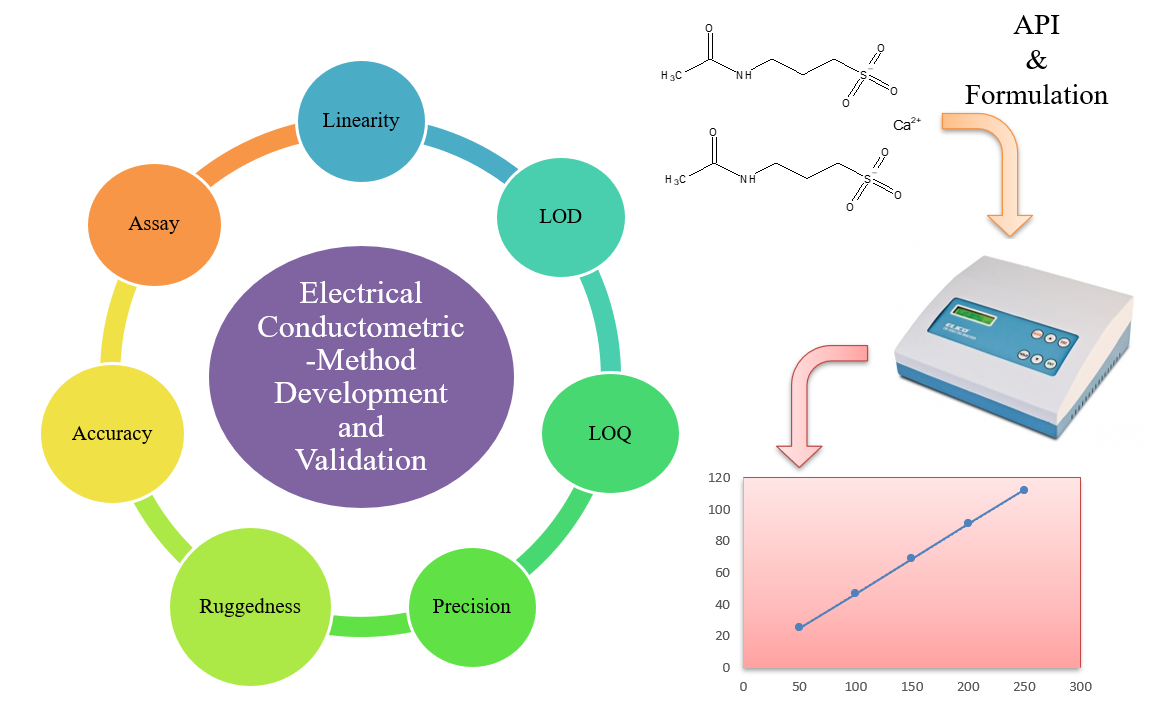
Conductometric method development and validation to estimate acamprosate calcium in API and marketed formulation
Rahul K. Yadav, Meenaxi M. Maste, Shailendra S. Surywanshi, Utkarsh Shastri
DOI: 10.7324/JAPS.2021.1101111Pages: 082–086
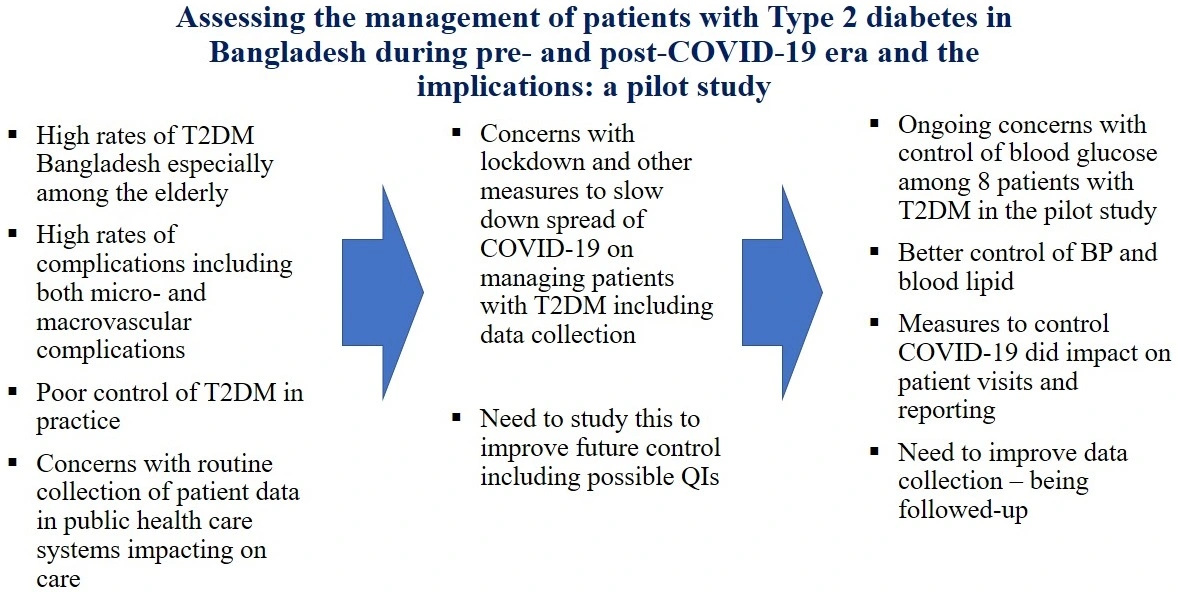
Assessing the management of patients with type 2 diabetes in Bangladesh during pre- and post-COVID-19 era and the implications: A pilot study
Farhana Akter, Mainul Haque, Sanira Akter, Gias Uddin, Naim Chy, Francis Kalemeera, Amanj Kurdi, Kona Chowdhury, Brian Godman
DOI: 10.7324/JAPS.2022.120506Pages: 088-097
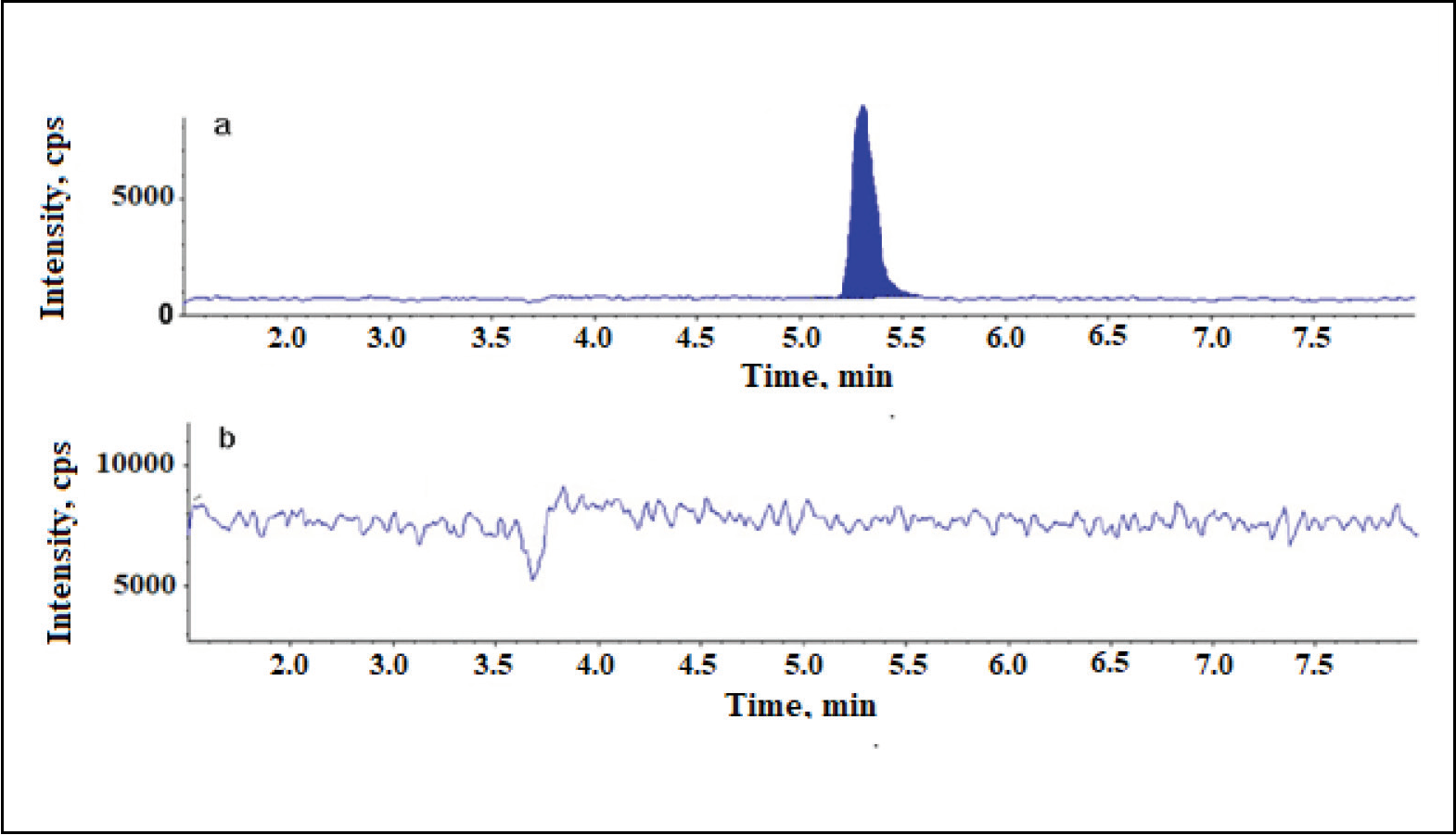
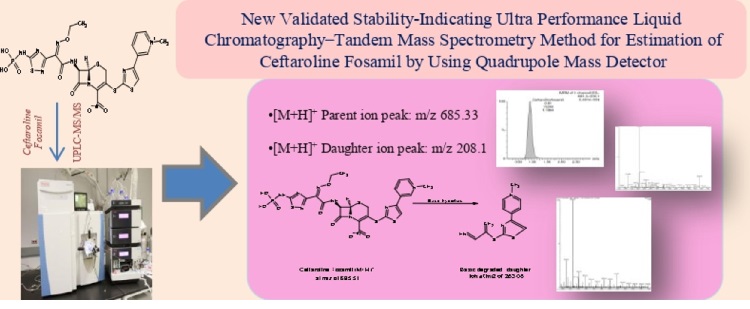
Newly validated stability-indicating ultra-performance liquid chromatography-tandem mass spectrometry method for the estimation of Ceftaroline Fosamil by using a quadrupole mass detector
Jabeen, Bangalore Venkatappa Suma
DOI: 10.7324/JAPS.2022.120621Pages: 215-223
Adherence to international perioperative antimicrobial prophylaxis guidelines in three general hospitals: A cross-sectional study
Hala ZI Alagha, Manal Shehata Zourab
DOI: 10.7324/JAPS.2023.35467Pages: 081-088

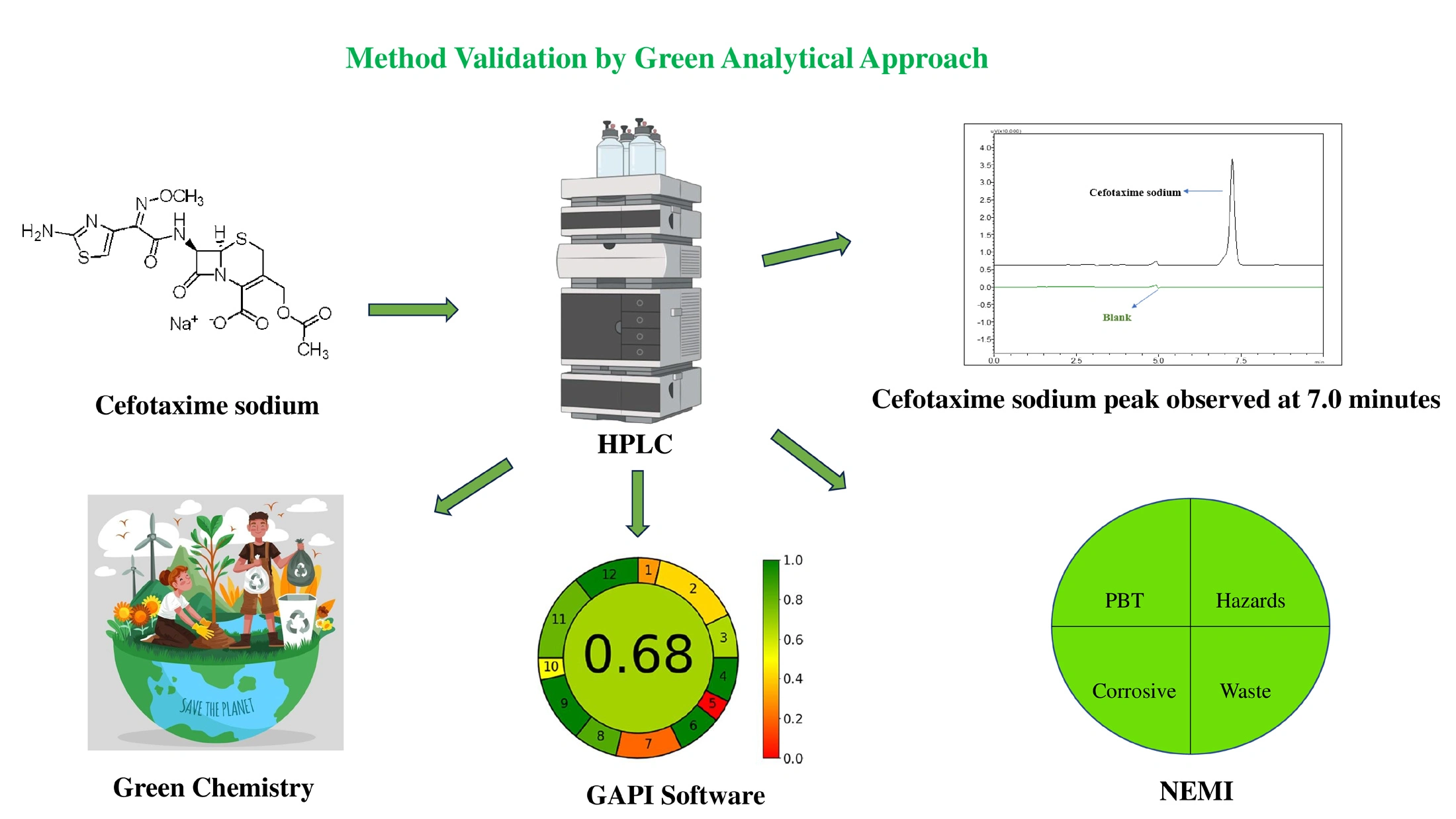
RP-HPLC method for quantification of cefotaxime sodium by using design of experiment, a green analytical approach: Analytical method development, validation, and application
Akhil Nair, H. Raghu Chandrashekhar, Usha Y. Nayak
DOI: 10.7324/JAPS.2024.192430Pages: 098-112
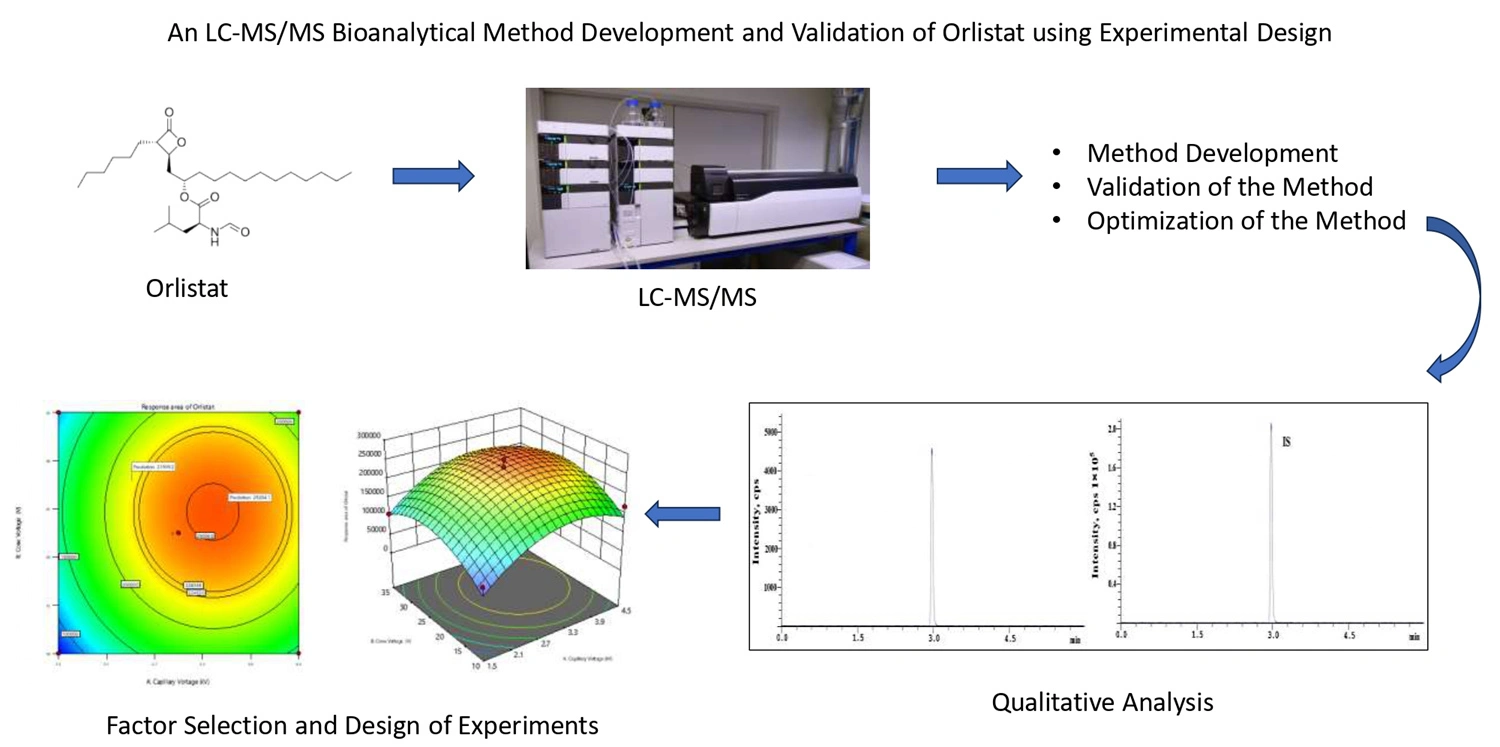
Development and validation of a new LC-MS/MS method for the determination of orlistat in biological matrices using experimental design
Rubina Kauser, Sunil Kumar Chaitanya Padavala, Venkatesan Palanivel
DOI: 10.7324/JAPS.2024.191461Pages: 196-204