INTRODUCTION
Cefotaxime sodium (CTX) is a beta-lactam antibiotic that contains a beta-lactam ring (Fig. S1; Supplementary Materials). It is classified as a third-generation cephalosporin with a chemical formula of C16H16N5 NaO7S2. The Food and Drug Administration (FDA) has approved CTX to treat anaerobic, Gram-negative, and Gram-positive bacterial infections. The antibiotic is commonly used to treat various conditions, including urinary tract infections, skin infections, intra-abdominal infections, joint infections, gynecological infections, lower respiratory tract infections, and septicemia. CTX is administered through the parenteral route. Several methods are available for the analysis and quantification of CTX. Literature reported methods for the determination of CTX, along with obtained analytical parameters are given in Table 1. The United States Pharmacopeia and Europe Pharmacopeia have established a method using a gradient of phosphate buffer and methanol for 60 minutes at a flow rate of 1.0 ml/minute. However, this method has a long run time and requires harmful solvents such as methanol, which does not align with green analytical chemistry (GAC) principles [1].
To the best of our knowledge, no article has been published till now for HPLC method development and validation of CTX using design of experiment (DoE), greenness tools, and GAC being a more sustainable, precise, fast, environment-friendly approach. Guidelines by organizations such as European Medicines Agency (EMA), US-FDA, and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) are inclined even to detect minute levels of impurities, leaving no room for any detrimental impact on pharmaceutical safety and efficacy. These minute impurities can affect the patients. Concisely, the ultimate aim is to develop analytical methods capable of detecting even minute amounts of impurities; these methods will be highly favored [12]. One of the most effective ways to achieve this is through analytical Quality by Design (QbD). The FDA’s revised Current Good Manufacturing Practice has resulted in a more cost-effective and robust method, following its original release in the twenty-first century and the updated ICH guidelines Q8 (R2) [13]. QbD is widely used in the quantitative measurement of pharmaceuticals [14,15]. DoE is the foundation of the QbD approach, representing a structured method for examining the relationships between factors affecting various processes and their outcomes using a range of designs, including the Central Composite Design and the full factorial design [16].
 | Table 1. Literature reported methods for the determination of cefotaxime sodium along with obtained analytical parameters. [Click here to view] |
The pharmaceutical industry widely employs high-performance liquid chromatography (HPLC) as the most effective and flexible analytical technique for quantitative analysis of mixture components. However, a majority of HPLC methods do not incorporate green practices. The term “green analytical chemistry” originated in the 2000s [17,18] and focuses on developing methods with minimal environmental impact while maintaining safety for analysts [19]. Ideally, green chemistry advocates for avoiding harmful solvents and reagents or reducing their use to a minimum with shorter analysis times, as well as using energy-efficient instruments [20]. A variety of reliable tools, such as the National Environmental Method Index (NEMI), Analytical Eco-Scale, Green Analytical Procedure Index (GAPI), and the newly developed “Analytical Greenness metric” (AGREE) [21]. These methods are designed to deliver comprehensive information about the methodology required from sampling to analysis. These methods provide comprehensive information on the methodology, from sampling to analysis, and it is recommended to utilize these tools to obtain a detailed report on the method’s greenness.
The primary objective of this study is to develop and validate an RP-HPLC method for the quantitative determination of CTX using a DoE approach and a green analytical approach.
MATERIAL AND METHODS
Standards and reagents
The materials utilized in this study were obtained from various sources. Chromatographic grade acetonitrile and analytical grade ammonium acetate were bought from Sigma-Aldrich, while HPLC-grade solvents and triethylamine (purity ≥ 99%) were procured from Molychem, Mumbai, India. The water used in the experiment was produced in our laboratory. Yarrow Chem Products, Mumbai, India, supplied the CTX.
Equipment and software
The HPLC analysis was carried out using a Shimadzu LC-2010CHT model equipped with a PDA detector, autosampler, and column oven. The dual-wavelength UV detector was used to measure the absorbance at 235 nm. The data acquisition and processing were performed using Lab Solution software. A Sartorius sensitive electronic balance was used to weigh the samples, while a GT Sonic Professional Ultrasonic Cleaner (Servewell Instruments, India) was used to degass the mobile phase. A pH meter (µ Controller based pH System 361) with a glass electrode (Systronics, India) was used to measure the pH of the buffer solution. The chromatographic separation was carried out using a Venusil XBP C8 column (5 um × 4.6 × 250 mm), Agela Technologies. The mobile phase filtration was done using a Millipore® glass filter with a pore size of 0.22 µm connected to a glass vacuum filtration system. The design-expert software (version 9) (Stat-Ease Inc., USA) was used for method optimization.
Method development
Chromatographic conditions
In the conducted research, a binary mixture of acetonitrile as the organic phase and ammonium acetate as the aqueous phase, in a ratio of 15:85, was employed as the mobile phase. The pH of the ammonium acetate solution was adjusted to 6.12 using triethylamine (TEA). Isocratic elution was achieved by maintaining a constant flow rate of 1.0 ml/minute. The sample injection volume was consistently set at 20 µl, with detection carried out at a wavelength of 235 nm.
Selection of wavelength
PDA detector was meticulously calibrated across a spectral range of 200–400 nanometres to identify the ambient conditions that exhibited the greatest sensitivity. This customization process ensured optimal performance and precise measurements in the chosen experimental context.
Selection of suitable column
The Venusil XBP C8 column, with specific dimensions of 4.6 × 250 mm, was employed to create an analytical method.
Selection of mobile phase and pH
The focus of this research was on the crucial factors associated with the use and selection of environmentally friendly solvents to minimize their waste. Capello et al. developed a comprehensive framework that examined approximately 26 solvents, including eco-friendly options such as simple alcohols (e.g., ethanol) and alkanes (e.g., heptane). Conversely, the use of solvents such as dioxane, formaldehyde, acids, and tetrahydrofuran was discouraged due to their high toxicity levels. In terms of solvent selection, a mixture of methanol and water was more favorable than pure alcohol. Moreover, water emerged as the greenest solvent, while benzene and carbon tetrachloride were identified as the least green options. Other factors considered in solvent selection included solubility and polarity. However, the primary emphasis was on selecting a green solvent to develop a sustainable method [22,23]. For instance, the method used by the United States Pharmacopeia and Europe Pharmacopeia to analyze CTX involves the use of methanol as an organic solvent and phosphate buffer. Although methanol is a green solvent, the long run time of 60 minutes and flow rate of 1.0 ml/minute result in significant solvent waste, making it less desirable from an environmental perspective [1].
General procedures
Primary stock and working solution preparation
In a 10 ml volumetric flask, 10 mg of CTX was dissolved in double distilled water to create a stock solution with 1 mg/ml concentration, equivalent to 1,000 µg/ml or 1000,000 ng/ml. The resulting stock solution was stored in a refrigerator when not in use. Subsequent dilutions were prepared from this stock solution at 25, 50, 100, 250, 500, 1,000, 2,500, 5,000, and 10,000 ng/ml concentrations. It is recommended that stock solutions be utilized within 7 days of preparation and stored appropriately in a refrigerator.
Method validation
Linearity and linearity range
In the present study, a total of eight distinct concentrations of CTX (Cefotaxime) were employed for the construction of calibration curves and for the examination of method linearity. The concentrations of CTX ranged from 25 ng/ml to 10,000 ng/ml. A calibration curve was generated by plotting the concentration of the drug against the peak area. To ascertain the linearity of the developed method, an analysis of least square regression was conducted.
Sensitivity
The study utilized the parameters of limit of detection (LOD) and limits of quantification (LOQ) to assess the sensitivity of the analytical method. These parameters correspond to signal-to-noise ratios of 3:1 and 10:1, respectively. The determination of LOD and LOQ values involved the analysis of the standard deviation (SD) response to the blank sample and the gradient (G) of the linearity plot. The calculations for LOD and LOQ were based on the following equations [24]:
These equations were employed to establish the sensitivity thresholds of the analytical approach, providing essential insights into the detection and quantification limits of the method under investigation.
Accuracy
The accuracy of the method was rigorously evaluated through the analysis of three distinct concentrations of CTX, namely low-quality control (LQC), middle-quality control (MQC), and high-quality control (HQC). Specifically, samples with CTX concentrations of 100, 5,000, and 8,000 ng/ml were subjected to HPLC for quantification. The accuracy of the method was determined by computing the SD, peak area, and percentage relative standard deviation (% RSD) of the results obtained from the analysis of these samples. This meticulous approach enabled a thorough assessment of the method’s precision and reliability, thereby ensuring the validity and credibility of the research findings.
Precision (intra-day and inter-day)
Three discrete concentrations of CTX (100, 5,000, and 8,000 ng/ml) were evaluated within the same day (intra-day) across various time intervals, with the analysis repeated on the subsequent day. SD and percentage RSD values were employed to assess the precision of the developed methodology [25].
System suitability
In the present investigation, the standard solution of CTX, with a concentration of 5,000 ng/ml, was analyzed systematically, with a total of six replications. The peak area measurements, which served as the basis for the analysis, were employed to calculate the percentage RSD. This statistical measure is commonly used to assess the precision and reproducibility of analytical methods, particularly in chemical and biological sciences [26].
Robustness
The present study scrutinized the prevailing methodology through incremental adjustments in flow rate (0.8 ± 0.1 ml/minute), injection volume (20 ± 2 µl), oven temperature (25°C ± 5°C), and mobile phase ratio (85% ± 5%) as recommended by Design Expert v9.0. The percentage % RSD was calculated to assess the analyte recovery [27].
Selectivity and specificity
The standard concentration of CTX was rigorously examined in accordance with the established protocol to assess the chromatogram, the method’s sensitivity in detecting the analyte amidst potential interference from process impurities, and the specificity of the developed analytical method for CTX. The evaluation of the chromatogram was conducted to ensure the method’s ability to produce clear and distinct peaks for the analyte, while the sensitivity of the method was assessed by determining the lowest concentration of CTX that could be reliably detected. The specificity of the method was evaluated by determining its ability to accurately detect CTX in the presence of potential impurities or interfering substances. The results of this investigation provide a comprehensive assessment of the analytical method’s performance in detecting and quantifying CTX, which is crucial for developing reliable and accurate analytical methods in this field [28].
Stability of solution
A standard CTX stock solution was meticulously formulated and injected at various intervals of time. Post-injection, the stock solution was consistently stored in a refrigerated environment. It is imperative to document any alterations in the chromatogram following each run, as this serves as an indicator of the drug’s stability [28].
Degradation studies
Forced degradation studies were executed in accordance with the guidelines stipulated in ICH Q1A (R2) to ascertain the stability and specificity of the optimized analytical methodology under various environmental conditions. The drug under investigation was subjected to a series of stress tests, encompassing basic sodium hydroxide (NaOH), oxidation (H2O2), acidic (HCl), and thermal stress, to elucidate its stability characteristics.
A standard sample of the drug was prepared at a concentration of 100 µg/ml or 100,000 ng/ml and was subjected to all the aforementioned hydrolysis conditions to observe the degradation patterns. The samples were subsequently neutralized and diluted, in accordance with the standard procedure, before being injected into the HPLC system for analysis. These studies were crucial in identifying the degradation products and validating the suitability of the proposed analytical methodology [28].
Acidic hydrolysis
In this research, an investigation into the acid degradation of CTX was conducted. The primary stock solution of CTX was prepared with a concentration of 100 µg/ml or 100,000 ng/ml. To this solution, 5 ml of 0.1N HCl was added and heated to 80°C for 6 hours using a water bath. After the 6 hours’ completion, the acidic solution was neutralized by adding 5 ml of 0.1N NaOH. At appropriate intervals, aliquots of the sample were extracted and subjected to HPLC analysis following dilution to a concentration of 1 µg/ml or 1,000 ng/ml.
Base hydrolysis
A study on the base hydrolysis of CTX was conducted by preparing a primary stock solution of CTX with a concentration of 100 µg/ml or 100,000 ng/ml. Subsequently, 5 ml of 0.1N NaOH was introduced to the stock solution and the mixture was heated to 80°C using a water bath for a duration of 30 minutes. Following this incubation period, the basic solution was neutralized by the addition of 5 ml of 0.1 HCl. Samples were extracted at specific time intervals and subsequently subjected to HPLC analysis after appropriate dilution (1 µg/ml or 1,000 ng/ml).
Oxidative degradation
The oxidative degradation of CTX was conducted by preparing a primary stock solution with a concentration of 100 µg/ml (100,000 ng/ml). To this solution, 5.0 ml of 30% hydrogen peroxide was added and allowed to react without disturbance for a period of 24 hours. Subsequently, the solution was diluted to 1 µg/ml (1,000 ng/ml) and subjected to analysis via HPLC. This process was implemented to evaluate the degradation of CTX under oxidative conditions.
Heating degradation
The heating degradation process was executed by boiling the sample at 105°C for 5 minutes, followed by a cooling period. The sample was subsequently diluted to a concentration of 1 µg/ml or 1,000 ng/ml, and then subjected to analysis using HPLC.
Statistical analysis
A One-way Anova was performed for the proposed method with a confidence interval of 95 on the robustness data. Values of F value, p value, R2 (coefficient of determination), equation with coded factors, predicted values, observed values, and relative error were observed.
Method applicability
Formulation of β-Cyclodextrin nanosponges loaded with CTX
In the synthesis of β-Cyclodextrin nanosponges, β-Cyclodextrin and Diphenyl Carbonate (DPC) were utilized as precursors. The precursors were combined in a molar ratio of 1:4 and transferred to a round bottom flask (RBF). The RBF was then heated on a heating plate at a temperature of 100°C with constant stirring for 5 hours, during which the melting of the mixture was observed. After 5 hours, the solid mixture obtained was grounded in a mortar and washed with water and acetone to remove unreacted DPC. The resulting solid was then dried in a vacuum oven at a temperature of 65°C for 72 hours and subsequently stored for further use [29], loaded with CTX using a solvent immersion technique.
Determination of total drug content in β-Cyclodextrin nanosponges loaded with CTX
To determine the CTX content in β-Cyclodextrin nanosponges, a precisely weighed quantity of the formulation (equivalent to 5 mg) was dissolved in methanol and subjected to centrifugation at a rate of 10,000 rpm for 10 minutes. Following centrifugation, the supernatant was extracted and diluted with the mobile phase, and the resulting solution was analyzed using optimized analytical conditions in HPLC to assess recovery. This process was repeated in triplicate to calculate the mean drug content [30].
In- vitro release studies
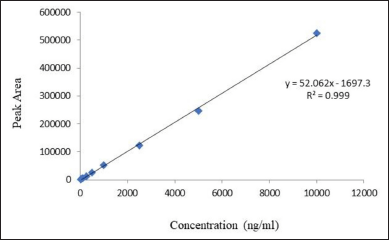
The in-vitro release study was conducted using a rigorous methodology to ensure the accuracy and reliability of the results. The experiment was executed using a dialysis bag with a molecular weight cut-off of 12 kDa, procured from HiMedia. The pure drug and β-Cyclodextrin nanosponges, loaded with an equivalent amount of CTX (5 mg) and (1.5 mg), respectively, were placed in separate dialysis bags. The two ends of each bag were securely tied to prevent any leakage of the contents. The dialysis bags were subsequently immersed in a beaker containing 50 ml of phosphate buffer with a pH of 7.2, maintained at a temperature of 37°C ± 5°C. The beaker was then placed in an orbital rotary shaker, set at a rotation per minute (rpm) of 120, to facilitate the release of the drug. At predetermined time intervals (0.5, 1, 2, 4, 5, 6, 12, 24, and 48 hours), a sample of 1 ml was withdrawn from the release media and replaced with an equal volume of fresh media to maintain sink conditions. Finally, the drug release was calculated by employing the linear regression equation obtained from the calibration curve of CTX. The equation, y = 52.062x—1697.3, was used to determine the peak area (y) corresponding to the amount of CTX (x) in the sample.
RESULTS AND DISCUSSION
Design of experiment
Selection of important potential variables
In the present investigation, water was selected as the solvent for sample preparation due to its environmentally benign and sustainable properties. This choice reflects an adherence to green chemistry principles emphasizing waste reduction, energy conservation, and environmental protection. The ratio of the mobile phase was adjusted to minimize the use of the organic phase, thereby enhancing the environmental friendliness of the analytical method. Although organic solvents are often necessary for effective compound extraction and analysis, they pose significant environmental risks, including greenhouse gas emissions and resource depletion. By prioritizing water as the solvent and minimizing the organic phase, this research aims to develop analytical methods that are effective, reliable, and environmentally sustainable. Consequently, the study contributes to the broader objective of fostering environmentally responsible scientific practices [31]. The methodology employed in this study utilizes acetonitrile and methanol as solvents, resulting in minimal waste generation of 10 grams per run, significantly below the allowable limit of 50 grams. This contrasts with the previously reported method, which produces 60 grams of waste per run, exceeding the permissible limit. Additionally, the proposed method demonstrates a markedly reduced run time of 10 minutes per run compared to the reported method’s 60-minute duration. Although this shorter run time may lead to higher power consumption, it allows for the analysis of more samples within a shorter period, thereby increasing overall process efficiency. The developed method can analyze six samples per hour, highlighting its potential for high-throughput analysis and presenting an environmentally friendly alternative to existing methods. Moreover, the retention time for each sample in the proposed method is only 5 minutes, facilitating rapid and efficient analysis, with the pH maintained at 6.12, well within the acceptable range of 2–12, indicating broad applicability.
Study of various factors using full factorial design (FFD)
In the context of this research, a FFD (24) methodology was employed to optimize and render the analytical technique more environmentally sustainable, with a reduced reliance on solvents and reagents. This method encompassed two levels (+1 and −1), four factors, and four responses. The factors under consideration were flow rate, mobile phase ratio, oven temperature, and injection volume. The responses, namely peak area, tailing factor, retention time (tR), and theoretical plates, were selected to evaluate the method’s performance and its capacity for efficient separation. The software suggested a total of 32 runs, as detailed in Table 2. The upper and lower limits for each factor were set at 0.7 and 0.9 ml/minute for flow rate, 18 and 22 µl for injection volume, 26°C and 23°C for oven temperature, and 89% and 80% for mobile phase (buffer%). These limits were determined based on preliminary experiments to ensure the optimal performance of the analytical method. The study aimed to enhance the method’s efficiency and environmental sustainability by employing this FFD approach.
Analysis of response
The responses were systematically recorded according to the criteria specified in Table S1. These responses were subsequently analyzed to develop statistically significant models (p-value < 0.05) illustrating the relationship between the responses and the variables under investigation. To assess the validity and reliability of these models, analysis of variance (ANOVA) testing was conducted, followed by an examination of diagnostic figures for potential transformation suggestions and improvements in adjusted and predicted R². The significant terms that underwent ANOVA testing, generating p-values (considering p-value < 0.05 as significant), are detailed in Table S1 along with the response equation derived from the DoE approach. A Pareto chart and perturbation plots were used to investigate the impact of these variables further, and the coefficients of the equation terms quantified the effect sizes. A half-normal plot identified the significant factors influencing each response, while contour plots in Figure 1 (A–D) visually depicted the effects of flow rate and mobile phase ratio on peak area, tailing factor, tR, and theoretical plates, showing that peak area decreases as flow rate and mobile phase (buffer%) increase, while tailing factor increases, tR decreases, and theoretical plates decrease. These findings provide valuable insights into the complex relationships between the investigated variables and responses.
 | Table 2. Full factorial design of possible variable. [Click here to view] |
The analysis of Pareto charts and perturbation plots, illustrated in Figures 2 and 3, indicates that the flow rate and mobile phase (%) buffer significantly affect peak area, tR, and theoretical plate count. A reduction in flow rate and mobile phase (%) buffer increases peak area. Additionally, flow rate negatively correlates with tR and theoretical plate count, where a higher flow rate decreases both metrics. The mobile phase ratio (% buffer) adversely impacts peak area and theoretical plate count, with higher buffer percentages leading to increased tailing factors, suggesting broader, and less symmetrical peaks. Furthermore, the temperature of the column oven influences the tailing factor, indicating its role in peak shape and symmetry. These findings are crucial for optimizing HPLC conditions to achieve effective separation and quantification of target analytes.
Optimization
During the optimization process, the principles of GAC were rigorously adhered to, with a particular emphasis on minimizing the use of hazardous solvents and reducing run time without compromising the efficiency of the separation process. The proposed method has met all the established criteria, including a minimum of 2,000 theoretical plates, a RSD of greater than 2%, and a tailing factor of less than 2. Furthermore, the method produces minimal waste, with only 10 grams being generated during each run. The detailed data generated by the DoE method is presented in Table S2. The selected solution, which has a flow rate of 0.8 ml/minute, a mobile phase ratio of 80% (buffer), a column oven temperature of 27°C, and an injection volume of 20 µl, has a desirability value of 1.000, indicating its suitability for the intended application.
Point prediction and confirmation
The predictive accuracy and reliability of the models that corresponded to the correct responses were examined. A reliable model should exhibit observed data that falls within the confidence and tolerance intervals (CI and TI). The observed values of both PI and TI were determined to be within the 95th percentile of low and high values, suggesting the validation of the models and the expectation that future data will remain within acceptable bounds (see Table S3, supplemental materials).
Mobile phase composition, pH
In light of the various factors considered, we determined that utilizing a mobile phase system consisting of ammonium acetate and acetonitrile, in conjunction with isocratic elution, was the most suitable option. The decision to employ an ammonium acetate buffer, as opposed to water, was predicated on the extended separation time and the observed unsatisfactory resolution. To achieve optimal resolution, the pH was adjusted to 6.12, given the significant impact that pH has on elution time.
Wavelength selection
The sensitivity of HPLC results is notably contingent upon optimizing the wavelength utilized. The importance of selecting the appropriate wavelength cannot be overstated, as it significantly impacts the peak resolution, the area under peak (AUP), and the tailing factor. Specifically, the utilization of a wavelength of 235 nm (λmax) has been demonstrated to be advantageous for its ability to enhance sensitivity while minimizing noise. The AUP and tailing factor are critical indicators of sensitivity and noise in HPLC analysis [32].
Column selection
The Venusil XBP C8 column exhibited favorable peak shape characteristics with reduced tR. Optimal peak separation, plate count, and shape were observed in the C8 column.
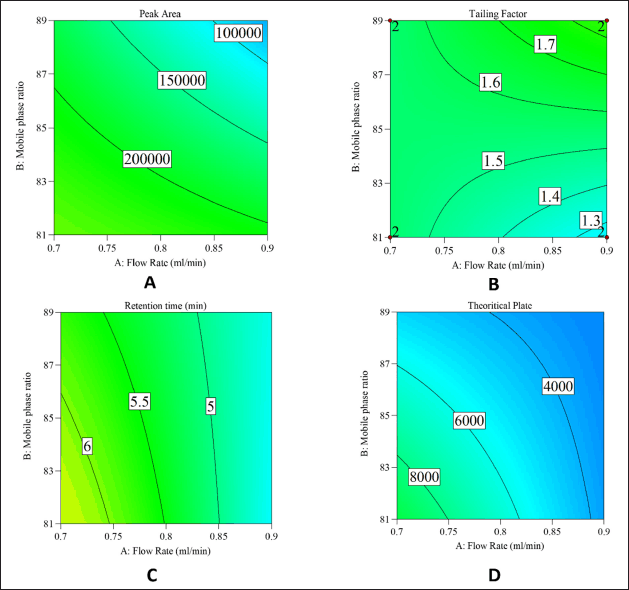 | Figure 1. Shows the contour plot for, A- peak area, B- tailing factor, C-retention time and D- theoretical plates. [Click here to view |
Method validation
Linearity and linearity range
The linearity of an analytical method is characterized by its ability to generate test outcomes that exhibit a direct or quantitative correlation with the concentrations of analytes within a specified range of concentrations [33]. The linear regression analysis conducted on the dataset encompassing the measured concentration range of 25 to 10,000 ng/ml reveals a robust linear relationship, as evidenced by a coefficient of determination (R2) of 0.999 and a p-value of 0.001 (Fig. 4). This analysis not only substantiates the linearity range but also indicates that the residuals of the responses fall within the bias limits of 5%, thereby suggesting that most residuals are typically encompassed within this range.
LOD and LOQ
The LOD and LOQ for CTX were determined to be 13.95 ng/ml and 42.30 ng/ml, respectively. These diminutive nanomolar thresholds underscore the exceptional sensitivity, effective separation, and precise quantification capabilities of the method employed [26,34].
Accuracy
The recovery and RSD values for a series of quality control samples of CTX were determined and utilized for accuracy studies. The findings of the accuracy evaluation revealed that the percentage recovery of CTX ranged from 100% to 104%, while the RSD values were between 0.03% and 0.100%. These results demonstrate a high level of accuracy for the analytical method employed in the HPLC analysis of CTX, as the RSD values were significantly lower than the acceptable limit of 2%.
Precision
Precision, in analytical chemistry, refers to the degree of agreement among multiple measurements obtained from homogenous samples under controlled conditions. The present study evaluated the inter-day and intraday precision of an analytical method by calculating the percentage RSD values. The results revealed that the RSD values for the interday and intraday precision investigations were consistently low, ranging between 0.028 and 1.159. These values are significantly below the acceptable limit of 2% for LQC, MQC, and HQC concentration levels. The low percentage RSD values further underscore the high precision of the developed analytical approach, indicating a consistent and reliable method for measuring the analyte of interest
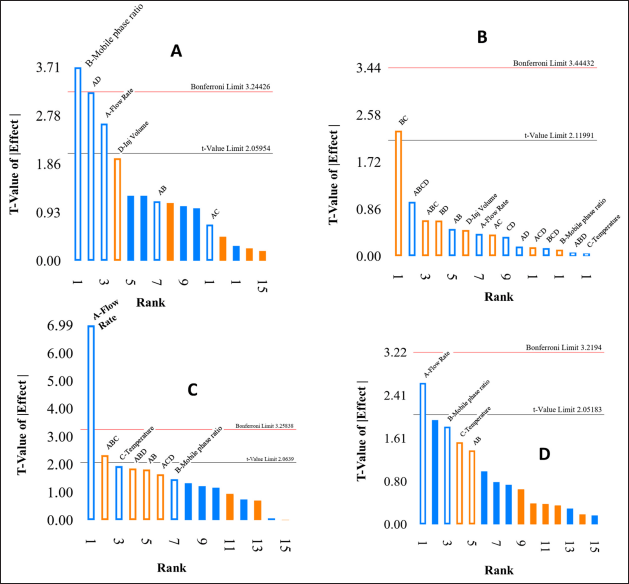 | Figure 2. Pareto charts for a-peak area, b- tailing factor, c- Retention time and d-theoretical plates, A- Flow rate, B- Mobile phase ratio (% buffer), C- Column oven temperature and D- Injection volume. [Click here to view] |
System suitability
The present investigation entailed the examination of six samples, all possessing an identical concentration of CTX. To assess the consistency and reliability of the analytical results, the following parameters were determined for each sample: tR, tailing factor, peak area, and % RSD. The % RSD values were found to be 0.076, within the acceptable agreement limit of less than 2%, indicating high precision and reproducibility in the analysis. Furthermore, the various evaluations observed no significant differences in peak area, tR, and tailing factor.
Robustness
In the experimental investigation, it was observed that there was no discernible variation in the analytical parameters, specifically the peak area and retention duration of CTX, despite manipulating the flow rate, pH, oven temperature, and wavelength, as recommended by the DoE. The supplementary materials provide a comprehensive overview of the various parameters evaluated, including the peak area, tailing factor, tR, and theoretical plates (Table S1).
Selectivity and specificity
Figure 5 displays the pure chromatogram of the CTX compound, which was obtained using a highly selective and specific analytical method. This method exhibited no interference from process-related impurities, indicating its suitability for the analysis of CTX.
Stability of the solution
An investigation was conducted on a singular standard sample at various time intervals (3, 6, 9, and 24 hours). Observations revealed a consistent lack of significant alterations in tR, peak area, and tailing factor across all time points, indicating high sample stability. Percentage% RSD values obtained, all of which fall below 2%, demonstrate conformity with established acceptable thresholds.
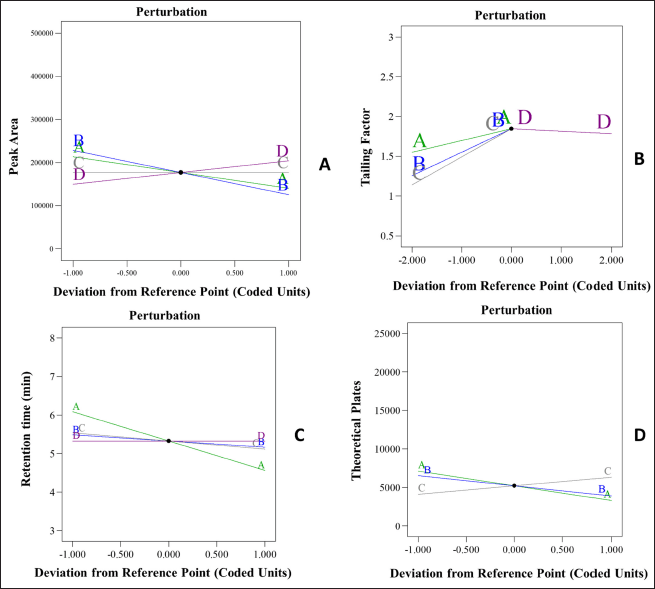 | Figure 3. Perturbation plots for, A- peak area, B-tailing factor, C- Retention time and D-theoretical plates. [Click here to view] |
 | Figure 4. Calibration curve of Cefotaxime sodium. [Click here to view] |
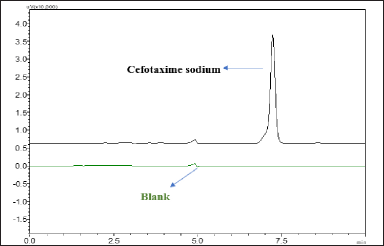 | Figure 5. Overlay of chromatogram of A) pure cefotaxime sodium and B) blank mobile phase at 7.0 and 5.0 minutes respectively, showing clear separation and no interference from each other. [Click here to view] |
Forced degradation studies
Forced degradation studies were conducted following the guidelines set forth by the ICH Q1A (R2), under various stress conditions, including acid and base hydrolysis, oxidative degradation, and thermal degradation. The objective of these studies was to thoroughly understand the behavior of the drug CTX under different conditions, thereby elucidating potential degradation pathways during the formulation development and storage of CTX-loaded nanosponge. These investigations are crucial for guiding scientists and researchers in selecting appropriate excipients, determining optimal storage temperatures, and assessing the impact of pH on the formulation. Addressing these factors is essential for developing a stable formulation that maintains its therapeutic efficacy over time. Additionally, these studies allow for the proposal of various strategies to enhance CTX stability during formulation, storage, and analytical processes. Such comprehensive knowledge is indispensable for ensuring the longevity and effectiveness of the CTX-loaded nanosponge.
Acidic hydrolysis
The chromatogram of CTX, which has undergone acid hydrolysis by applying 0.1N HCL, is presented in Figure S2 of the supplementary material. Notably, several degradant peaks were detected, which can be attributed to the decarboxylation process of the drug.
Basic hydrolysis
The chromatogram of Ciguatoxin (CTX) following exposure to alkaline hydrolysis is depicted in Figure S2 of the supplementary materials, utilizing a 0.1N NaOH concentration. Notably, analogous degradant peaks were detected, mirroring those observed during acid hydrolysis.
Oxidative degradation
The chromatogram of CTX, which underwent oxidative degradation using a 3% hydrogen peroxide solution, is depicted in Figure S2 (Supplementary Material). The presence of degradant peaks was discernible within the chromatogram.
Heating Oxidation
Figure S2 (Supplementary material) illustrates the chromatogram of CTX after exposure to thermal degradation via boiling under reflux for 5 minutes at a temperature of 80°C. The resulting chromatogram depicts a substantial degradation of the drug, with the emergence of distinct peaks indicative of the formation of degradation products. This finding underscores the sensitivity of CTX to heat and the potential for degradation under elevated temperature conditions.
Statistical analysis
In Table 3, a comprehensive exposition of the One-way ANOVA is presented, encompassing key statistical parameters such as the F value, p-value, R2 (coefficient of determination), the equation incorporating coded factors, predicted values, observed values, and the relative error.
Method applicability
ß- Cyclodextrin nanosponges
β-Cyclodextrin: a versatile nanocarrier for drug delivery
β-Cyclodextrin, a cyclic oligosaccharide, holds approval from the FDA for diverse pharmaceutical applications. Primarily utilized as a drug carrier in forms such as micelles, nanoparticles, hydrogels, and micelles, β-Cyclodextrin nanosponges offer enhanced loading capacity, solubility, and stability for drugs and guest molecules. Through polymer cross-linking modifications, β-Cyclodextrin transforms into a highly porous, branched nanometric matrix known as β-Cyclodextrin nanosponges. These structures, characterized by carbonate bridges and a lipophilic cavity, establish a network of hydrophilic channels that augment drug loading capacity, enhance bioavailability, and improve stability. Cavities and multiple pores resulting from crosslinking further contribute to the increased loading capacity. These attributes collectively position β-Cyclodextrin nanosponges as efficacious and versatile nanocarriers for drug delivery.
In this study, cefotaxime-loaded β-Cyclodextrin nanosponges were synthesized. The RP-HPLC method was employed to quantify the cefotaxime loading in β-Cyclodextrin nanosponges. The technique demonstrated specificity, sensitivity, and absence of interference from blank peaks. Recovery rates fell within acceptable limits, affirming the method’s efficacy in accurately analyzing the cefotaxime content within the β-Cyclodextrin nanosponges. This established method is a reliable tool for assessing the drug content in β-Cyclodextrin nanosponges, showcasing its potential for further pharmaceutical applications.
Analysis of CTX-loaded ß- Cyclodextrin nanosponges
The analysis of CTX-loaded β-Cyclodextrin nanosponges, equivalent to a dosage of 5 mg, was conducted. The resulting recovery was 70% ± 0.100%. The chromatogram peak for nanosponges loaded with CTX is depicted in Figure 6. In this research paper, the analysis of CTX-loaded β-Cyclodextrin nanosponges, which are equivalent to a dosage of 5 mg, was conducted. The results of the analysis revealed a recovery rate of 70% ± 0.100%. This indicates that the nanosponges could effectively deliver the drug, with a high degree of consistency. The chromatogram peak for the nanosponges loaded with CTX is presented in Figure 6. This graphical representation provides a visual representation of the drug’s presence within the nanosponges. The peak in the chromatogram serves as evidence of the successful loading of the drug onto the nanosponges. In summary, the analysis of CTX-loaded β-Cyclodextrin nanosponges has shown promising results, with a high recovery rate and successful loading of the drug. This research has implications for developing nanosponges as a drug delivery system, particularly for drugs such as CTX.
Drug release studies (in vitro) of CTX-loaded nanosponges
A comprehensive investigation was conducted to examine the drug release profile of CTX in a pH 7.2 phosphate buffer for both pure CTX drug and CTX-loaded β-Cyclodextrin nanosponges. This particular pH level was selected based on the fact that the formulation will be administered intravenously, and the basic blood pH is approximately 7.4. The results demonstrated that the pure CTX drug exhibited a complete release of 100% within 5 hours. In contrast, the CTX-loaded nanosponges displayed a more gradual and sustained release pattern, with a total release of 98% achieved over a duration of 24 hours. This observation suggests that the CTX-loaded nanosponges provide a more controlled and extended release of the drug in a basic pH environment, potentially enhancing the therapeutic efficacy and reducing the frequency of dosing (Fig. 7).
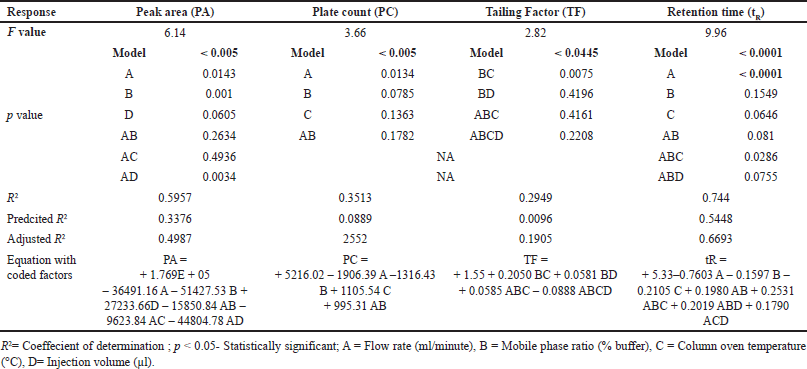 | Table 3. Result of Anova using 24 full factorial design for robustness testing. [Click here to view] |
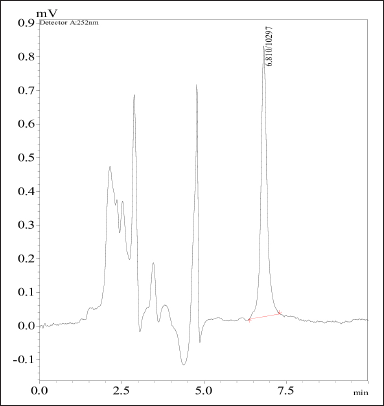 | Figure 6. Chromatogram peak of cefotaxime-loaded nanosponges. [Click here to view] |
Assessment of greenness
In the realm of analytical procedures, it is imperative to advocate for environmentally sustainable chromatographic practices throughout the entire analytical process, spanning from sample collection to separation. Given the inherent variability among these methods, the necessity for robust evaluation techniques becomes apparent. In recent years, numerous methodologies have emerged dedicated to assessing the ecological footprint of analytical procedures. Leveraging these tools, the proposed methodology underwent a comparative analysis with established practices to evaluate its environmental sustainability.
 | Figure 7. Release study of Cfs. [Click here to view] |
Greenness assessment using NEMI tool
The impetus for the inception of an environmentally sustainable methodology stemmed from the utilization of a qualitative framework. This approach is founded upon a pictorial representation segmented into four distinct quadrants, each representing a parameter. The four quadrants of the diagram represent different categories of chemical substances. Precisely, the first quadrant corresponds to persistent, bioaccumulative, and toxic (PBT) substances, the second quadrant encompasses hazardous chemicals, the third quadrant pertains to corrosive substances, and the fourth quadrant signifies waste materials. If the first quadrant is shaded in green. In that case, it indicates that the reagents utilized do not fall under the classification of toxic substances as defined by the Toxic Release Inventory (TRI) of the Environmental Protection Agency (EPA) [35]. In accordance with the regulations for the second quadrant, hazardous substances on the TRI list are prohibited. Compliance with this prohibition is essential for safety within the area. For the third quadrant, the pH of the medium must be maintained between 2 and 12. In the fourth quadrant, waste generation should be kept below 50 grams. The NEMI pictogram indicates that the proposed method is environmentally friendly, meeting green classification criteria in all quadrants. Reagents and solvents used are not on the PBT list, although acetonitrile and methanol are classified as hazardous on the TRI list [36]; The process maintains a pH of 6.12, making it noncorrosive, and generates only 8 grams of waste, significantly below the 50-gram limit, thus ensuring safety and environmental sustainability.
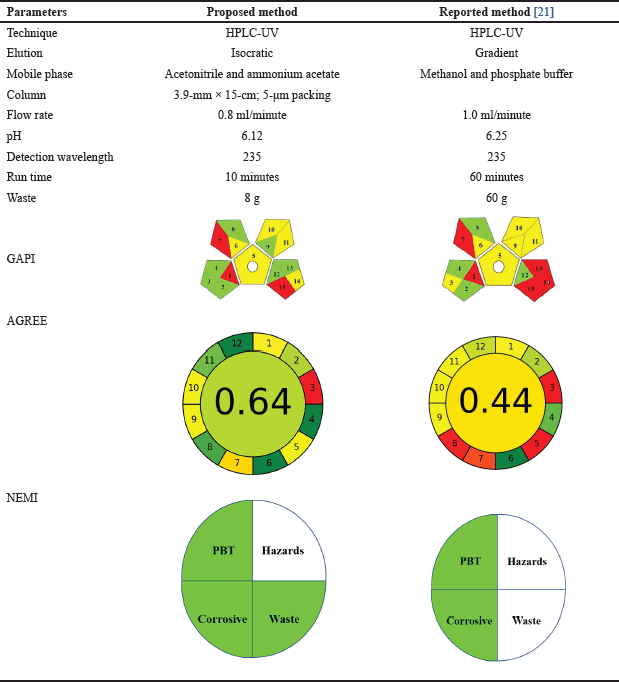 | Table 4. Comparison between the proposed method and reported method for the analytical quantification of cefotaxime sodium. [Click here to view] |
Greenness assessment using GAPI tool
The integration of NEMI and ESA tools has resulted in GAPI, a semi-quantitative assessment tool for evaluating the environmental sustainability of analytical methods. GAPI uses a visual, three-color scale to compare different techniques across fifteen parameters, including collection processes, reagent safety, waste treatment, and instrumentation. Each step is rated as red (high impact), yellow (medium impact), or green (low impact). Assessments revealed that solvent and reagent usage exceeded 10 ml with minimal health hazards, and the chart’s lower left corner indicated minimal waste production. However, the presence of red zones in this area highlighted the high ecological impact due to offline sampling techniques away from production sites [37]. Table 4 comprehensively compares the reported methodologies and the proposed approach. For a thorough understanding of all GAPI parameters, kindly refer to Table S4 in the Supplementary Material.
Greenness assessment using AGREE tool
Pena-Pereira and colleagues introduced a pioneering software application for evaluating the environmental sustainability of analytical methodologies in June 2020 [38]. The software in question adheres to the principles and guidelines established by the Generalized Annotated Constraint (GAC) framework [18]. The software under examination adheres to the GAC framework principles and guidelines. This adherence ensures operational consistency, reliability, and robustness. The GAC framework offers a robust foundation for software development, aligning with the latest research and best practices. Following the GAC framework, the software effectively addresses complex problems and delivers accurate results [39]. The software generates a pictogram divided into twelve segments with adjustable widths based on their importance. Each segment is color-coded from deep red (0) to deep green (1), with the cumulative score displayed centrally. Designed for simplicity, flexibility, inclusivity, and clarity, the tool was downloaded from AGREE articles on May 19, 2023 [40]. Critical data from the proposed and reported methodologies were documented through the twelve stages of the GAC (Glycerolysis of Alginate Calcium) process. These data were visualized as pictograms for ease of comprehension. The pictograms, available in a designated supplementary material folder, are summarized in Table 4, providing a direct comparison for analysis.
CONCLUSION
In conclusion, the HPLC analysis of CTX was performed using a highly selective, environmentally benign, straightforward, sensitive, stable, and robust methodology. The application of the QbD approach, supported by expert software design, achieved optimal results with minimal feasible runs. The selected parameters and responses met acceptable limits, validating the proposed procedure according to International Council for Harmonisation (ICH) guidelines. The environmental impact was assessed using GAPI, AGREE, and NEMI tools, confirming the method’s environmental sustainability. This developed methodology is cost-effective, accurate, and suitable for determining Cefotaxime in Cefotaxime-loaded nanosponges.
ACKNOWLEDGMENTS
The authors express their profound gratitude to the Indian Council of Medical Research (ICMR) for granting a Senior Research Fellowship (SRF) to Mr. Akhil Nair (3/1/2(3)/U.R.O./2022-NCD-II) and for the financial assistance under reference number 35/13/2020-Nano/BMS. The support from ICMR was instrumental in the successful completion of this research.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the journal’s website: Link Here [https://japsonline.com/admin/php/uploadss/4382_pdf.pdf].
DECLARATIONS OF COMPETING INTEREST
The authors declare no financial or personal relationships that could have influenced the study’s outcome. There are no conflicts of interest, such as financial holdings or personal relationships, affecting the research’s impartiality. This declaration ensures the integrity and transparency of the research process. It upholds the highest standards of academic scholarship.
REFERENCES
1. USP Monographs: Cefotaxime sodium [Internet]. [cited 2023 May 18]. Available from: http://www.pharmacopeia.cn/v29240/usp29nf24s0_m14080.html
2. Ling SSN, Yuen KH, Barker SA. Simple liquid chromatographic method for the determination of cefotaxime in human and rat plasma. J Chromatogr B. 2003 Jan 5;783(1):297–301. CrossRef
3. Rudnicki K, Sobczak K, Kaliszczak M, Sipa K, Powa?ka E, Skrzypek S, et al. Voltammetric study of cefotaxime at the macroscopic and miniaturized interface between two immiscible electrolyte solutions. Microchim Acta. 2021 Nov 9;188(12):413. CrossRef
4. Melekhin AO, Tolmacheva VV, Goncharov NO, Apyari VV, Dmitrienko SG, Shubina EG, et al. Multi-class, multi-residue determination of 132 veterinary drugs in milk by magnetic solid-phase extraction based on magnetic hypercrosslinked polystyrene prior to their determination by high-performance liquid chromatography – tandem mass spectrometry. Food Chem. 2022 Sep 1;387:132866. CrossRef
5. Molecules | Free Full-Text | Green chemometric determination of cefotaxime sodium in the presence of its degradation impurities using different multivariate data processing tools; GAPI and AGREE Greenness Evaluation [Internet]. [cited 2024 Apr 27]. Available from: https://www.mdpi.com/1420-3049/28/5/2187 CrossRef
6. Nabi SA, Laiq E, Islam A. Selective separation and determination of cephalosporins by tlc on stannic oxide layers. Acta Chromatogr 14(14) 92–101.
7. Salem H, Samir E. Determination of cefotaxime, cefoperazone, ceftazidime and cefadroxil using surface plasmon resonance band of silver nanoparticles. Braz J Pharm Sci. 2018 Nov 29;54:e17565. CrossRef
8. Martínez-Piernas AB, Plaza-Bolaños P, Gilabert A, Agüera A. Application of a fast and sensitive method for the determination of contaminants of emerging concern in wastewater using a quick, easy, cheap, effective, rugged and safe-based extraction and liquid chromatography coupled to mass spectrometry. J Chromatogr A. 2021 Sep 13;1653:462396. CrossRef
9. Kumar ACH, Gurupadayya BM, Sloka SN. Determination and validation of cefadroxil, ceftriaxone and cefotaxime by using n-bromosuccinamide in human plasma and pharmaceutical dosage form. Int J Res Pharm Sci. 2011 May 31;2(2):206–12.
10. Wang R, Jia ZP, Fan JJ, Jun M, Hua X, Zhang Q, et al. Separation and determination of cefotaxime enantiomers in injections by capillary zone electrophoresis. Die Pharmazie – Int J Pharm Sci. 2009 Mar 1;64(3):156–60.
11. Omar MA, Abdelmageed OH, Attia TZ. Kinetic spectrofluorimetric determination of certain cephalosporins in human plasma. Talanta. 2009 Feb 15;77(4):1394–404. CrossRef
12. Kelani KM, Elzanfaly ES, Saad AS, Halim MK, El-Zeiny MB. Different greenness assessment perspectives for stability-indicating RP-HPLC method used for the assay of isoxsuprine hydrochloride and four nephrotoxic and hepatotoxic photothermal degradation products. Microchem J. 2021 Dec 1;171:106826. CrossRef
13. El-Sayed HM, Hashem H. Quality by design strategy for simultaneous HPLC determination of bromhexine HCl and its metabolite ambroxol HCl in dosage forms and plasma. Chromatographia. 2020 Sep 1;83(9):1075–85. CrossRef
14. Kannaiah KP, Sugumaran A. Environmental benign AQbD based estimation of ketoconazole and beclomethasone by RP-HPLC and multi-analytical UV spectrophotometric method. Microchem J. 2022;172:106968. CrossRef
15. Wadhwa G, Krishna KV, Dubey SK, Taliyan R. Development and validation of RP-HPLC method for quantification of repaglinide in mPEG-PCL polymeric nanoparticles: QbD-driven optimization, force degradation study, and assessment of in vitro release mathematic modeling. Microchem J. 2021 Sep 1;168:106491. CrossRef
16. Politis SN, Colombo P, Colombo G, Rekkas DM. Design of experiments (DoE) in pharmaceutical development. Drug Dev Ind Pharm. 2017;43(6):889–901. CrossRef
17. Anastas PT, Kirchhoff MM. Origins, current status, and future challenges of green chemistry. Acc Chem Res. 2002;35(9):686–94. CrossRef
18. Ga?uszka A, Migaszewski Z, Namie?nik J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. Trends Anal Chem. 2013 Oct 1;50:78–84. CrossRef
19. Nishio T, Kanazawa H. Green bioanalytical chemistry. In: Handbook of green analytical chemistry [Internet]. Hoboken, NJ: John Wiley & Sons, Ltd; 2012 [cited 2023 Jun 30]. pp. 425–47. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/9781119940722.ch20 CrossRef
20. Mohamed HM. Green, environment-friendly, analytical tools give insights in pharmaceuticals and cosmetics analysis. Trends Anal Chem. 2015 Mar 1;66:176–92. CrossRef
21. El-Maraghy CM. Implementation of green chemistry to develop HPLC/UV and HPTLC methods for the quality control of fluconazole in presence of two official impurities in drug substance and pharmaceutical formulations. Sustain Chem Pharm. 2023 Jun 1;33:101124. CrossRef
22. Capello C, Fischer U, Hungerbühler K. What is a green solvent? a comprehensive framework for the environmental assessment of solventsof solvents. Green Chem. 2007 Aug 28;9(9):927–34. CrossRef
23. Alfonsi K, Colberg J, Dunn PJ, Fevig T, Jennings S, Johnson TA, et al. Green chemistry tools to influence a medicinal chemistry and research chemistry based organisation. Green Chem. 2008 Jan 3;10(1):31–6. CrossRef
24. Khurana RK, Rao S, Beg S, Katare OP, Singh B. Systematic development and validation of a thin-layer densitometric bioanalytical method for estimation of mangiferin employing analytical quality by design (AQbD) Approach. J Chromatogr Sci. 2016 May;54(5):829–41. CrossRef
25. Palmer AC, Sorger PK. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell. 2017 Dec 14;171(7):1678–91.e13. CrossRef
26. Younis SE, El-Nahass SA, Soliman SA, Youssef RM. Simultaneous micro-determination of eplerenone and torsemide in their combined tablets using HPTLC-Dual wavelength spectrodensitometric and spectrophotometric methods. Microchem J. 2020 Jul;156:104861. CrossRef
27. Goto T, Yoshida Y, Kiso M, Nagashima H. Simultaneous analysis of individual catechins and caffeine in green tea. J Chromatogr. 1996 Oct 18;749(1):295–9. CrossRef
28. Mostafa F. Al-Hakkani. HPLC analytical method validation for determination of cefotaxime in the bulk and finished pharmaceutical dosage form. SCE. 2020 Mar 30;33–42. CrossRef
29. Asela I, Donoso-González O, Yutronic N, Sierpe R. β-Cyclodextrin-based nanosponges functionalized with drugs and gold nanoparticles. Pharmaceutics. 2021 Apr 8;13(4):513. CrossRef
30. Almutairy BK, Alshetaili A, Alali AS, Ahmed MM, Anwer MK, Aboudzadeh MA. Design of olmesartan medoxomil-loaded nanosponges for hypertension and lung cancer treatments. Polymers. 2021 Jan;13(14):2272. CrossRef
31. P?otka J, Tobiszewski M, Sulej AM, Kupska M, Górecki T, Namie?nik J. Green chromatography. J Chromatogr. 2013 Sep;1307:1–20. CrossRef
32. Shah P, Patel J, Patel K, Gandhi T. Development and validation of an HPTLC method for the simultaneous estimation of Clonazepam and Paroxetine hydrochloride using a DOE approach. J Taibah Univ Sci. 2017 Jan 1;11(1):121–32. CrossRef
33. Shabir G. Step-by-step analytical methods validation and protocol in the quality system compliance industry. J Valid Technol [Internet]. 2004 [cited 2023 Jun 30]. Available from: https://www.semanticscholar.org/paper/Step-by-step-analytical-methods-validation-and-in-Shabir/68718039ed36445c5e02c1100f21632f6858eff1
34. Sharma T, Khurana RK, Jain A, Katare Op, Singh B. Development of a validated liquid chromatographic method for quantification of sorafenib tosylate in the presence of stress-induced degradation products and in biological matrix employing analytical quality by design approach. Biomed Chromatogr. 2018;32(5):e4169. CrossRef
35. Emergency Planning and Community Right-to-Know Act Section 313. Guidance for reporting toxic chemicals: polycyclic aromatic compounds category.
36. US EPA O. TRI-listed chemicals [Internet]. 2013 [cited 2023 May 21]. Available from: https://www.epa.gov/toxics-release-inventory-tri-program/tri-listed-chemicals
37. P?otka-Wasylka J. A new tool for the evaluation of the analytical procedure: green analytical procedure index. Talanta. 2018;181:204–9. CrossRef
38. Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE—Analytical GREEnness metric approach and software. Anal Chem. 2020;92(14):10076–82. CrossRef
39. First report of columnea latent viroid (CLVd)in Gloxinia gymnostoma, G. nematanthodes and G. purpurascens in a botanical garden in Denmark—Nielsen—2010—New Disease Reports— Hoboken, NJ: Wiley Online Library [Internet]. [cited 2023 May 21]. Available from: https://bsppjournals.onlinelibrary.wiley.com/doi/10.5197/j.2044-0588.2010.022.004
40. Garrigues S, de la Guardia M. Non-invasive analysis of solid samples. Trends Anal Chem. 2013 Feb 1;43:161–73. CrossRef