Development and validation of dissolution procedures
Bhavesh Vaghela, Rajan Kayastha, Nayana Bhatt, Nimish Pathak, Dashrath Rathod
Pages: 50-56

Development of validated liquid chromatographic method for estimation of levocetirizine from pharmaceutical dosage forms
Chaitanya Prasad MK, Vidyasagar G, Sambasiva Rao KRS, Madhusudhanareddy Induri, Ramanjeneyulu S
Pages: 95-97

Physicochemical quality evaluation of amoxicillin capsules produced in compounding pharmacies at Diadema, Sβo Paulo, Brazil
Fúlvio Gabriel Corazza, Blanca Elena Ortega Markman, Paulo César Pires Rosa
DOI: 10.7324/JAPS.2015.501205Pages: 029-034

Validated Ultraviolet-Spectrometric Method for Determination of Sofosbuvir in Tablets Formulation
Mohamed A. El Hamd, Ramadan Ali, Adel A. Marzouk, Osama H. Abdelmageed
DOI: 10.7324/JAPS.2017.70214Pages: 114-119

Quality Assessment and Ecotype Distinction for Panax quinquefolius L. from China and Canada by 1H NMR and Chemometrics
Caimei Gu, Zenghui Wang, Labin Wu, Linfang Huang
DOI: 10.7324/JAPS.2017.70504Pages: 018-023

Simultaneous determination of valsartan, amlodipine besylate and hydrochlorothiazide in tablets by near infrared
Natana Becker, Gabriela R. Foresti, Willian R. R. Almeida, Karine F. Nicorena, Marco F. Ferrão, Fabiana E. B. Silva
DOI: 10.7324/JAPS.2017.71111Pages: 074-078

Validation of methodology for assay, pharmaceutical equivalence, and comparative dissolution profile for tablets containing amlodipine besylate
Renata Micheli Martinez, Jenifer Freitas da Silva, Larissa Regina Jorge, Rhye Lessa Ishikawa, Ana Paula Novelli, Talita Laiane Cardoso Cezar, Sandra Regina Georgetti, Marcela Maria Baracat, Rúbia Casagrande
DOI: 10.7324/JAPS.2019.91112Pages: 093-100

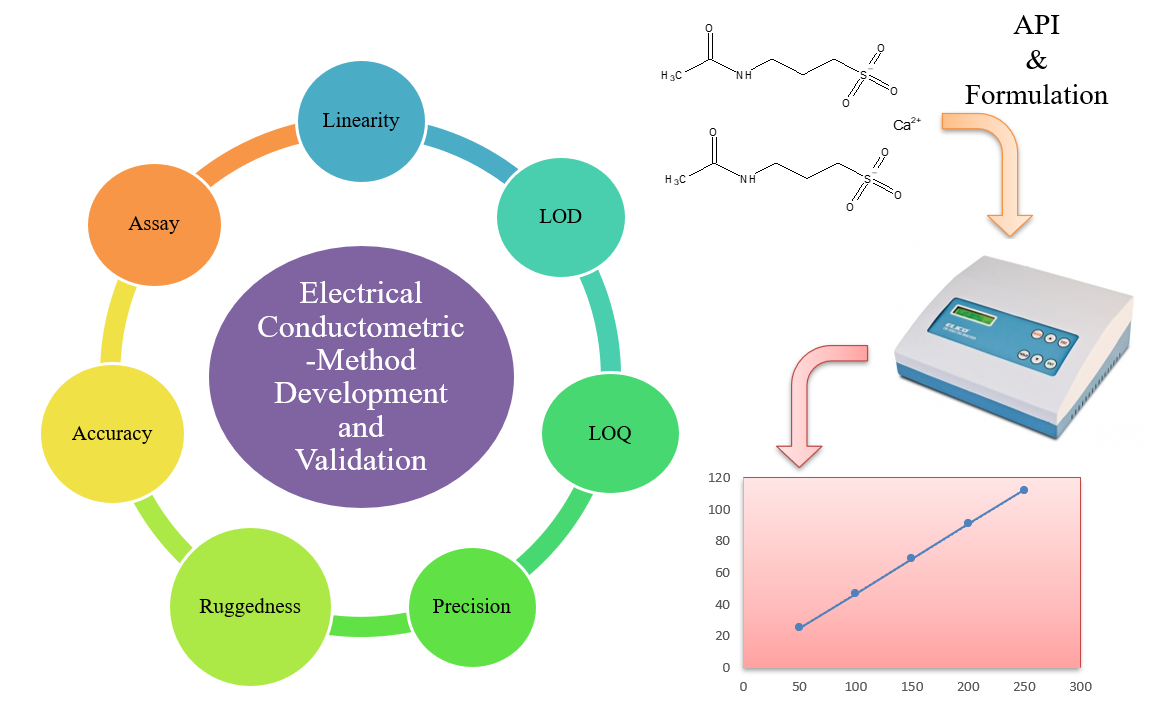
Conductometric method development and validation to estimate acamprosate calcium in API and marketed formulation
Rahul K. Yadav, Meenaxi M. Maste, Shailendra S. Surywanshi, Utkarsh Shastri
DOI: 10.7324/JAPS.2021.1101111Pages: 082–086
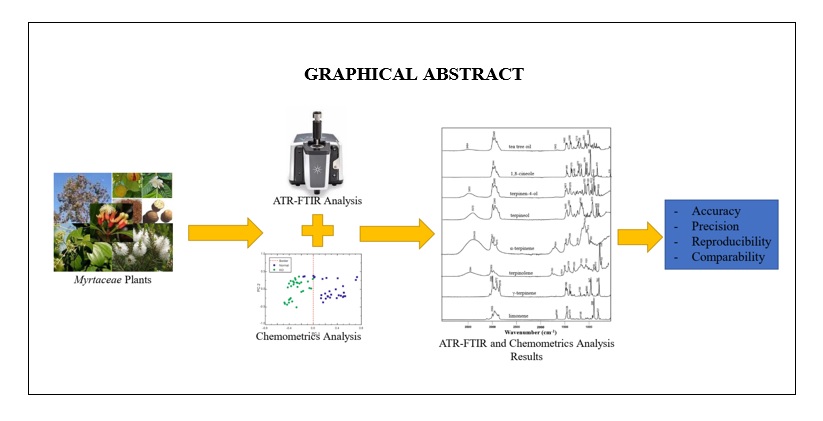
The combination of ATR-FTIR and chemometrics for rapid analysis of essential oil from Myrtaceae plants – A review
Islamudin Ahmad, Jihan Azmi Nur Fikri, Ayun Erwina Arifianti, Sarini Abdullah, Abdul Munim
DOI: 10.7324/JAPS.2022.120604Pages: 030-042
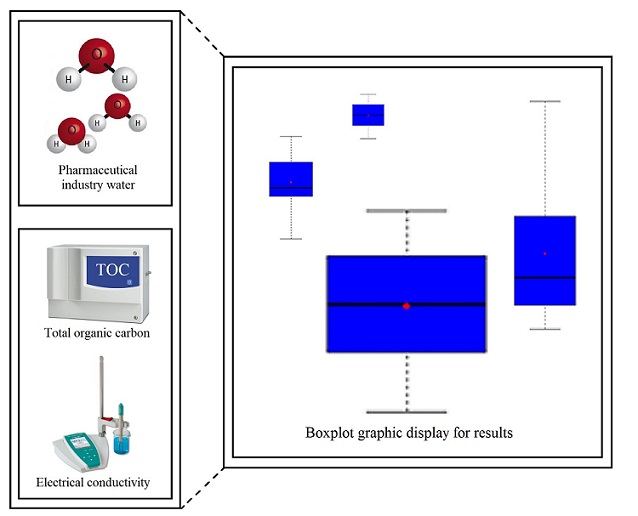
Electrical conductivity and total organic carbon analysis of water in Brazilian industrial pharmaceutical formulations
Daniela Toledo de Matos, Flávio Silva de Carvalho, Fernando Machado dos Santos
DOI: 10.7324/JAPS.2023.130118Pages: 187-192
Quality by design approach for the formulation of bilayer tablets of domperidone and itopride in gastro-esophageal reflux disease
Roshani Prajapati, Bhavna Kumar, Jagannath Sahoo, Shailendra Shakya, Diwya Kumar Lal
DOI: 10.7324/JAPS.2024.168489Pages: 169-181
