Recent analytical strategies on "Date-Rape" Drugs and its metabolites
Chanbasha Basheer
Pages: 21-28

High Performance Liquid Chromatography (HPLC) Method Development and Validation Indicating Assay for Ciprofloxacin Hydrochloride
Sani A. Ali, Chijioke C. Mmuo, Rafat O. Abdulraheem, Sikirat S. Abdulkareem, Emmanuel T. Alemika, Musa A. Sani, Mohammed Ilyas
Pages: 239-243

RP-HPLC Method Development for Estimation of Sildenafil Citrate in Tablets and in Seminal Fluid
Nitin Sharma, Praveen Rajput, Arti Thakkar, G. S. Sarma
DOI: 10.7324/JAPS.2012.2615Pages: 172-178

Development and Validation of RP-HPLC-PDA Method for Simultaneous Estimation of Baclofenand Tizanidine in Bulk and Dosage Forms
Buchi N. Nalluri, K. Sushmitha, B. Sunandana, D. Prasad Babu
DOI: 10.7324/JAPS.2012.2714Pages: 111-116

Artemisia herba alba & Artemisia monosperma: The Discovery of the first potential Egyptian plant sources for the Pharmaceutical Commercial Production of Artemisinin and Some of Its Related Analogues
El Motaz Bellah El Maggar
DOI: 10.7324/JAPS.2012.2709Pages: 77-91

Simultaneous Estimation of Ramipril and Amlodipine in Bulk and tablet Dosage form by RP-HPLC Method
Praveen S. Rajput, Amanjot Kaur, Navdeep Kaur Gill, Karan Mittal, Ganti Subrahmanya Sarma
DOI: 10.7324/JAPS.2012.2724Pages: 160-165

RP-HPLC-PDA method development and validation for the analysis of Tadalafil in bulk, pharmaceutical dosage forms and in-vitro dissolution samples
Aziz Unnisa, Yogesh Babu, Santosh kumar suggu, Siva Chaitanya
DOI: 10.7324/JAPS.2014.41213Pages: 072-076

Isolation and Identification of Potential Antineoplastic Bioactive Phenolic Compounds in Malaysian Honeys
Norjihada Izzah Ismail, Mohammed Rafiq Abdul Kadir, Razauden Mohamed Zulkifli
DOI: 10.7324/JAPS.2015.501011Pages: 059-066

Simultaneous determination of ciprofloxacin hydrochloride and metronidazole in spiked human plasma by ultra performance liquid chromatography-tandem mass spectroscopy
Ramzia El-bagary, Asmaa Ahmed El-Zaher, Ehab Elkady, Asmaa Abdelkerim Mandour
DOI: 10.7324/JAPS.2016.60307Pages: 041-047

Liquid Chromatographic Assay for the Analysis of Kanamycin sulphate nanoparticles in Rat after intramuscular administration: Application to a Pharmacokinetic Study
Sanaul Mustafa, V. Kusum Devi
DOI: 10.7324/JAPS.2016.60809Pages: 057-066

A Study of Method Development, Validation and Forced Degradation for Quantification of Buprenorphine Hydrochloride in a Microemulsion Formulation
Dhanashree Arun Mundhey, Vishal V. Rajkondawar, Anwar S. Daud, Nidhi P. Sapkal
DOI: 10.7324/JAPS.2016.601022Pages: 159-169

Development and Validation of Content Uniformity Analytical Procedure of Glipizide Extended Release Tablet
Ilma Nugrahani, Indhah Fatmawati, Slamet Ibrahim
DOI: 10.7324/JAPS.2016.601228Pages: 192-196

Quality evaluation of Dendrobium based on ultra-performance liquid chromatography (UPLC) and chemometrics
Caimei Gu, Xiang Zhang, Labin Wu, Xue Jiang, Linfang Huang
DOI: 10.7324/JAPS.2017.70103Pages: 017-023

A Liquid Chromatography/Tandem Mass Spectrometric Method for Determination of Captopril in Human Plasma: Application to a Bioequivalence Study
Eman S. Elzanfaly, Hanan A. Merey
DOI: 10.7324/JAPS.2017.70202Pages: 008-015

Simultaneous quantification of ramipril, glimepiride and metformin in human plasma by ultra-performance liquid chromatography – tandem mass spectrometry
Eman S. Elzanfaly, Sherif A. Abdel-Gawad
DOI: 10.7324/JAPS.2017.70711Pages: 062-069

Effect of Piperine on Pharmacokinetics of Rifampicin and Isoniazid: Development and Validation of High Performance Liquid Chromatography Method
Amit Singh, Smita Verma, Nouh M H Al Jarari, Ajay Pal Singh, Neeraj Kumar Fuloria, Shivkanya Fuloria, Pradeep Kumar Sharma, Chhotelal
DOI: 10.7324/JAPS.2018.8311Pages: 072-081

A sensitive analytical liquid chromatography-tandem mass spectrometry method for the estimation of Topiramate in bulk and pharmaceutical formulation
R. Sangamithra, S. T. Narenderan, S. N. Meyyanathan, Prachi Sharma, Mohire Sourabh Sanjay, B. Babu, M. Kalaivani
DOI: 10.7324/JAPS.2020.103014Pages: 109-112
Isolation, characterization, and validation of RP-HPLC method for the quantification of quercetin in Huberantha senjiana leaf extract
Rajakannu Pandiyan, Kaliappan Ilango
DOI: 10.7324/JAPS.2020.10515Pages: 110-118

Method validation for the simultaneous estimation of three-bioactive components in combined extracts of three hepatoprotective plants using RP-HPLC method
Sharuti Mehta, Anil Kumar Sharma, Rajesh Kumar Singh
DOI: 10.7324/JAPS.2021.110714Pages: 127-131

Study on chloroquine containing ability of minicells derived from Lactobacillus acidophilus ATCC 4356
Tu My Ho, Yen Thi Hai Tran, Tu Hoang Khue Nguyen
DOI: 10.7324/JAPS.2024.22371Pages: 091-096
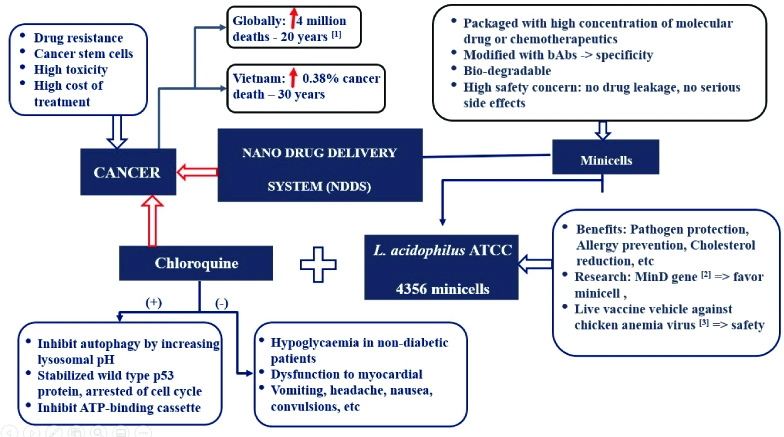
Identification and validation of potential genotoxic impurity in Rizatriptan by ultra performance liquid chromatography
Veeraswami Boddu, Rama Rao Rayala
DOI: 10.7324/JAPS.2024.152121Pages: 212-220
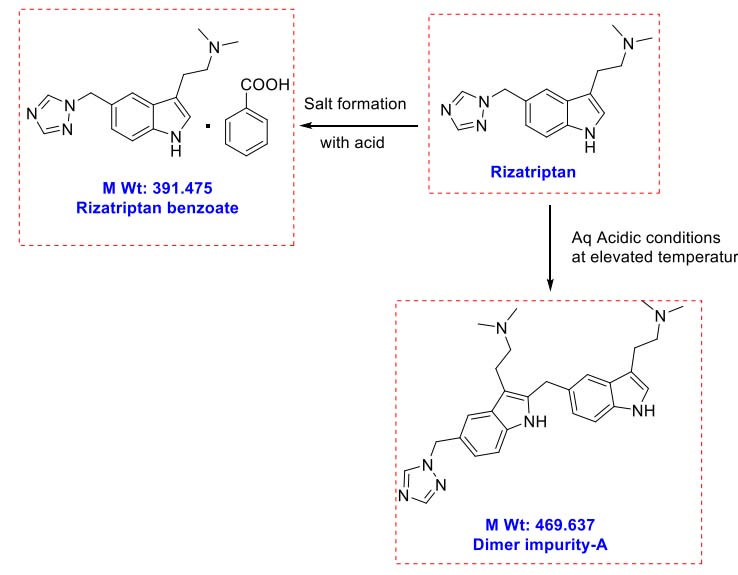
Stability-indicating HPLC method optimization using quality by design with design of experiments approach for quantitative estimation of organic related impurities of Doripenem in pharmaceutical formulations
N. V. V. D. Praveen Boppy, Sharath Babu Haridasyam, Niroja Vadagam, Naveen Sara, Karthik Sara, Eswarlal Tamma
DOI: 10.7324/JAPS.2024.190386Pages: 114-126
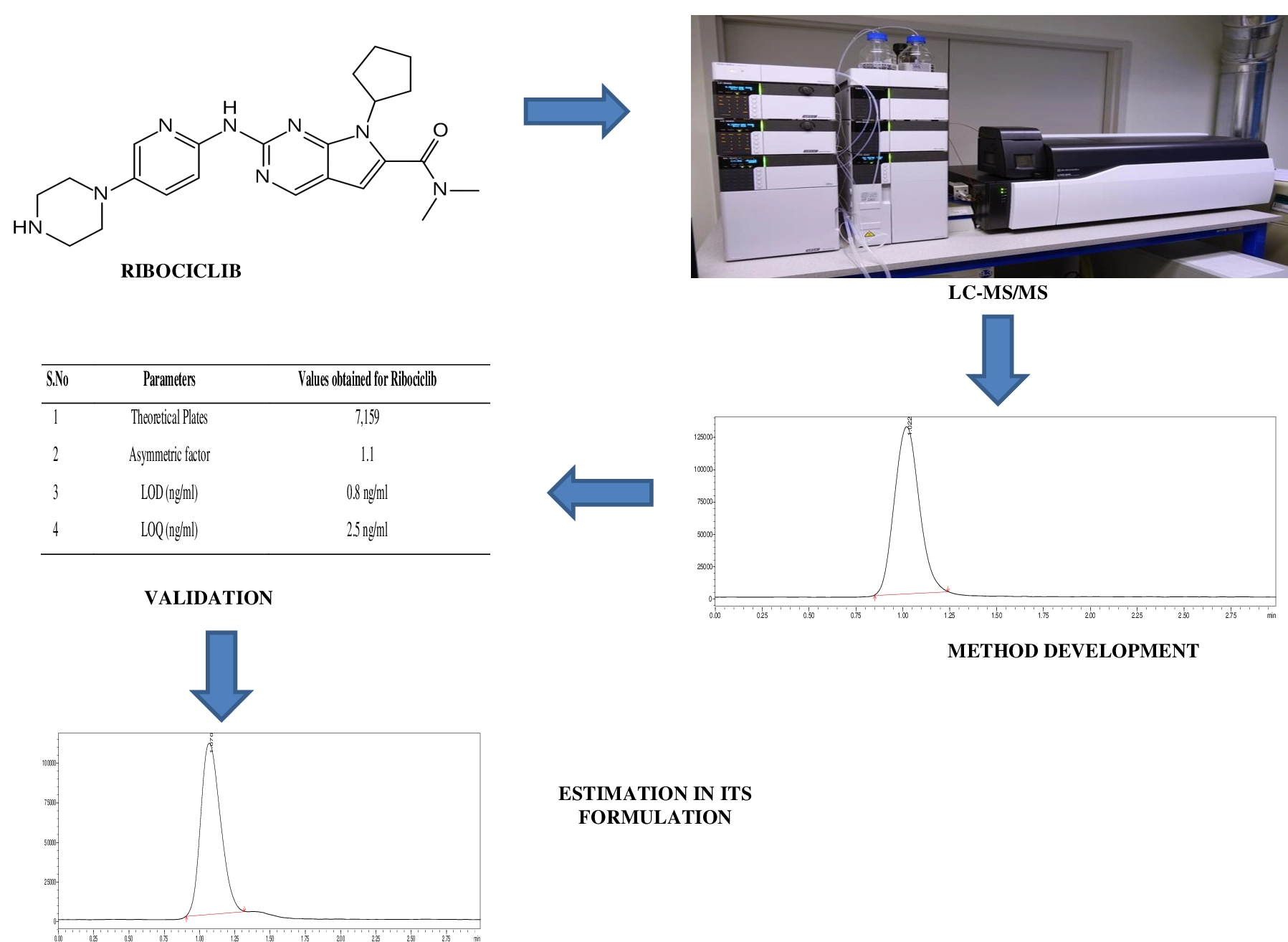
A sensitive liquid chromatography tandem mass spectrometric method development and validation for ribociclib and its formulation
J. Ramesh, B. Babu, R. Sangamithra, D. Anandha Jothi, S.N. Meyyanathan, B. Gowramma
DOI: 10.7324/JAPS.2024.178562Pages: 227-232
Development of a validated RP-HPLC/PDA method for the quantitative estimation of tepotinib in tablet dosage form
Sumalatha Chepyala, Srinivas Medidi, Jitender Kumar Malik
DOI: 10.7324/JAPS.2024.184366Pages: 064-071
_.jpg)