INTRODUCTION
Topiramate is a hexose derivative that is 2,(3:4),5-di-O-isopropylidene-beta-D-fructopyranose, in which hydroxy group is converted to the corresponding sulfamate ester. Topiramate increases receptor activation for gamma-amino butyric acid (GABA)-A at the sites of non-benzodiazepines and decreases the activity of glutamate in kainate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors. Moreover, it is proposed that mesolimbic activity should also be reduced. Topiramate also blocks sodium-based voltage channels to inhibit seizure spread. Structurally, Topiramate presents a very high concentration of O2, and it does not resemble a normal therapeutic agents. It reduces the action of excitatory neurotransmitter through the AMPA receptor and kainite receptor (Drug Information, 2019). It is available commercially as tablet (25, 50, 100, and 200 mg). Topiramate is an anticonvulsant drug and is used to treat antiepileptic and prevent migraine headache. Topiramate contains not less than 98.0% and not more than 102% of C12H21NO8S. Melting range is 120 - 130 °C. As per the literature survey, a few analytical methods were reported by high-performance liquid chromatography (Kumari et al., 2015; Mahadev et al., 2017; Reddy et al., 2015) for the estimation of Topiramate in bulk and pharmaceutical dosage forms and liquid chromatography-mass tandem spectrometry (LC-MS-MS) (Das 2013; Goswami et al., 2009; Park et al., 2008) methods are reported for the determination in biological samples. However, the reported method had low sensitivity and selectivity with long run time. Hence, the objective is to develop a simple, rapid, highly sensitive, and economical method for the determination of Topiramate in its formulations by LC-MS-MS.
METHODS AND MATERIALS
Chemicals
The working standard Topiramate was obtained from the Indian Pharmacopoeia Commission, New Delhi. Chemicals (ammonium acetate) and solvents (acetonitrile and formic acid) were of LC-MS grade procured from SD Fine Chemicals. Ultrapure water was obtained from Milli-Q RO system.
Instrument and chromatographic conditions
Shimadzu LC-MS/MS 8,030 system was equipped with electrospray ionization interface, SIL-20AC autosampler, CBM-20 alite controller, CTO-20AC column oven, LC-20AD pump, and SPD-M20 PDA detector and operated using Lab Solutions data station.
A stationary phase consisting of Zorbax RP-C18 column (50 mm × 4.6 mm i.d., 5 μ) was selected, and ammonium acetate and acetonitrile were used as a mobile phase at the ratio of 15:85 v/v and pumped at a flow rate of 0.5 ml/min. 10 μl of injection volume was employed at an ambient column temperature.
Preparation of stock and working solution
About 10 mg of Topiramate was weighed and dissolved into 10 ml of ethanol (1 mg/ml). From the above stock solution, 1 μg/ml of working concentration was prepared. Further, from the working standard, calibration standard ranging from 1 to 1,000 ng/ml of Topiramate was prepared. Three different quality control (QC) levels were prepared, i.e., low (LQC; 3 ng/ml), medium (MQC; 100 ng/ml), and high (HQC; 900 ng/ml) in the mobile phase.
Optimization of mass range
The optimization of mass conditions was performed using standard Topiramate (1,000 ng/ml). The transition was obtained at m/z 338.05 (precursor ion) → 77.95 (product ion) and m/z 338.05 → 95.90 which were used to monitor Topiramate (Fig. 1).
Method validation
As per the ICH guidelines, analytical method was validated using various validation parameters such as specificity, linearity range, accuracy and precision, Detection limit (LOD) and quantification limit (LOQ), robustness, and system suitability (ICH, 1996).
Linearity
Six different concentrations of Topiramate ranging from 1 to 1,000 ng/ml were used to determine the linearity. The linearity curve was used to measure the correlation coefficient (R2), slope (m), and intercept values.
Specificity
The method specificity was determined based on the ability of the method to determine the analyte in the presence of interferences.
Accuracy and precision
As per the ICH guidelines, the accuracy and precision of the method were determined at three QC levels by analyzing six replications. Recovery of the method was used to determine the accuracy of the method. Intra- and interday precision was carried out to determine the method precision, and the results were recorded in terms of percent relative standard deviation (% RSD).
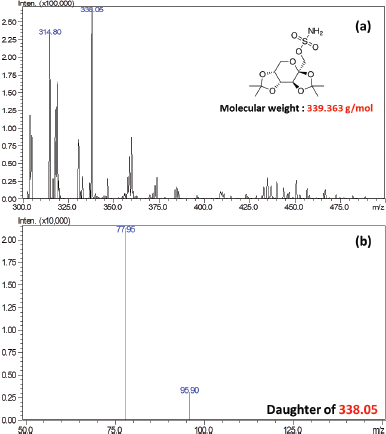 | Figure 1. (a) Mass scan spectra and (b) product ion scan spectra for Topiramate in the negative mode. [Click here to view] |
Detection limit and quantification limit
LOD and LOQ were calculated by signal-to-noise ratio. Signal-to-noise ratio of 3:1 was used to determine the LOD, and signal-to-noise ratio of 10:1 was used to determine the LOQ.
Robustness
For the determination of robustness, chromatographic parameters such as flow rate, column temperature, wavelength detection, injection volume, mobile phase, and pH were studied.
RESULTS AND DISCUSSION
Specificity
At the retention time of Topiramate, there were no potential interference peaks found (Fig. 2a). Therefore, the method was found to be selective and highly sensitive.
Accuracy and precision
The method accuracy was determined by recovery studies for three QC samples. The recovery ranged between 93.3% and 99.7% with % RSD ranging from 0.10% to 1.85% for precision study (Table 1).
Linearity
The linearity curve was determined at six different concentration levels, and the regression equation was found to be 0.999 for the concentration ranging from 1 to 1,000 ng/ml. The standard deviations (SDs) of calibration curve results were found to be within the limits, with the regression equation of y = 1,055.x + 1,287 (Fig. 3).
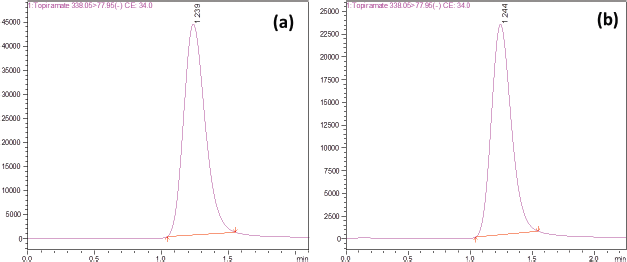 | Figure 2. Multiple reaction monitoring (MRM) chromatogram of Topiramate (a) standard and (b) sample formulation. [Click here to view] |
.png) | Table 1. Accuracy and precision studies of Topiramate. [Click here to view] |
.png) | Figure 3. Six-point calibration curve of Topiramate (1–1,000 ng/ml). [Click here to view] |
LOD and LOQ
The LOD and LOQ were obtained at 0.5 and 1.0 ng/ml for the developed method, respectively, based on the minimum level of peak area and signal-to-noise ratio.
Robustness
The conditions such as flow rate (0.2, 0.5, and 0.8 ml/min), pH (5.5, 6.0, and 6.5), and acetonitrile percentage (75%, 85%, and 90%) were studied to determine the robustness of the method. The % RSD was calculated for flow rate (1.10%, 0.82%, and 0.25%), pH (1.20%, 0.91%, and 0.33%), and acetonitrile percentage (1.30%, 0.79%, and 0.22%) which were found to be within the limit and demonstrated that the developed method was found to be robust (Table 2).
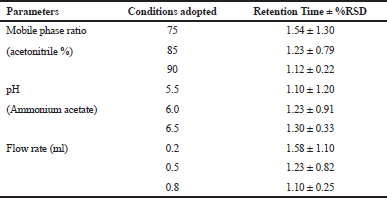 | Table 2. Robustness studies. [Click here to view] |
System suitability
The system suitability study was carried out to determine parameters such as tailing factor (T), number of theoretical plates (N), and retention factor (Rt), as per the ICH guidelines. The results were found to be within the limits (Table 3).
Estimation of Topiramate dosage form
Twenty tablets of Topiramate tablet were accurately weighed and crushed into powder, and the weight equivalent to 10 mg was taken. The powder was dissolved using 5 ml of organic solvent by sonication for 15–20 minutes and filtered through 0.45-μm membrane, and the volume was made up to 10 ml using organic solvent (ethanol). From the resulting solution (1 mg/ml), the QC samples (3, 100, and 900 ng/ml) were prepared by appropriate dilution and analyzed (Table 4 and Fig. 2b).
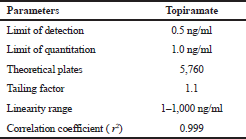 | Table 3. System suitability. [Click here to view] |
.png) | Table 4. Recovery studies for the Topiramate formulation. [Click here to view] |
CONCLUSION
An accurate, simple, precise, rapid, selective, and cost-effective method was successfully developed and applied for the quantitative determination of Topiramate in bulk and commercial formulation. The newly developed and validated method will be useful for the clinical pharmacokinetic, biopharmaceutical, and bioequivalence studies due to their high sensitivity.
ACKNOWLEDGMENT
The authors gratefully acknowledge the Indian Pharmacopoeia Commission for providing Topiramate in the form of gift.
CONFLICT OF INTEREST
None
REFERENCES
Das GK. Estimation of Topiramate in human plasma using LC-MS/MS. Asian J Pharm Clinical Res 2013; 6(7):217–20.
Drug Information. [Online]. Available via http://www.drugs.com (Accessed 10 August 2019).
Goswami D, Kumar A, Khuroo AH, Monif T, Rab S. Bioanalytical LC-MS/MS method validation for plasma determination of Topiramate in healthy Indian volunteers. Biomed Chromatogr 2009; 23:1227–41. CrossRef
ICH. Q3B validation of analytical procedures: methodology, International Conference on Harmonization, November 1996. [ONLINE] Available via: https://www.fda.gov/downloads/drugs/guidances/ucm073384.pdf [Accessed 20 August 2019].
Kumari R, Shyamala, Sharma JVC. Stability indicating method development and validation of Topiramate using RP-HPLC in bulk and pharmaceutical dosage forms. Eur J Pharm Med Res 2015; 2(7):264–88.
Mahadev BK, Moreshwar PM, Sanjay DS. Method development and validation by RP-HPLC for estimation of Topiramate in bulk and pharmaceutical dosage form. 2017; 10(7):843–9.
Park JH, Park YS, Lee MH, Rhim SY, Song JC, Lee SJ, Kim JM, Shaw LM, Kang JS. Determination of plasma Topiramate concentration using LC-MS/MS for pharmacokinetic and bioequivalence studies in healthy Korean volunteers. Biomed Chromatogr 2008; 22(8):822–9. CrossRef
Reddy SS, Suresh KV, Nagaraju PT, Venugopal K. Analytical method development and validation for estimation of Topiramate bulk and pharmaceutical dosage form by using HPLC method. World J Pharm Res 2015; 4(10):1043–60.