INTRODUCTION
Diabetes is an endocrine disorder marked with the hyperglycemic condition due to decreased insulin secretion, decreased activity of damaged insulin, or both [1]. The International Diabetes Federation stated that diabetes is the fastest-growing health problem in the 21st century [2]. In 2021, approximately, 537 million people suffer from this disease, and the number is projected to increase to 643 million by 2030, even up to 783 million by 2045. Diabetic patients tend to suffer complications, namely chronic wounds, and as much as 25% of them need to undergo amputation. Peripheric arterial disease, neuropathy, ischemic, and microbial infection are key factors in how diabetic patients develop chronic wounds [3]. These factors exacerbate the wounds by increasing the amount of reactive oxygen species (ROS) and hindering its healing process by keeping it at the chronic inflammation phase without showing any development toward the proliferation and remodeling phase [4].
Several topical therapeutic agents may be useful to treat chronic wounds caused by the diabetic condition. However, despite numerous efforts to achieve this, a truly effective therapeutical agent has never been obtained [5]. The administration of protein, antibiotic, and nucleic acid-based topical therapeutic agents may lead to the degradation of the therapeutic substances by endogenous enzymes, acute toxicity toward the affected tissues and organs, antimicrobial resistance, and abnormal immunological response [3]. Natural products offer a promising alternative therapy to treat diabetes-related chronic wounds due to their various pharmacological activities that can assist in wound healing. It has also been viewed as a safer option due to its lesser side effects [6].
Carvacrol (CAR) is the main component of the volatile oil extracted from oregano, thyme, and marjoram plant [7]. CAR is categorized into the phenolic monoterpenoids group, which exhibits various pharmacological activities such as antioxidant, anti-inflammatory, antimicrobial, and analgetic activities [8]; these activities are advantageous for aiding the healing process of diabetes-related chronic wounds. There are several types of research about the wound-healing activity of CAR [9] and the CAR containing volatile oil [10–12] as substances that may aid the wound-healing process significantly. However, the direct usage of CAR to the wound site is not ideal due to its hydrophobic, volatile nature, and the risk of dermal irritation due to the massive uncontrolled CAR release [13–15].
Recent developments have shown that nanostructured lipid carriers (NLCs) have garnered much attention as pharmaceutical carriers in wound healing treatment. NLC may overcome CAR’s hydrophobicity and volatility issue while also providing a controlled release profile, thus reducing its irritability issue [16,17]. Several types of research using NLC loaded with natural products, such as ferulic acid [18], rosemary oil [19], thymoquinone [20], protopanaxadiol [21], and curcumin + epidermal growth factor [22], to treat acute and chronic wounds have also been performed before. The result showed an improvement in the wound healing process compared to the control group and the free-form active substances.
To the best of the researchers’ knowledge, CAR-loaded NLC (CAR-NLC) research in supporting diabetes-related wounds has never been done before. Therefore, in this research, CAR will be formulated into the NLC system, which will then be characterized and evaluated regarding its activity in healing diabetes-related wounds. Theoretically, loading CAR into an NLC formulation shall improve its diabetic wound healing activity so that it can be developed as a promising alternative therapy.
MATERIALS AND METHODS
Materials
CAR with ≥98.5% purity was purchased from Shanghai Macklin Biochemical Co. Ltd. (Shanghai, China). Tween® 80, ascorbic acid, and analytical-grade methanol were purchased from Merck Co. (Darmstadt, Germany). TEGO® Care 165 was purchased from Evonik Co. (Essen, Germany). Beeswax (BW) was purchased from RAS Chemical (Bandung, Indonesia); 2,2-diphenyl-1-picrylhydrazyl (DPPH) was purchased from Sigma-Aldrich Co. (Steinheim, Germany). Bovine serum albumin (BSA) was purchased from Himedia Co. (Mumbai, India). Streptozotocin (STZ) was purchased from Santa Cruz Biotechnology Co. (Dallas, TX). Diclofenac sodium was purchased from PT. Brataco (Bandung, Indonesia). Analytical grade water (Onelab Waterone™) was purchased from PT. Jayamas Medica Industry (Sidoarjo, Indonesia). Analytical-grade ethanol was purchased from PT. Smart Lab Indonesia (Tangerang Selatan, Indonesia). The other reagents used in the study were of analytical grade.
Preparation of CAR-NLC
The CAR-NLC was produced by the hot homogenization-ultrasonication method as explained by Li and Ge [23] with some modifications. The lipid and aqueous phases were prepared separately. The lipid phase consists of CAR 1.5% (w/v), TEGO® Care 165 1.3% (w/v), and BW 3.5% (w/v), while the aqueous phase consists of Tween® 80 5.2% (w/v) and Onelab Waterone™. The lipid and aqueous phases were separately heated until they reached a temperature of 70°C ± 2°C for 10 minutes. While maintaining a similar temperature, the aqueous phase was poured into the lipid phase while being homogenized by the high-shear homogenizer (Ultra-Turrax® T-25, IKA-Works, Inc., Staufen im Breisgau, Germany) at 7,000 rpm for 8 minutes. Further particle size (PS) reduction was done using a probe-type ultrasonicator (CY-500 Ultrasonic Homogenizer, J.P. Selecta, Barcelona, Spain) at 70% amplitude for 7 minutes (with a pulse cycle of 45 seconds on and 15 seconds off) within an ice bath to prevent the temperature increase of the mixture during the sonication process. The obtained CAR-NLC was stored away to reach room temperature. The total volume of CAR-NLC obtained was 20 ml. The schematic figure according to our work, novelty, and all the procedures is shown in Figure 1.
Characterization of CAR-NLC
The particle size (PS) of CAR-NLC was analyzed with the dynamic light-scattering (DLS) method by using the photon correlation spectroscopy (Delsa™ Nano C Particle Analyzer, Beckman Coulter, Brea, CA), the polydispersity index (PDI) was used to portray the CAR-NLC PS distribution. The zeta potential (ZP) of CAR-NLC was analyzed with the electrophoretic light-scattering (ELS) method with similar instruments. For the determination of PS and PDI, the sample was diluted 20 times with Onelab Waterone™, put into disposable semi-micro cuvettes, and measured. For the ZP analysis, the sample was diluted 200 times with Onelab Waterone™, and then put into a flat surface cell and analyzed.
The indirect method determined the encapsulation efficiency (EE) and drug loading (DL) measurement of CAR inside the NLC. The CAR-NLC was centrifuged at 13,000 rpm for 30 minutes using the centrifugal ultrafilter device (10 kDa, Amicon Ultra, Millipore, Billerica, MA). The amount of unencapsulated CAR was determined by taking the filtrate and dissolving it in ethanol, then measuring the content using a UV-visible spectrophotometer (DU 7500i, Beckman Coulter, Brea, CA) at the wavelength 275 nm. The amount of CAR was determined by the calibration curve CAR in ethanol [15] at the concentration of 15–55 µg/ml. EE and DL were determined by using the following equations:
(1)
(2)
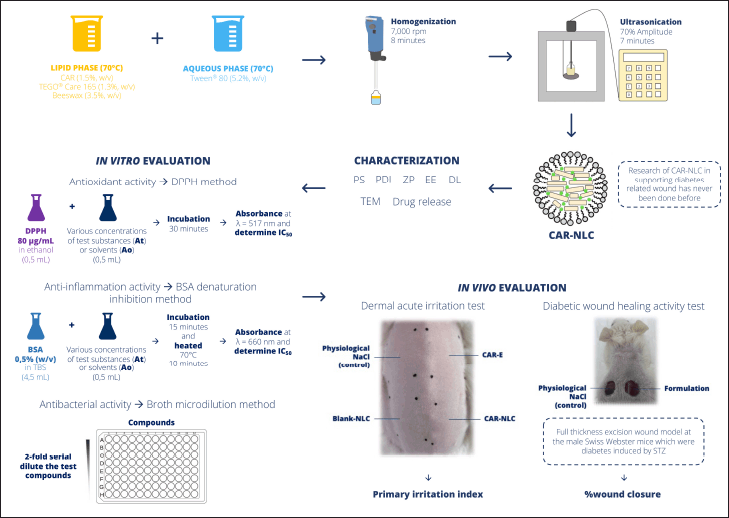 | Figure 1. The schematic figure is according to our work, novelty, and all the procedures. [Click here to view] |
The morphology of CAR-NLC was analyzed using transmission electron microscopy (TEM) (HT7700, Hitachi, Tokyo, Japan) at 100 kV. One drop of the CAR-NLC formula was loaded onto a carbon-coated copper grid with a mesh size of 200 nm. After that, negative staining was performed using the UranyLess EM Stain by applying 15 µl of the staining agent onto the grid containing the sample and letting it sit for 1 minute.
In vitro drug release study
CAR release study from NLC was carried out using the dialysis bag method as Galvão et al. [14] explained with some modifications. The cellulose-based dialysis bag (14 kDa MWCO, Ward’s Science, Rochester, NY) was activated before its usage as instructed by the producer’s instruction. The release medium was composed of a mixture of phosphate buffer (pH 7.4) and ethanol at 7:3 (v/v). CAR solution in its free form (CAR-free) 1.5% (w/v) was made with ethanol as the solvent. As a comparison toward conventional dosage form, CAR emulsion (CAR-E) was made by mixing CAR 1.5% (w/v) and Tween® 80 5.2% (w/v) at the mixing speed 1,500 rpm for 8 minutes at the temperature 70°C ± 2°C. Afterward, 1 ml of CAR-free, CAR-E, and CAR-NLC was each stored inside the dialysis bag and placed inside a vessel containing 20 ml of release medium. The system was maintained at 37°C ± 0.5°C and mixed constantly by magnetic stirrer at 200 rpm for 24 hours. As much as 400 µl of release medium was collected at the 0.25, 0.5, 0.75, 1, 2, 3, 4, 5, 6, 7, 8, and 24 hour time stamps in reference to the starting timestamp. Each time the release medium was sampled, it was then replaced with fresh release medium to maintain the sink condition. The collected samples were analyzed using UV-visible spectrophotometry (DU 7500i, Beckman Coulter, Brea, CA) at wavelength 275 nm. The amount of CAR was determined by a calibration curve of CAR within ethanol [15] at the concentration range of 15–55 µg/ml. The cumulative percentage of released CAR was obtained according to the following equation:
(3)
Antioxidant activity
The antioxidant activity was performed by determining the free radical scavenger capacity of DPPH according to the method explained by Putri et al. [24] with some modifications. First, a solution of DPPH in ethanol with a concentration of 80 µg/ml was prepared. Next, a series concentration of active substances within suitable solvents was made (ascorbic acid standard and CAR-free in ethanol, while CAR-E and CAR-NLC in Onelab Waterone™), then after it was reacted with the DPPH 80 µg/ml solution (each sample’s volume was 0.5 ml), incubated at room temperature for 30 minutes, and protected from light exposure. The absorbance value of each mixture was then measured by using UV-visible spectrophotometry (DU 7500i, Beckman Coulter, Brea, CA) at wavelength 517 nm (At). The formula for CAR-NLC at each concentration similar to the tested concentration was used as the blank solution because it also exhibited absorbance at 517 nm. The control solution (A0) was the mixture of blank solvent and the solution of DPPH 80 µg/ml with a similar treatment. The DPPH free radical scavenger activity of the tested substance was determined according to the DPPH scavenger percentage calculated from the following equation:
(4)
The free radical scavenger capacity of the test substance was calculated by constructing a curve of the free radical inhibition percentage of the test substance against the concentration; the result was displayed as the half-maximal inhibitory concentration (IC50).
BSA denaturation inhibitory activity
The anti-inflammation activity was evaluated by determining the capability of the test substance to inhibit albumin denaturation according to the method explained by Putri et al. [24] with some modifications. First, a solution of 0.5% (w/v) BSA in tris-buffer saline 0.05 M with pH adjusted to 6.3 with glacial acetic acid was produced. Then, solutions of the test substances in a series of concentrations in a suitable solvent were made (diclofenac sodium standard and CAR-free in methanol, while CAR-E and CAR-NLC in Onelab Waterone™) and reacted with the 0.5% BSA solution in the volume of 0.5 and 4.5 ml. The mixtures were then incubated at room temperature for 15 minutes and then heated for 10 minutes at 70°C ± 2°C inside a tube in a water bath. After it reached room temperature, the absorbance of each mixture was then measured by using UV-Visible spectrophotometry (DU 7500i, Beckman Coulter, Brea, CA) at wavelength 660 nm (At). The control solution (A0) was the mixture of the solvent from each test substance and 0.5% BSA solution with a similar treatment. The capability of the test substances to inhibit albumin denaturation was determined according to the albumin denaturation inhibition percentage calculated from the following equation:
(5)
The capacity of each tested substance to inhibit albumin denaturation was calculated by constructing a curve of the percentage of albumin denaturation inhibition by the test substance against its concentration, and the result was displayed as the IC50.
Antibacterial activity
The antibacterial activity study of CAR-free, CAR-E, and CAR-NLC was carried out by determining the minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) using the microdilution method as recommended by the Clinical and Laboratory Standards Institute (CLSI) [25]. Staphylococcus aureus (ATCC 6538) and Escherichia coli (ATCC 8939) were used as the tested strains. Tested strains were incubated in Mueller Hinton Agar (MHA) at 37°C for 24 hours, then the tested strains were suspended in Mueller Hinton Broth (MHB), and the concentration was adjusted to 108 CFU/ml by comparing it with the turbidity of 0.5 McFarland standard. The culture suspension was further diluted with MHB by the ratio 1:20 to produce a bacterial suspension of 106 CFU/ml.
In short, a 96-well microplate was filled with 100 µl MHB. Next, 100 µl of CAR-free (made in dimethyl sulfoxide (DMSO) 40% v/v), CAR-E, and CAR-NLC in the concentration of 4 mg/ml was added into the first well and mixed homogeneously. The DMSO solvent 40% (v/v) was added to the wells and examined as the control. Next, 100 µl of tested substances from the first-row wells was transferred to the second-row wells; the transfer was continued until the last row of wells. Each row of wells contains half of the previous wells’ samples. For each well, 10 µl of bacterial inoculum of 106 CFU/ml was added. The absorbance value of each well was determined by a microplate reader (Multiskan GO FC 357, Thermo Scientific, Waltham, MA) at wavelength 630 nm before and after 24 hours of incubation at 37°C [26]. The MIC was determined according to the smallest concentration of the tested substances, which yielded an increase in absorbance of not more than 0.200. To evaluate MBC, 20 µl of the samples from each well without bacterial growth from the MIC test was spread onto the MHA plate and incubated for 24 hours at 37°C. The MBC was determined as the lowest sample concentration without observed bacterial growth.
Dermal acute irritation test
The dermal acute irritation test was performed per the Regulation of Indonesian FDA Number 10 of 2022 [27]. All test procedure has received approval from the Ethical Committee of Institut Teknologi Bandung (No. KEP/I/2022/IX/H220822FF/UIDN). The study was carried out on three male albino rabbits, about 6 months old, weighing around 2 kg, purchased from a local rabbit farm at Lembang, Bandung, Indonesia. Before the study, the rabbits were acclimatized for 7 days at the laboratory and placed in individual cages. The rabbits were given a diet of vegetables (carrots and water celery) and drinking water ad libitum. The room used for the upkeep of the rabbits was kept at 25°C ± 3°C, relative humidity of 60%–70%, and an illumination cycle of 12 hours alternating light. At least 24 hours before testing, the rabbits’ fur was shaved, yielding an area approximately 10 × 15 cm on its posterior area as the place for applying the tested dosage form.
A preliminary study was conducted beforehand on one rabbit, exposing it to 0.5 ml of CAR-E, Blank-NLC (NLC formula without CAR addition), and CAR-NLC by applying three patches with an area of ±6 (2 × 3) cm2 on the tested area, then covering it with sterile gauze and nonirritant tape. As the control, 0.5 ml of physiological sodium chloride solution was also applied by a patch with a similar treatment. After 3 minutes, the skin area covered by the patches was examined. If there was no observed dermal reaction, then the examination was continued for 1 hour. Afterward, if there was still no observed serious dermal reaction (irritation), the examination continued until 4 hours, then the gradation of dermal injury was determined. If any irritation was observed after exposure for 3 minutes or 1 hour, then the test was aborted and all patches were taken off. The observation continued until 14 days unless any irritation was observed in the early examination stage.
Afterward, if there was no observed irritation after 4 hours of exposure, then the test was continued with a confirmatory test. Two additional albino rabbits were employed, each exposed to CAR-E, Blank-NLC, and CAR-NLC for 4 hours. The exposure site was covered with sterile gauze and nonirritant tape. The residue of the tested dosage form was immediately cleaned with a physiological sodium chloride solution after 4 hours of exposure. The control was done with 0.5 ml of physiological sodium chloride solution, which was also applied by a patch with a similar treatment. Any erythema or edema was observed on all test animals at the 1, 24, 48, and 72 hour time stamps after opening the wound dressing. To evaluate the reversibility, the animals were observed no less than 14 days after opening the wound dressing.
The primary irritation index (PII) was calculated according to the erythema and edema evaluation observed at the 24, 48, and 72 hour time stamps. PII was calculated by the following equation:
(6)
A is the sum of erythema and edema scores from each sample observation points of 24, 48, and 72 hour time stamps divided by the number of observations, B is the sum of erythema and edema score from each control group observation points of 24, 48, and 72 hour time stamps divided by the number of observation, and C is the number of animals tested. The irritation/corrosion category was estimated according to the criteria explained in the document of ISO 10993-10 [28].
In vivo diabetic wound healing activity
The in vivo diabetic wound healing activity of CAR-NLC was performed following the method explained by Shanmugapriya et al. [29] with some modifications. All test procedure has received approval from the Ethical Committee of Institut Teknologi Bandung (No. KEP/I/2022/IX/H220822FF/UPLD). The test subjects were Male Swiss Webster mice, aged 8–10 weeks, and weighing around 35–40 g, obtained from the Animal Laboratory of the School of Life Sciences and Technology, Institut Teknologi Bandung. Before the testing, the mice were acclimatized for 7 days at the laboratory and placed in individual cages. The mice were given a diet of laboratory standard pellets and drinking water ad libitum. The room used for the maintenance was maintained at 25°C ± 3°C, relative humidity of 60%–70%, and an illumination cycle of 12 hours alternating light.
Before the excision of the diabetic wound, the mice were diabetically induced first by administering repeated doses of STZ with a dose of 40 mg/kgBW a day intraperitoneally for 5 days. The mice fasting for 4 hours were given STZ injection dissolved in citrate buffer (50 mM, pH 4.5), which was sterilized by filtering it through a polytetrafluoroethylene membrane with 0.22 µm pore size. The drinking water of the mice was substituted with 10% sucrose solution for 5 days, starting from the initial day of diabetes induction, given in a manner of ad libitum. Meanwhile, the feeding pellets were still given ad libitum without any change. The blood glucose level of each mouse was examined 21 days after the diabetes induction [30]. The blood glucose level was checked with the digital blood glucose meter (Easy Touch® GCU ET-301, Bioptik Technology, Inc., Miaoli County, Taiwan). Only mice with blood glucose levels above 200 mg/dl were chosen and subjected to the next testing stage.
One day before the testing, the mice fur was shaved at the posterior area with an area of 6 × 3 cm2; the shaved area was then wrapped with sterile gauze to protect the skin. On the testing day, the shaved posterior area was sterilized with a 70% ethanol wipe and dried properly. The mice were given the anesthetic of ketamine/xylazine dosed 80/10 mg/kgBW administered intraperitoneally. Before the anesthetic procedure, the mice were sedated by administering atropine sulfate dosed 0.05 mg/kgBW subcutaneously. Afterward, two wounds (reaching the epidermis and dermis, until hypodermis) were created by an 8 mm diameter sterile biopsy punch. The wounds were then treated by four types of treatment: (1) CAR-E; (2) Blank-NLC; (3) CAR-NLC; and (4) liquid povidone-iodine 10%, given once daily. Each treatment group comprises six mice (as stated by the Federer equation). Each mouse received two treatments: the right-side wound was given the aforementioned tested substance, and the left-side wound was given physiological sodium chloride solution as a control. Each tested substance was administered 30 µl by micropipette.
Each wound was examined and photographed every 3 days for 15 days. The degree of wound closure was determined by the software ImageJ® 1.53 (Aspire Software International, Leesburg, VA). The wound area was calculated according to the following equation:
(7)
Histological analysis
The histological analysis was performed according to the methodology explained by Saporito et al. [31]. The mice were euthanized on the last day of examination, then the full-thickness biopsy was created at the wounded area, and the histological analysis was performed. The biopsy of intact skin was also drawn for comparison. The tissue sample was sectioned along the widest wound, fixed for 48 hours in 4% (w/v) neutral paraformaldehyde buffer, dehydrated with a series of ethanol gradients, cleaned by xylene, and embedded in liquid paraffin. A microtome was obtained from the biopsy and stained with hematoxylin and eosin (H&E). Each slice was examined under a light microscope (Olympus CX22, Olympus Medical Systems India Pvt. Ltd., Gurugram, Haryana) equipped with a digital camera.
Statistical analysis
The statistical analysis and data visualization were performed using GraphPad Prism v.8.0 (InStat 3.06, San Diego, CA). Student’s t-test and one-way analysis of variance were used to determine the statistical significance of the difference. The results were declared statistically significant if the p-value < 0.05.
RESULT AND DISCUSSION
Characterization of CAR-NLC
CAR was formulated within the NLC drug carrier system to overcome the problem of hydrophobicity, volatility, and irritant potency, thus rendering it ideal to be applied for wound healing treatment. A prior study performed by Khezri et al. [19] successfully developed an NLC formula to overcome the problem of hydrophobicity and volatility of rosemary oil to be applied for wound healing treatment. Moreover, a study done by Carbone et al. [18] exhibited that NLC formulation was able to overcome the toxicity problem of ferulic acid in its testing of in vitro wound healing treatment; it also improved the wound healing activity of ferulic acid compared to controls. These study results were about the NLC’s ability to regulate the active substance release and the nano-scaled NLC PS, which might offer improvements in the wound healing treatment.
The CAR-NLC formula exhibited PS, PDI, ZP, EE, and DL values of 174.5 ± 1.2 nm, 0.31 ± 0.04, −24.83 ± 5.51 mV, 98.46% ± 0.07%, and 42.20% ± 0.03%, respectively. CAR not only acts as the active substance, but it also serves as the liquid lipid in the NLC formulation. Therefore, elevated CAR concentration in the formula may decrease the viscosity of the formula’s internal phase, causing the lipid globules to be dispersed more easily during the hot homogenization process and further CAR-NLC PS reduction during the ultrasonication process, yielding small PS of the CAR-NLC formula [32]. The PDI parameter showed that the PS distribution of CAR-NLC was uniform, without any aggregation between particles occurring. In contrast, the ZP parameter showed that the CAR-NLC possessed improved stability in its liquid phase [16]. The EE and DL of the CAR-NLC formula also showed a high value, which can be attained by using the solid lipid and surfactant combinations that can provide high CAR loading capacity into NLC.
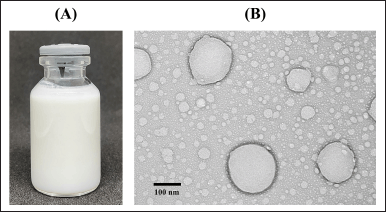 | Figure 2. Characterization of (A) CAR-NLC formula without dilution and (B) TEM image showing the morphology of the CAR-NLC formula at 30,000× magnification. [Click here to view] |
The physical appearance and morphology by TEM-imaging of the CAR-NLC formula are shown in Figure 2A and B, respectively. The CAR-NLC formula showed white and homogenous suspension (referring to Fig. 2A) and exhibited spherical or oval-shaped particles (referring to Fig. 2B) with a diameter of around 150–170 nm, following the PS data of the DLS instrument analysis. There was also no aggregation between particles observed from the TEM image.
In vitro drug release study
The cumulative percentage of released CAR from CAR-free, CAR-E, and CAR-NLC toward the release media as a function of time was shown in Figure 3. The CAR release from the CAR-NLC formula exhibited a biphasic manner with an immediate CAR release during the initial 4 hours, followed by a sustained CAR release for 24 hours. During the first 4 hours, the CAR-NLC formula showed a cumulative percentage of released CAR as much as 50.21% ± 0.55%; after 24 hours, it was 70.17% ± 3.11%. This behavior can be attributed to the immediate release of CAR attached to the NLC particle surface, continued by the gradual diffusion of the CAR within the NLC core across the NLC matrix until it reaches the surface or NLC erosion matrix [32]. Müller et al. [33] explained that a burst release behavior was expected from NLC particles formed from the hot homogenization method due to the redistribution effect of the active substance from the aqueous phase toward the lipid phase during the hot homogenization process, continued by temperature decrease until there was an accumulation of active substance on the surface of the NLC particles.
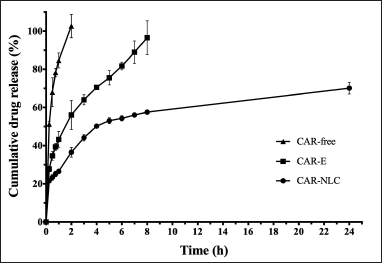 | Figure 3. In vitro drug release profile of CAR-free, CAR-E, and CAR-NLC. Data were expressed as mean ± standard deviation (n = 3). [Click here to view] |
The CAR release profile from the CAR-free and CAR-E were also tested in a similar condition compared to conventional drug formulation. The result showed that CAR-free and CAR-E exhibited a cumulative percentage of released CAR as much as 102.69% ± 6.14% after 2 hours and 96.59% ± 8.89% after 8 hours, respectively. It can be inferred that the CAR-NLC formula exhibited a controlled CAR release compared to the CAR-E and CAR-free form, which aids in wound healing. The biphasic profile of the active substance release of the NLC formula may provide an immediate release of the active substance at the start of the treatment, achieving the onset dosage quickly, followed by the sustained release phase, thus maintaining the active substance concentration to be within the therapeutic window during the treatment period [34]. Furthermore, the controlled release may also reduce the administration frequency, improving patients’ adherence [22].
Antioxidant activity
Diabetes-related chronic wounds experience an increase in the amount of ROS such as superoxide (O2−), hydrogen peroxide (H2O2), hydroxyl radicals (OH), and other reactive oxygen derivates, which were lethal and generated wide injury toward protein, DNA, and lipid, ultimately affecting the normal cellular function. Therefore, the ROS level within wounds must be regulated by small-molecule antioxidants [3].
CAR has been reported to exhibit antioxidant activity [8]. CAR possesses a phenol moiety that could donate a hydrogen atom to a free radical and then stabilize its polarity with electron delocalization [35]. The antioxidant activity of CAR-free, CAR-E, and CAR-NLC was evaluated by the DPPH method. This method is highly sensitive and widely used. The uncoupled electron from the radical DPPH• species will accept one hydrogen atom from an antioxidant, converting the radical DPPH• species into DPPH•–H species, leading to a shift in the absorbance spectrum [24,36]. The comparison of the IC50 value for the DPPH damping activity of CAR-free, CAR-E, CAR-NLC, and ascorbic acid standard is shown in Figure 4. A higher antioxidant activity was portrayed by a lower IC50 value. The IC50 value is the sample concentration able to dampen the DPPH radical by 50% within the solution. In this study, ascorbic acid was used as the antioxidant standard, which exhibited a very low IC50 value, 0.008 ± 0.001 mg/ml. Figure 4 shows the IC50 value of CAR-E (0.171 ± 0.022 mg/ml) and CAR-NLC (0.330 ± 0.029 mg/ml), which were significantly lower (p-value < 0.05) compared to the IC50 value of CAR-free (0.624 ± 0.018 mg/ml). According to this study result, it can be inferred that the CAR antioxidant activity was affected by the formulation factor, wherein if the CAR was formulated in the emulsion of NLC form, then its antioxidant activity was bound to be higher.
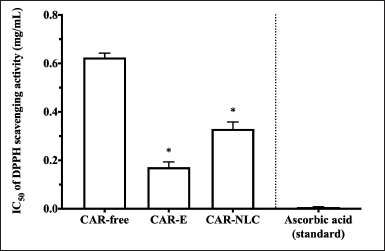 | Figure 4. DPPH scavenging activity of CAR-free, CAR-E, CAR-NLC, and ascorbic acid standard presented as IC50. Data were expressed as mean ± standard deviation (n = 3). *p-value < 0.05 was considered significant compared to CAR-free. [Click here to view] |
The higher antioxidant activity of CAR-E compared to CAR-free can be attributed to the addition of emulsifiers, as shown in the study done by Shu et al. [37]. They showed that there was an impact on the antioxidant activity after an emulsifier was added to the tested formula. Previous studies have also shown an improvement in the antioxidant activity of lutein and lemon oil in the form of microemulsion/nanoemulsion compared to its free form, which may be correlated to the synergic effect of the active substance and its emulsifier [38,39]. Meanwhile, the higher antioxidant activity of CAR-NLC compared to CAR-free can be attributed to the lipid-based drug delivery system, which acts as a secondary antioxidant with the mechanism of decreasing the oxidation rate while also scavenging the free radicals, as observed in previous studies regarding β-carotene loaded NLC and turmeric extract loaded NLC [40,41]. The synergic effect between the lipid matrix and CAR produced a higher antioxidant activity of CAR-NLC than CAR-free [42].
BSA denaturation inhibitory activity
Persistent inflammation hinders diabetes-related wounds from developing toward the proliferation and remodeling phase [4]. Prolonged inflammations are caused by the incessant presence of macrophages in large amounts at the injury site. These macrophages produce proinflammation cytokines such as tumor necrosis factor-alpha, interleukin-1β, and ROS, which lead to abnormal fibroblast and keratinocyte cell apoptosis, accompanied by a decrease in angiogenesis [3]. Therefore, the administration of anti-inflammatory agents at the chronic wound site may assist the healing process.
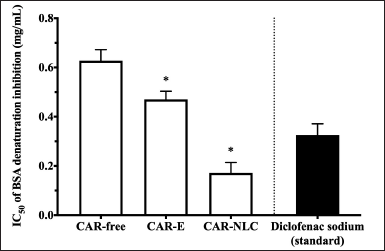
CAR has been reported to exhibit an anti-inflammatory activity before [8]. The heat-induced BSA denaturation inhibition method evaluated CAR-free, CAR-E, and CAR-NLC’s anti-inflammatory activity, considering that inflammation can be marked with protein denaturation [43]. In vivo protein denaturation stimulates the production of inflammation, stimulating autoantigens, which may cause tissue damage. Several anti-inflammatory drugs exhibited the ability to inhibit heat-induced albumin denaturation [24,44]. The comparison of IC50 value for heat-induced BSA denaturation inhibition activity of CAR-free, CAR-E, CAR-NLC, and diclofenac sodium standard is depicted in Figure 5. Stronger anti-inflammatory activity was indicated by a lower IC50 value, where the IC50 is defined as the sample concentration that inhibited BSA denaturation within the solution by as much as 50%. Diclofenac sodium was used as the nonsteroidal anti-inflammatory drug standard with an IC50 value of 0.325 ± 0.045 mg/ml. It has been summarized in Figure 5 that the IC50 value of CAR-E (0.470 ± 0.033 mg/ml) and CAR-NLC (0.171 ± 0.043 mg/ml) was significantly lower (p-value < 0.05) than that of CAR-free (0.628 ± 0.045 mg/ml). Furthermore, the IC50 value of CAR-NLC was lower than the diclofenac sodium standard, indicating the potential of CAR-NLC as an anti-inflammatory agent. From this study, it is observed that the CAR activity to inhibit albumin denaturation was affected by the formulation factor, wherein if the CAR was formulated into an emulsion or NLC form, then its activity to inhibit albumin denaturation shall increase.
Proteins will adhere to the surface of nanoparticles within a system, forming a nanoparticle-protein complex. The nanoparticle composition and its surface physicochemical characteristics will affect this protein bond. Hydrophobic and charged particles will bind faster to proteins than hydrophilic and uncharged particles. Furthermore, the smaller the PS, the larger the surface area to volume ratio; thus, the amount of protein able to bind on the particle surface will be considerable [45]. The CAR-NLC particles are composed of hydrophobic CAR and BW, exhibiting negative ZP value and small PS. Therefore, the improvement of CAR albumin denaturation inhibition activity after formulated in NLC may be attributed to the synergic effect between CAR and NLC matrix, which increased its interaction with albumin.
Antibacterial activity
Diabetes-related chronic wound healing often experiences disturbances that mainly pertain to microbial infection. The presence of bacterial biofilm leads to prolonged inflammation due to cytokine stimulation and accumulation of free radicals [3]. S. aureus and E. coli are Gram-positive and Gram-negative bacteria, respectively, most often identified in diabetes-related chronic wounds [46]. The administration of antimicrobial agents onto the infected wound site was deemed necessary for the healing process. Using natural products such as plant-derived essential oils has long been thought to be a safer treatment choice than synthetic products as antimicrobial agents [47].
 | Figure 5. Inhibition of BSA denaturation by CAR-free, CAR-E, CAR-NLC, and diclofenac sodium standard presented as IC50. Data were expressed as mean ± standard deviation (n = 3). *p-value < 0.05 was considered significant compared to CAR-free. [Click here to view] |
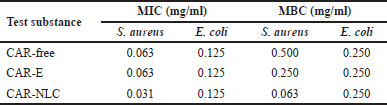
CAR has been reported to possess an antibacterial activity [8]. In this study, the antibacterial activity of CAR-free, CAR-E, and CAR-NLC was evaluated using the broth microdilution method per the CLSI recommendation. The MIC and MBC values toward the S. aureus and E. coli tested strains are depicted in Table 1. In this study, the solvent DMSO used to dissolve CAR-free has been observed to exert an inhibitory effect toward S. aureus and E. coli tested strains at the concentration of 10% (v/v). However, the MIC and MBC values of CAR-free were obtained using DMSO at a concentration below 10% (v/v), hence rendering the obtained test values to be valid. The MIC values of CAR-free toward S. aureus and E. coli tested strains were 0.063 and 0.125 mg/ml, respectively. Meanwhile, the MBC values of CAR-free toward S. aureus and E. coli tested strains were 0.500 and 0.250 mg/ml, respectively. The antimicrobial activity of CAR can be attributed to CAR’s lipophilic interaction with the phospholipid membrane component, causing a drastic structural change in its membrane altering its integrity. The distortion of the physical structure leads to expansion, destabilization, and increase of membrane fluidity, eventually increasing the microbial membrane’s passive permeability [7].
The encapsulation of CAR into an emulsion and NLC formulation may decrease the MIC and/or MBC value toward S. aureus, especially if CAR was encapsulated in an NLC system. Inversely, the encapsulation of CAR into an emulsion and NLC formulation did not affect the MIC and MBC value toward E. coli. The MIC and MBC values of CAR after encapsulation within the NLC system toward S. aureus decreased two times from 0.063 to 0.031 mg/ml, and eight times from 0.500 to 0.063 mg/ml, respectively. Similar antibacterial activity improvement was also observed in a turmeric extract-loaded NLC study. This improvement may be attributed to the fact that NLC could deliver antibacterial substances toward the cell membrane layer of bacteria. Numerous pores on the cell membrane of bacteria act as the substance transfer site. The smaller the nanoparticle size, the easier it is to enter said pores and release the encapsulated substance. NLC also acts as a delivery agent for hydrophobic antimicrobial substances, carrying it toward the bacteria in an aqueous phase [41]. Furthermore, NLC also increased the retention time of the active substance within the bacteria, thus reducing the amount of active substance necessary to give rise to a similar effect in its free form [48]. CAR release from the NLC matrix exhibited a biphasic behavior, facilitating the immediate amount to reach MIC and MBC of the active substance, followed by its prolonged release for 24 hours during the incubation time to maintain the activity of the active substance. This particular active substance release model effectively reduces the administration frequency while preventing antimicrobial resistance.
 | Table 1. Antibacterial activities of CAR-free, CAR-E, and CAR-NLC presented as MIC and MBC. [Click here to view] |
Dermal acute irritation test
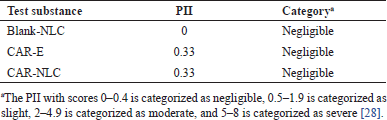
A dermal acute irritation test was performed to determine whether the Blank-NLC, CAR-E, and CAR-NLC may irritate the rabbit skin after a single administration. The preliminary test of the three tested substances did not show any corrosive effect or severe irritation. Therefore, the confirmation test was performed to determine the PII score. The PII scores of Blank-NLC, CAR-E, and CAR-NLC are shown in Table 2. According to the confirmation test, the three tested substances exhibited a PII score of near zero (<0.4), confirming that the tested substances were categorized as negligible irritants according to ISO 10993-10, 2010 [16]. Although CAR had the potential to cause dermal irritation when used in its pure form, the CAR-NLC formula used CAR in the smallest concentration possible while providing a controlled release of CAR, thus reducing the dermal irritation effect and optimizing the pharmacological effect of CAR at the appropriate dosage.
 | Table 2. The PII of Blank-NLC, CAR-E, and CAR-NLC and their category. [Click here to view] |
In vivo diabetic wound healing activity
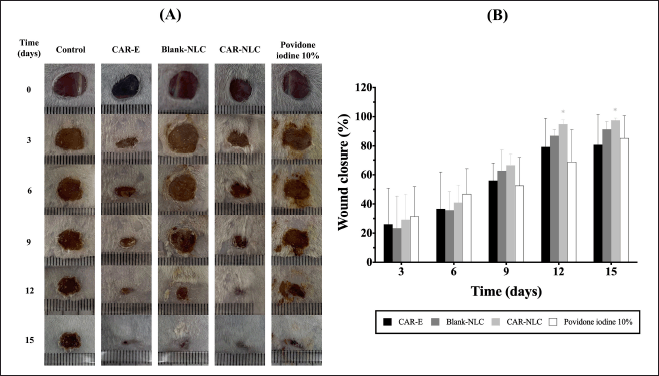
The in vivo diabetic wound healing activity of the CAR-NLC formula was evaluated using the full-thickness excision wound model in the male Swiss Webster mice, which were diabetes induced by STZ. Figure 6A and B show that the wound treated by the CAR-NLC formula showed a significantly higher (p-value < 0.05) cumulative wound closure percentage (94.97% ± 2.87%) compared to its control (80.19% ± 9.48%) at day 12 of observation. Moreover, wounds administered by the CAR-NLC formula exhibited the highest cumulative wound closure percentage (97.56% ± 1.48%) compared to the other tested substance groups (80.88% ± 20.74% for the CAR-E group, 91.36% ± 5.17% for the Blank-NLC group, and 85.34% ± 15.46% for the povidone-iodine 10% group), and significantly different (p-value < 0.05) compared to its control (80.97% ± 6.29%) at the last day of observation (day 15). The group treated by CAR-E and Blank-NLC showed an improved but insignificant (p-value > 0.05) wound healing activity compared to the control group at every observation time stamp. It can be inferred from this test that the CAR-NLC formula improved the diabetic-related wound healing activity, especially from the intermediate phase until the complete healing point.
In general, wounds will heal with or without any additional treatment. Diabetes may affect every multifactorial wound healing process, thus rendering the acute wound chronic [49]. Diabetes-related chronic wounds showed an increase in ROS amount, microbial infection, and persistent inflammation, which do not show any improvement toward the proliferation and remodeling phase [3,4]. CAR possesses antioxidant, anti-inflammatory, and antimicrobial activity [8], which can assist chronic wounds in reverting to normal wound healing. From this test, NLC was able to improve CAR’s antioxidant, anti-inflammatory, and antimicrobial activity to improve its effectiveness in chronic wound healing. NLC may also assist the wound healing process due to its occlusive attribute, protecting against microbial infection [50].
 | Figure 6. Diabetic wound healing activity of CAR-E, Blank-NLC, CAR-NLC, and povidone-iodine 10%. (A) Wound images after treatment with different formulations. (B) %wound closure calculated as the percentage area of the initial wounds. Data were expressed as mean ± standard deviation (n ≥ 5). *p-value < 0.05 was considered significant compared to their respective control. [Click here to view] |
NLC is an effective and nontoxic drug delivery system for various active substances. It has been reported before that NLC was able to protect the active substance against instability while also providing a controlled drug release. NLC is the appropriate drug delivery for topical administration, especially on open wounds. Recent research on natural product-loaded NLC for treating acute and chronic wound healing has been performed before and the results were promising [51]. According to a study done by Carbone et al. [18], NLC may lower the toxicity of natural products. Free ferulic acid at a similar concentration as the tested NLC formulation could not encourage wound closure at the in vivo test. Moreover, it exhibited a severe toxicity-related effect compared to the untreated cells. This result was associated with the ability of NLC to control the release of ferulic acid. The release of ferulic acid in its solution form will be massive because it hinders cell metabolism. According to Chen et al. [52], the lipid nanoparticle may support the wound healing process due to its ability to release a continuous stream of drugs in a certain amount, thus prolonging the contact time of drugs on the skin surface, providing good compatibility, and occlusive attribute. Sun et al. [21] have formulated protopanaxadiol within an NLC system to improve the diabetic ulcer wound. The NLC formulation of protopanaxadiol may encourage diabetic wounds to heal regularly. This may occur due to the controlled release of active substances from the NLC matrix. At initial administration, the NLC system released a large amount of active substance, thus providing the necessary concentration for the active substance to exert its desired activity at the wound site. This was then followed by a prolonged release of the active substance, thus maintaining the effective concentration necessary at the wound site.
The CAR-NLC exhibited excellent pharmacological activity in treating diabetic wounds. However, further studies are required to explicitly elucidate the cellular and molecular mechanism underlying CAR-NLC-mediated wound healing. Furthermore, it is essential to conduct clinical trials to develop these findings into pharmaceutical preparations for treating diabetic wounds in patients.
Histological analysis
At the end of the diabetic wound healing activity study (day 15), skin sections of the intact skin, wound treated with physiological sodium chloride, wound treated with CAR-E, wound treated with Blank-NLC, wound treated with CAR-NLC, and wound treated with povidone-iodine 10% were stained using H&E to be further analyzed. The microphotographs of the skin sections are shown in Figure 7.
The intact skin (Fig. 7A) has complete skin properties such as epidermis (bracket), dermal papillae (black arrow), bundles of collagen (yellow arrow), dermal appendages (blue arrow), and vacuoles/blood vessels (red arrow). Compared with the wound treated with physiological sodium chloride as control (Fig. 7B), the epidermis part was replaced by a necrotic tissue (brown arrow), while the bundles of collagen, the dermal papillae, dermal appendages, and vacuoles/blood vessels were not seen. Meanwhile, wounds treated with CAR-E (Fig. 7C), Blank-NLC (Fig. 7D), CAR-NLC (Fig. 7E), and povidone-iodine 10% (Fig. 7F) showed better recovery compared with control. They showed the development of the epidermis, dermal papillae, bundles of collagen, and vacuoles/blood vessels, whereas the dermal appendages were not developed. Excellent wound recovery was shown by the wounds treated with CAR-NLC as they showed the development of a thick epidermis, a great number of dermal papillae, large bundles of collagen, and a few vacuoles/blood vessels. Therefore, it can be inferred from the histological analysis that the CAR-NLC formula can indeed improve diabetes-related wound healing, as shown by the microscopical improvement of skin properties.
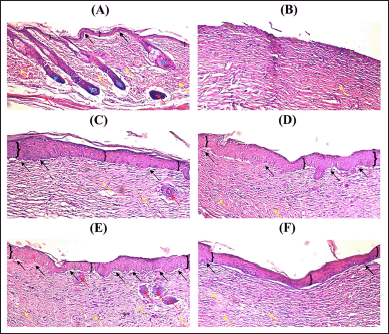 | Figure 7. Microphotographs of (A) intact skin, (B) wound treated with physiological sodium chloride as control, (C) wound treated with CAR-E, (D) wound treated with Blank-NLC, (E) wound treated with CAR-NLC, and (F) wound treated with povidone-iodine 10%, stained with H&E, taken at 15th day of treatment, at 100× magnification. Bracket: epidermis, black arrow: dermal papillae, yellow arrow: bundles of collagen, blue arrow: dermal appendages, red arrow: vacuoles/blood vessels, and brown arrow: necrotic tissue. [Click here to view] |
CONCLUSION
The CAR-NLC formula was successfully constructed by using the hot homogenization–ultrasonication method in this research. The CAR-NLC formula exhibited excellent activities according to the in vitro and in vivo tests. The antioxidant, anti-inflammation, and antibacterial activity of the CAR-NLC formula were improved from the free CAR form and/or its emulsion form according to the in vitro test. Moreover, the in vivo diabetic wound healing activity test result showed that the CAR-NLC formula was able to close the diabetic-related wound significantly better than the control. This study offers a promising alternative therapy to treat diabetes-related wounds by combining natural products with NLC. It provides valuable insights into the potential use of the NLC system compared to traditional formulations for encapsulating other natural products whose pharmacological activities are hindered by their inadequate physicochemical properties, instability, and irritant properties.
ACKNOWLEDGMENT
The authors would like to thank the Research Center of Nanoscience and Nanotechnology from Institut Teknologi Bandung for assisting with the TEM imaging and Mr. Irfan from Padjajaran University for preparing the histological slide preparations.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
The authors would like to thank the Directorate General of Higher Education, Research, and Technology, Ministry of Education, Culture, Research, and Technology, the Republic of Indonesia, for funding research through the Master’s Thesis Research grant scheme year 2022 (Grant number: 083/E5/PG.02.00.PT/2022).
CONFLICTS OF INTEREST
The authors declare no conflicts of interest in this research.
ETHICAL APPROVALS
The in vivo experiments were conducted after being approved by the Ethical Committee of Institut Teknologi Bandung (No. KEP/I/2022/IX/H220822FF/UIDN (for the dermal acute irritation test) and No. KEP/I/2022/IX/ H220822FF/UPLD (for the in vivo diabetic wound healing activity)).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Noor S, Zubair M, Ahmad J. Diabetic foot ulcer—a review on pathophysiology, classification and microbial etiology. Diabetes Metab Syndr Clin Res Rev. 2015;9(3):192–9. CrossRef
2. International Diabetes Federation. IDF diabetes atlas. 10th ed. Brussels, Belgium: IDF; 2021. Available from: http://www.diabetesatlas.org/
3. Ezhilarasu H, Vishalli D, Dheen ST, Bay BH, Kumar Srinivasan D. Nanoparticle-based therapeutic approach for diabetic wound healing. Nanomaterials. 2020;10(6):1234. CrossRef
4. Choudhury H, Pandey M, Lim YQ, Low CY, Lee CT, Marilyn TCL, et al. Silver nanoparticles: advanced and promising technology in diabetic wound therapy. Mater Sci Eng C. 2020;112:110925. CrossRef
5. Baltzis D, Eleftheriadou I, Veves A. Pathogenesis and treatment of impaired wound healing in diabetes mellitus: new insights. Adv Ther. 2014;31:817–36. CrossRef
6. Hajialyani M, Tewari D, Sobarzo-Sánchez E, Nabavi SM, Farzaei MH, Abdollahi M. Natural product-based nanomedicines for wound healing purposes: therapeutic targets and drug delivery systems. Int J Nanomed. 2018;13:5023–43. CrossRef
7. Keawchaoon L, Yoksan R. Preparation, characterization and in vitro release study of carvacrol-loaded chitosan nanoparticles. Colloids Surf B Biointerfaces. 2011;84(1):163–71. CrossRef
8. Galvão JG, Santos RL, Lira AAM, Kaminski R, Sarmento VH, Severino P, et al. Stearic acid, beeswax and carnauba wax as green raw materials for the loading of carvacrol into nanostructured lipid carriers. Appl Sci. 2020;10(18):6267. CrossRef
9. Gunal MY, Heper AO, Zaloglu N. The effects of topical carvacrol application on wound healing process in male rats. Pharmacogn J. 2014;6:10–4. CrossRef
10. Süntar I, Akkol EK, Tosun A, Keles H. Comparative pharmacological and phytochemical investigation on the wound-healing effects of the frequently used essential oils. J Essent Oil Res. 2014;26(1):41–9. CrossRef
11. De Oliveira MLM, Bezerra BMO, Leite LO, Girão VCC, Nunes-Pinheiro DCS. Topical continuous use of Lippia sidoides Cham. essential oil induces cutaneous inflammatory response, but does not delay wound healing process. J Ethnopharmacol. 2014;153(1):283–9. CrossRef
12. Süntar I, Akkol EK, Keles H, Oktem A, Baser KHC, Yesilada E. A novel wound healing ointment: a formulation of Hypericum perforatum oil and sage and oregano essential oils based on traditional Turkish knowledge. J Ethnopharmacol. 2011;134(1):89–96. CrossRef
13. Laothaweerungsawat N, Neimkhum W, Anuchapreeda S, Sirithunyalug J, Chaiyana W. Transdermal delivery enhancement of carvacrol from Origanum vulgare L. essential oil by microemulsion. Int J Pharm. 2020;579:119052. CrossRef
14. Galvão JG, Santos RL, Silva ARST, Santos JS, Costa AMB, Chandasana H, et al. Carvacrol loaded nanostructured lipid carriers as a promising parenteral formulation for leishmaniasis treatment. Eur J Pharm Sci. 2020;150:105335. CrossRef
15. Santos EH, Kamimura JA, Hill LE, Gomes CL. Characterization of carvacrol beta-cyclodextrin inclusion complexes as delivery systems for antibacterial and antioxidant applications. LWT Food Sci Technol. 2015;60(1):583–92. CrossRef
16. Vairo C, Collantes M, Quincoces G, Villullas S, Peñuelas I, Pastor M, et al. Preclinical safety of topically administered nanostructure lipid carriers (NLC) for wound healing application: biodistribution and toxicity studies. Int J Pharm. 2019;569:118484. CrossRef
17. Tamjidi F, Shahedi M, Varshosaz J, Nasirpour A. Nanostructured lipid carriers (NLC): a potential delivery system for bioactive food molecules. Innov Food Sci Emerg Technol. 2013;19:29–43. CrossRef
18. Carbone C, Caddeo C, Grimaudo MA, Manno DE, Serra A, Musumeci T. Ferulic acid-NLC with lavandula essential oil: a possible strategy for wound-healing? Nanomaterials. 2020;10(5):898. CrossRef
19. Khezri K, Farahpour MR, Mounesi Rad S. Accelerated infected wound healing by topical application of encapsulated rosemary essential oil into nanostructured lipid carriers. Artif Cells Nanomed Biotechnol. 2019;47(1):980–8. CrossRef
20. Alexander HR, Syed Alwi SS, Yazan LS, Zakarial Ansar FH, Ong YS. Migration and proliferation effects of thymoquinone-loaded nanostructured lipid carrier (TQ-NLC) and thymoquinone (TQ) on in vitro wound healing models. Evid Based Complement Altern Med. 2019;2019. CrossRef
21. Sun D, Guo SY, Yang L, Wang YR, Wei XH, Song S, et al. Silicone elastomer gel impregnated with 20(S)-protopanaxadiol-loaded nanostructured lipid carriers for ordered diabetic ulcer recovery. Acta Pharmacol Sin. 2020;41(1):119–28. CrossRef
22. Lee HJ, Jeong M, Na YG, Kim SJ, Lee HK, Cho CW. An EGF- and curcumin-co-encapsulated nanostructured lipid carrier accelerates chronic-wound healing in diabetic rats. Molecules. 2020;25(20):4610. CrossRef
23. Li B, Ge ZQ. Nanostructured lipid carriers improve skin permeation and chemical stability of idebenone. AAPS PharmSciTech. 2012;13:276–83. CrossRef
24. Putri TN, Bachtiar A, Hayun DH. Synthesis, antioxidant, and anti-inflammatory activity of morpholine mannich base of AMACs ((2E, 6E)-2-((4-hydroxy-3-[morpholin-4-yl-)methyl]phenyl)methylidene)-6-(phenylmethylidene) cyclohexan-1-one) and its analogs. J Appl Pharm Sci. 2018;8(5):19–25. CrossRef
25. Clinical Laboratory Standards Institute (CLSI). Broth microdilution method in: methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 10th ed. Wayne, PA: CLSI Standard M07; 2015.
26. He J, Huang S, Sun X, Han L, Chang C, Zhang W, et al. Carvacrol loaded solid lipid nanoparticles of propylene glycol monopalmitate and glyceryl monostearate: preparation, characterization, and synergistic antimicrobial activity. Nanomaterials. 2019;9(8):1162. CrossRef
27. Indonesian FDA. Regulation of Indonesian FDA number 10 of 2022 about guideline of in vivo preclinical toxicity study. Jakarta, Indonesia: Indonesian FDA; 2022. pp 1–220.
28. International Organization for Standardization (ISO). ISO 10993-10 tests for irritation and skin sensitization. Geneva, Switzerland: ISO 10993-10; 2007. pp 1–11.
29. Shanmugapriya K, Kim H, Kang HW. A new alternative insight of nanoemulsion conjugated with κ-carrageenan for wound healing study in diabetic mice: in vitro and in vivo evaluation. Eur J Pharm Sci. 2019;133:236–50. CrossRef
30. Wu KK, Huan Y. Streptozotocin-induced diabetic models in mice and rats. Curr Protoc Pharmacol. 2008;40(1):5–47. CrossRef
31. Saporito F, Sandri G, Bonferoni MC, Rossi S, Boselli C, Cornaglia AI, et al. Essential oil-loaded lipid nanoparticles for wound healing. Int J Nanomed. 2018;13:175–86. CrossRef
32. Kraisit P, Sarisuta N. Development of triamcinolone acetonide-loaded nanostructured lipid carriers (NLCs) for buccal drug delivery using the Box-Behnken design. Molecules. 2018;23(4):982. CrossRef
33. Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:131–55. CrossRef
34. Pivetta TP, Simões S, Araújo MM, Carvalho T, Arruda C, Marcato PD. Development of nanoparticles from natural lipids for topical delivery of thymol: investigation of its anti-inflammatory properties. Colloids Surf B Biointerfaces. 2018;164:281–90. CrossRef
35. Carpena M, Nuñez-Estevez B, Soria-Lopez A, Garcia-Oliveira P, Prieto MA. Essential oils and their application on active packaging systems: a review. Resources. 2021;10(1):7. CrossRef
36. Soni K, Rizwanullah M, Kohli K. Development and optimization of sulforaphane-loaded nanostructured lipid carriers by the Box-Behnken design for improved oral efficacy against cancer: in vitro, ex vivo and in vivo assessments. Artif Cells Nanomed Biotechnol. 2018;46(sup1):15–31. CrossRef
37. Shu X, Zhang L, Liao W, Liu J, Mao L, Yuan F, et al. Nanostructured lipid carriers (NLCs) stabilized by natural or synthetic emulsifiers for lutein delivery: improved physicochemical stability, antioxidant activity, and bioaccessibility. Food Chem. 2023;403:134465. CrossRef
38. Jalali-Jivan M, Abbasi S. Novel approach for lutein extraction: food grade microemulsion containing soy lecithin & sunflower oil. Innov Food Sci Emerg Technol. 2020;66:102505. CrossRef
39. Liu T, Gao Z, Zhong W, Fu F, Li G, Guo J, et al. Preparation, characterization, and antioxidant activity of nanoemulsions incorporating lemon essential oil. Antioxidants. 2022;11(4):650. CrossRef
40. Rohmah M, Rahmadi A, Raharjo S. Bioaccessibility and antioxidant activity of β-carotene loaded nanostructured lipid carrier (NLC) from binary mixtures of palm stearin and palm olein. Heliyon. 2022;8(2):08913. CrossRef
41. Karimi N, Ghanbarzadeh B, Hamishehkar H, Mehramuz B, Kafil HS. Antioxidant, antimicrobial and physicochemical properties of turmeric extract-loaded nanostructured lipid carrier (NLC). Colloids Interface Sci Commun. 2018;22:18–24. CrossRef
42. Keivani Nahr F, Ghanbarzadeh B, Samadi Kafil H, Hamishehkar H, Hoseini M. The colloidal and release properties of cardamom oil encapsulated nanostructured lipid carrier. J Dispers Sci Technol. 2020;42(1):1–9. CrossRef
43. Osman NI, Sidik NJ, Awal A, Adam NAM, Rezali NI. In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L. and total phenolic content analysis for potential anti-inflammatory use in gouty arthritis. J Intercult Ethnopharmacol. 2016;5(4):343–9. CrossRef
44. Kedi PBE, Meva FE, Kotsedi L, Nguemfo EL, Zangueu CB, Ntoumba AA, et al. Eco-friendly synthesis, characterization, in vitro and in vivo anti-inflammatory activity of silver nanoparticle-mediated Selaginella myosurus aqueous extract. Int J Nanomed. 2018;13:8537–48. CrossRef
45. Aggarwal P, Hall JB, McLeland CB, Dobrovolskaia MA, McNeil SE. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv Drug Deliv Rev. 2009;61(6):428–37. CrossRef
46. Macdonald KE, Boeckh S, Stacey HJ, Jones JD. The microbiology of diabetic foot infections: a meta-analysis. BMC Infect Dis. 2021;21(1):1–10. CrossRef
47. Sugumar S, Ghosh V, Nirmala MJ, Mukherjee A, Chandrasekaran N. Ultrasonic emulsification of eucalyptus oil nanoemulsion: antibacterial activity against Staphylococcus aureus and wound healing activity in Wistar rats. Ultrason Sonochem. 2014;21(3):1044–9. CrossRef
48. Piran P, Kafil HS, Ghanbarzadeh S, Safdari R, Hamishehkar H. Formulation of menthol-loaded nanostructured lipid carriers to enhance its antimicrobial activity for food preservation. Adv Pharm Bull. 2017;7(2):261–8. CrossRef
49. Bernal-Chávez S, Nava-Arzaluz MG, Quiroz-Segoviano RIY, Ganem-Rondero A. Nanocarrier-based systems for wound healing. Drug Dev Ind Pharm. 2019;45(9):1389–402. CrossRef
50. Naderi N, Karponis D, Mosahebi A, Seifalian AM. Nanoparticles in wound healing; from hope to promise, from promise to routine. Front Biosci Landmark. 2018;23(6):1038–59. CrossRef
51. Fauzian F, Garmana AN, Mauludin R. Applications of nanotechnology-based drug delivery system for delivering natural products into acute and chronic wounds: a review. Biointerface Res Appl Chem. 2023;13:1–19. CrossRef
52. Chen X, Peng LH, Shan YH, Li N, Wei W, Yu L, et al. Astragaloside IV-loaded nanoparticle-enriched hydrogel induces wound healing and anti-scar activity through topical delivery. Int J Pharm. 2013;447(1–2):171–81. CrossRef