INTRODUCTION
Ceritinib, a second-generation Anaplastic Lymphoma Kinase (ALK) inhibitor, has the potential to treat ALK-positive metastatic non-small cell lung cancer [1]. Ceritinib is classed as a Class IV medication by the biopharmaceutical classification system (BCS), which implies it has low solubility and permeability, leading in limited oral bioavailability. Its aqueous solubility is pH-dependent; it is well soluble at low pH levels (11 mg/ml at pH 1) but poorly soluble at neutral pH levels (0,0002 mg/ml at 6.8 pH) [2]. Ceritinib functions as an apical efflux transporter and glycoprotein substrate. Ceritinib’s highest concentrations were found 4–6 hours after oral dosing. Oral absorption was estimated to be ≥25% based on metabolite percentages in the faeces.
However, ceritinib’s categorization as a Class IV drug in the [BCS and the associated challenges pertaining to its bioavailability, mainly attributed to its limited aqueous solubility, introduce complexities in its clinical application [3]. While Ceritinib exhibits exceptional efficacy, its suboptimal solubility in aqueous solutions can result in unpredictable absorption within the gastrointestinal tract, potentially affecting the drug’s overall therapeutic performance.
In response to these solubility challenges, ongoing research endeavors and pharmaceutical innovations ex; solid dispersions, lipid formulations, nanoparticles, and nanostructured lipid carriers (NSLCs), have explored various techniques and strategies to enhance ceritinib’s solubility. These strategies include using solubilizing agents, lipid formulations, nanoparticle drug delivery systems, and amorphous solid dispersions, each of which is designed to address and alleviate Ceritinib’s solubility constraints.
Bioavailability, the percentage of a given medication that enters systemic circulation, is an important component in the efficacy of pharmacological formulations [4]. Poorly water-soluble medicines, especially those designated as BCS Class IV, pose major hurdles to ensuring acceptable bioavailability [5]. These drugs often exhibit low dissolution rates and variable absorption, which can limit their therapeutic efficacy. NSLCs are emerging as a promising and novel technique for overcoming these challenges and increasing the bioavailability of poorly soluble medications [6].
NSLCs represent a specialized lipid-based drug delivery system designed to improve drug solubility and absorption [7]. They consist of a mixture of solid and liquid lipids, which are used to encapsulate drug molecules in nano-sized particles. The unique structural properties of NSLCs offer several advantages for enhancing drug bioavailability [8]. This includes increased drug loading, protection of the drug from degradation, sustained release, and improved drug solubilization in the gastrointestinal tract. Additionally, NSLCs can target specific tissues or cells, thereby enhancing drug delivery and reducing potential side effects.
The use of NSLCs has shown significant potential in addressing the bioavailability challenges associated with BCS Class IV drugs, which often include poorly soluble compounds used in the treatment of various diseases, such as cancer, cardiovascular disorders, and infectious diseases [9]. By encapsulating these drugs within NSLCs, their dissolution and absorption profiles can be optimized, leading to increased therapeutic effectiveness. Furthermore, the controlled release properties of NSLCs can help minimize fluctuations in plasma drug levels, reducing the need for frequent dosing and improving patient compliance.
NSLCs enhance drug solubility by dispersing drugs in lipid matrices, overcoming solubility challenges of hydrophobic compounds. Their nanoscale structure provides a large surface area for drug incorporation, improving dissolution rates and bioavailability. NLCs offer a versatile platform for formulating poorly soluble drugs, addressing a critical limitation in drug development and enhancing therapeutic efficacy.
MATERIALS AND METHODS
Ceritinib was obtained as a gift sample from MSN Laboratories Pvt.Ltd. Hyderabad. Glyceryl monostearate, egg lecithin, poloxamer 188, and capmul MCM were purchased from S.D. Chemicals Hyderabad. All chemicals and solvents used in the study are analytical grade and are used exactly as received.
QbD approach for optimization—using central composite design
The features of Ceritinib Nanostructured Lipid Carriers (C-NSLCs) were investigated by utilizing the Design Expert software (Version 13.0) with the central composite design of the response surface technique. As shown in Table 1, Three independent elements were considered: the concentration of capmul (0.1% w/w–0.15% w/w) (factor A), the concentration of egg lecithin (0.15%w/w–0.25% w/w) (factor B), and the concentration of poloxamer 188 (0.2%w/w–0.37%w/w) (factor C), whereas particle size (response 1) and entrapment efficiency (response 2) were regarded as dependent factors. The target responses were chosen to be minimal particle size and maximum entrapment efficiency. The optimized formula was then created utilizing the optimal independent variables. As given in Table 2, the central composite design resulted in 15 distinct formulations. The particle size and entrapment efficiency measurements were carried out three times to get the precise values. The concentration ranges of Capmul, egg lecithin, and Poloxamer 188 were selected based on preliminary studies indicating their critical impact on the stability and performance of NSLCs. These specific ranges ensure an optimal balance between surfactant properties and lipid matrix integrity, aiming to achieve minimal particle size and maximum entrapment efficiency. Additionally, the chosen ranges align with reported effective concentrations in existing literature, facilitating reliable and reproducible formulation outcomes.
Method of preparation of C-NSLCs
C-NSLCs were prepared by a modified homogenization method followed by probe sonication. Egg lecithin was dissolved in a 1:1 ratio of methanol and chloroform which was vapourised for 20 minutes to completely evaporate the solvent and resulted in the formation of egg lecithin film. Glyceryl monostearate and capmul were added to the above mixture. Then the drug was added to this mixture. The poloxamer solution was heated to the same temperature and added to that. The mixture was homogenized at 2,000 rpm for 10 minutes with continuous heating at 60°C to obtain a coarse emulsion. Maintaining at the same temperature ensures the stability of the product. Then this mixture was subjected to probe sonication for 15 minutes at 50°C. Then, the mixture was cooled to room temperature to precipitate lipids into solid form [10].
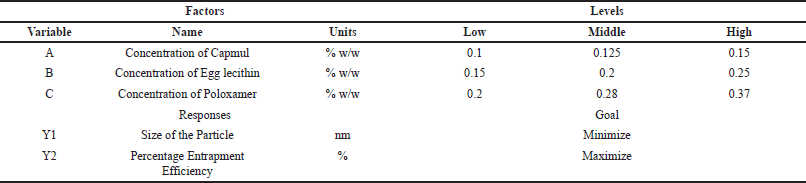 | Table 1. List of independent and dependent variables in central composite design (CCD). [Click here to view] |
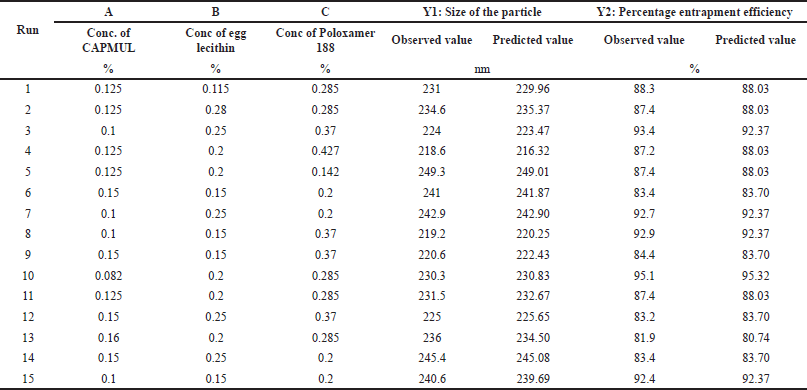 | Table 2. DoE and measured responses for optimization of the design. [Click here to view] |
Size of the Particle, PDI & Zeta-potential
The formulations’ particle size and PDI were determined using dynamic light scattering on a Zetasizer (Malvern Panalytical Ltd., Malvern, UK) Nano ZS after suitable dilution (1:100) with distilled water. The technology detected fluctuations in the intensity of light (Fluctuations refer to the variations in light intensity caused by the Brownian motion of lipid nanoparticles, which are used to calculate particle size and distribution) [11]. The zeta potential of the optimized C-NSLCs was evaluated using a zeta sizer. The particle size and zeta potential were determined by diluting the sample [12].
Entrapment efficiency
Entrapment efficiency was measured by acidifying the formulation with 0.1 N HCl (as Ceritinib shows maximum solubility in acidic pH) to cause aggregation and separation of lipid nanoparticles, which were subsequently centrifuged at 10,000 rpm for 30 minutes with a high-speed centrifuge. The supernatant was removed, and the pellets were resuspended in 10 ml of methanol before being analyzed at 320 nm using a UV spectrophotometer [13].
Drug excipient compatibility studies
FTIR is a useful technique to check and confirm any interaction that may occur between excipients and drugs. The FTIR spectrum of Ceritinib and other excipients used were recorded by FTIR (FTIR-4,800, Shimadzu). Briefly, 1 mg of solid sample along with 100 mg of KBr was compressed into a disc. For the liquid sample, sample drops were dripped onto NaCl or KBr aperture plate and sandwiched it under other aperture plates by forming a thin liquid membrane. Then, the sample was scanned for absorbance between 4,000 and 400 cm–1. The obtained spectra were matched with each other [14].
Thermal analysis data were recorded using a differential Scanning Colorimeter (DSC 204 Fl.Phoenix) for the pure drug sample and all the excipients used in the formulation [15].
Scanning electron microscopy
The surface morphology of optimized C-NSLCs was studied using SEM (Model JSM-7,800F, JEOL Ltd., Tokyo, Japan). The sample was dusted on double-sided tape onto an aluminum stub coated with gold by using a cold sputter coater in an SEM chamber of thickness 400Ao The graphs were recorded with a voltage of 15Kv electron beam [16].
In-vitro drug release studies
The dialysis membrane approach was used to conduct in-vitro drug release experiments on both the optimized formulation and the marketed medication. The dialysis membrane, which has a molecular weight of 12,000–14,000, was soaked in water overnight with 0.01M HCl as the dissolution medium. The donor compartment holds 150 mg of the optimized formulation, while the receptor compartment has 100 ml of release media at 37°C ± 0.5°C. Approximately 1 ml of the sample solution was collected from the receptor compartment at intervals of 1–12 hours, diluted with dissolving buffer, and analyzed using a UV spectrophotometer at 320 nm [17].
Evaluation of drug release kinetics
To establish the mechanism and manner of drug release, the dissolution data from the optimized formulation was fitted into several kinetic equations such as zero order, first order, Korsemeyer-Peppas, and Higuchi. The release data from the NSLCs were determined by the curve fitting method. Curve fitting involves selecting a mathematical model (e.g., zero-order, first-order) that best describes drug release behavior. Model parameters are adjusted using nonlinear regression to minimize the difference between experimental data and model predictions. Goodness-of-fit metrics like R-squared and RMSE assess the accuracy of the fitted curve. Visualization of the fitted curve alongside experimental data validates the model’s suitability. Interpreting fitted parameters provides insights into the release mechanism and kinetics, aiding formulation optimization for controlled drug delivery systems.
Study to determine the stability
NSLCs of Ceritinib were prepared to increase the bioavailability of the drug. Particle size (that determines the solubility) and entrapment efficiency (that determines the drug available) are important to be maintained throughout the shelf life of the product. Hence, optimized C-NSLCs were tested for stability (Model ICH-256, Memmert GmbH + Co. KG, Schwabach, Germany) at three different temperatures as per ICH guidelines, 25°C ± 2°C/ 60%±5% RH, ambient temperature and 40°C ±2oC/ 75%±5% RH. Change in the particle size and entrapment efficiency were monitored for 6 months [18].
Pharmacokinetic studies
The study protocol was approved by Institutional Animal Ethic Committee of St. Pauls College of Pharmacy, Hyderabad, India (Approval no.: SPCP/2021-22/CEU/01.18). 18 male Wistar rats were selected. Animals were fasted over night before conducting the study and access to the water was given. For the pharmacokinetic study rats were assigned randomly into three groups (n = 6) as shown in Table 7.
C-Suspension and C-NSLCs were administered to Group II and Group III, respectively (46.5 mg/kg). Blood samples were collected at pre determined time intervals upto 24 hours into 200 µl solution of 4% sodium citrate from retro orbital plexus. Centrifugation was done to separate the plasma at 5,000 rpm and 4oC for 10 minutes. 400 µl of methanol was added to the plasma, vortexed for 2 minutes, and centrifuged for 20 minutes at 800 rpm. Quantification of the drug was done using the HPLC method. The amount of drug present in each sample was calculated using a plasma concentration-time profile [19].
RESULTS And DISCUSSION
Optimization of the study design
The response surface methodology—central composite design can investigate several variables at distinct levels in only a few number of experimental runs. The effect of independent variables on dependent variables is shown in table-the two dependent variables particle size (Y1) and percentage entrapment efficiency (Y2) ranged from 218.6 nm–249.3 nm and 81.9%–93.4%, respectively. The observed as well as predicted values are given in the Table 2. A mathematical relationship between different variables for formulation of C-NSLCs was established using Design Expert software version 13 (Stat ease., Inc., USA).
In polynomial equation, the positive sign depicts an effect that supports the optimization and the negative sign depicts an adverse relationship between variables. The higher values of the correlation coefficients for dependent variables (Particle size and entrapment efficiency) showed a good fit.
These equations illustrate the measurable effects of independent factors on dependent variables. One factor and higher coefficients reflect interaction terms and linear relationships. (Table 3). ANOVA revealed statistically significant results (p ≤ 0.0001) for both polynomial equations.
Effect on size of the particle(Y1)
The effect of particle size can be represented by the following regression equation:
Y1= 232.67 + 1.09A + 1.61B – 9.72C
Y- Particle Size, A- Concentration of Capmul, B- Concentration of egg lecithin, and C- Concentration of Poloxamer.
The 3D response surface plots and contour plots illustrated the link between dependent and independent variables. Poloxamer 188 reduces particle size by stabilizing the nanoparticle surface, preventing aggregation, while egg lecithin increases particle size due to its larger molecular structure forming a thicker lipid layer. Capmul’s negligible effect suggests its concentration range does not significantly influence the particle size within the studied parameters as shown in Figure 1.
Effect on percentage entrapment efficiency (Y2)
The effect of entrapment efficiency can be represented by the following regression equation:
Y2 = 88.03 – 4.33A – 0.1401B + 0.1218C
Y- Entrapment efficiency, A- Concentration of Capmul, B- Concentration of egg lecithin, and C- Concentration of Poloxamer.
As illustrated in Figure 2, Factors A and B had negative impact on %EE whereas Factor C have positive impact. Ceritinib is lipid soluble which gets entrapped in the lipid mixture and show good %EE. Further Capmul in the lipid mixture increases drug dissolution and also helps in preventing separation and crystallisation of Ceritinib from lipid blend. The %EE of NSLCs reduced greatly with an increment in liquid concentration. This may be due to higher concentrations of Capmul and egg lecithin likely interfere with Ceritinib entrapment by increasing drug solubilization in the lipid matrix and promoting lipid precipitation, respectively. Poloxamer 188 enhances %EE by stabilizing the lipid matrix and preventing drug crystallization and separation.
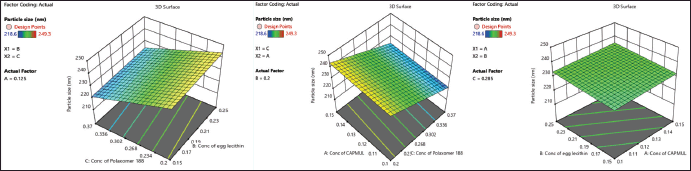 | Figure 1. 3D plots showcasing the effect of concentration of Capmul, egg lecithin, and Poloxamer on particle size of NSLCs. [Click here to view] |
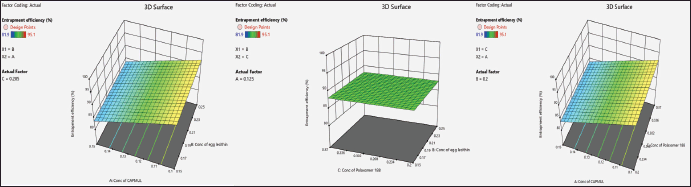 | Figure 2. 3D plots showcasing the effect of concentration of Capmul, egg lecithin, and poloxamer on percentage entrapment efficiency of NSLCs. [Click here to view] |
Particle size, poly dispersity index (PDI), and zeta potential (ZP)
The Z average size and PDI of C-NSLCs were obtained at 214.23 ± 3.15 and 0.15 ± 0.04, respectively (Fig. 3). The zeta potential was obtained at 0.79 ± 0.7 mv (Fig. 4). Smaller particle sizes indicate better miscibility and longer systemic circular time (Fig. 4). As a result of this the drug can reside for longer time in the body and it can be absorbed better. The low PDI score indicates the homogeneity of nanoparticle dispersion. The zeta potential reflects the stability of the nanoformulation. The positive ZP value indicates a longer residence period and less absorption by the reticuloendothelial system in the blood circulation system. All the values of particle size, PDI and ZP indicates the thermodynamic stability of the prepared formulation. The low PDI is due to Poloxamer 188 and Capmul, which enhance nanoparticle stability and uniformity, preventing aggregation. The positive ZP results from egg lecithin, providing electrostatic stabilization and reducing particle aggregation. This positive charge also minimizes reticuloendothelial system uptake, ensuring longer systemic circulation. These factors together contribute to the thermodynamic stability and efficient performance of the C-NSLCs formulation.
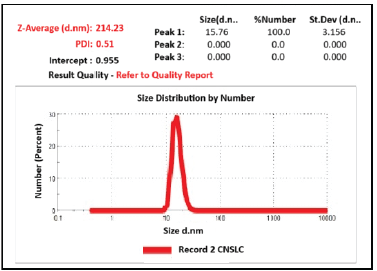 | Figure 3. Particle size and PDI of optimized C-NSLCs. [Click here to view] |
Entrapment efficiency
The average entrapment efficiency was found to be 92.63% which was in the range between 81.9% and 95.1%. In contrast to hydrophilic drugs, lipophilic drugs are intrinsically more soluble in lipids. As a result of their lipophilic properties, they get increasingly entrapped inside the lipids. Ceritinib being the lipid-soluble drug had shown better entrapment in the lipids taken.
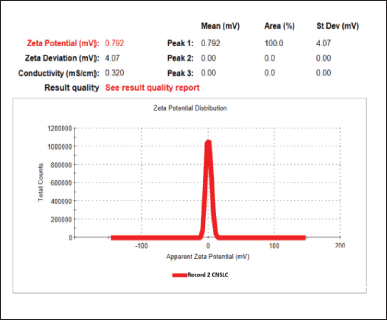 | Figure 4. Zeta Potential of optimized C-NSLCs. [Click here to view] |
FTIR analysis
To determine drug-excipient compatibility, FTIR analysis was used. Drug purity was confirmed by the presence of major peaks at 3,400.31cm–1, 1,500.90 cm–1, 1,138 cm–1, 778.36 cm–1, 2,936.83 cm–1, 2,808.36 cm–1, and 1,532.27 cm–1 in FTIR analysis of pure Ceritinib (Peak values comply with that of the values of the given functional groups). Peaks at the same wave numbers were also identified in the spectra of the physical combination of Ceritinib, glyceryl monostearate, egg lecithin, poloxamer 188, and capmul, as illustrated in Figure 5a and b.
DSC
Figure 6 shows Ceritinib’s temperature profile as measured by DSC. The melting point of the drug is marked by a prominent endothermic peak at 179oC in the thermogram of pure drug. The two endothermic peaks at 65oC (Capmul & Glyceryl Monostearate) and 176oC in the thermogram of a physical combination, are lipid and drug peaks.
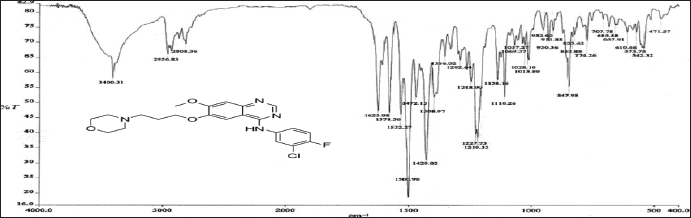 | Figure 5a. FTIR spectrum of pure Ceritinib drug. [Click here to view] |
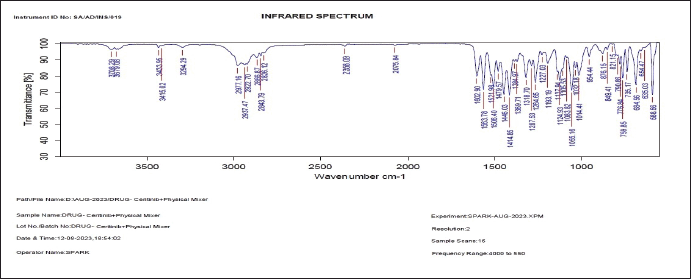 | Figure 5b. FTIR spectrum of pure Ceritinib and physical mixture of the excipients used. [Click here to view] |
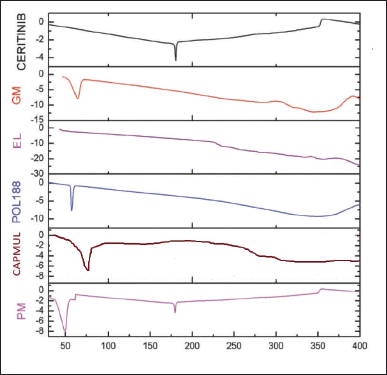 | Figure 6. Differential scanning thermograms of the pure drug and the other excipients used in the formulation of C-NSLCs. [Click here to view] |
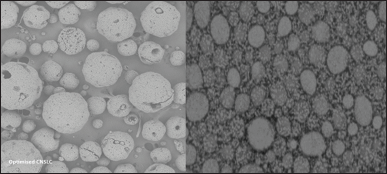 | Figure 7. SEM pictures of optimized formulation of Ceritinib Nano structured lipid carriers. [Click here to view] |
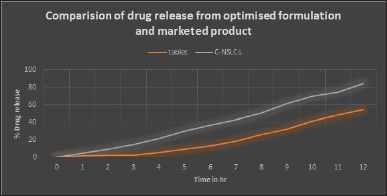 | Figure 8. Comparision of drug release from optimised C-NSLCs and marketed tablet. [Click here to view] |
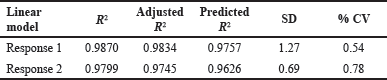 | Table 3. Results of experimental design. [Click here to view] |
 | Table 4. Ceritinib release mechanism from optimized NSLCs. [Click here to view] |
SEM analysis
SEM measurements showed a spherical shape with a homogeneous and somewhat narrow particle size range (Fig. 7). Spherical particles might be readily absorbed as compared to deformed ones thus help in better bioavailability than the non-spherical particles. Some particles deviated from spherical shape may be due to the lipid transformation during drying process.
Drug release studies
Figure 8 illustrates the release of Ceritinib from optimized C-NSLCs and the marketed tablet (Zykadia). The drug release from NSLCs in the given acidic dissolution medium at optimum temperature was found to be 84.6% at the twelvth hour and that from marketed product was found to be 54.2% as shown in Figure 8. The enhanced drug release of Ceritinib from the optimized C-NSLCs, reaching 84.6% at the twelvth hour, compared to 54.2% from the marketed tablet, can be attributed to several formulation factors. The incorporation of Capmul and Poloxamer 188 improves drug solubility and stability within the lipid matrix, enhancing the dissolution rate. The small particle size and low PDI achieved through optimized lipid and surfactant concentrations increase the surface area for drug release. The positive zeta potential aids in prolonged systemic circulation, reducing premature clearance. Overall, these factors collectively enhance the bioavailability and sustained release profile of Ceritinib in the NSLC formulation [20].
Drug release kinetics
The drug release mechanism and order were discovered by fitting the in vitro release data of optimized NSLCs to several kinetic equations. The first order plot showed rapid initial drug release, with an R2 that was similar to linear. The zero order graph clearly showed diminished linearity. The use of mathematical models such as Korsemeyer peppas and Higuchi plots revealed non-fickian diffusion (n = 0.864 in Korsemeyer peppas) with drug release via erosion and linked diffusion (Table 4).
Non-Fickian diffusion in NSLCs improves drug release kinetics and control, resulting in customised release profiles and enhanced drug loading capacity, which are critical for optimising therapeutic effects in pharmaceutical applications.
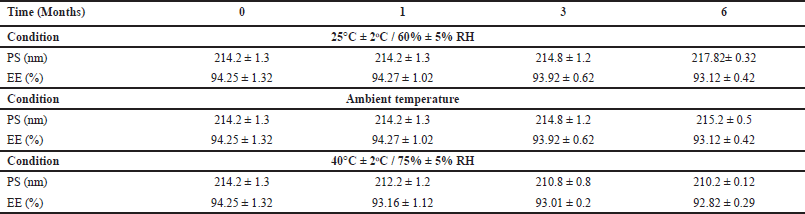 | Table 5. Stability studies. [Click here to view] |
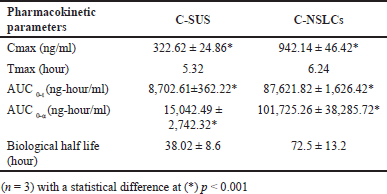 | Table 6. Pharmacokinetic parameters of Ceritinib Suspension (C-SUS) and C-NSLCs. [Click here to view] |
 | Table 7. Grouping of animals for the pharmacokinetic study. [Click here to view] |
Stability studies
Table 5 shows that after 6 months of stability testing under three settings, there was no significant variation in particle size or entrapment efficiency (p < 0.005). There is not much difference in the values of particle size and entrapment efficiency during the study period.
Pharmacokinetic studies
Plasma concentration-time profile and various pharmacokinetic parameters viz. Cmax (maximum concentration of the drug in the blood stream after administration), Tmax (time taken for the drug to reach maximum concentration), AUC0-t (Area under the curve from time zero to measurable point t), AUCt-α (Area under the curve from time zero to measurable point extrapolated to infinity), biological half life were estimated using optimized C-NSLCs and C-Sus after an oral administration of dose equivalent to 46.5 mg/kg has been presented in Table 6.
The Cmax of C-NSLCs was significantly higher than that of C-Sus. The other pharmacokinetic parameters were also found to be significantly more (p < 0.05) for optimized C-NSLCs than C-SUS. This may be due to the drug incorporated in the lipids and formulated in nano size there may be improvement in both solubility and permeability of the drug showing better bioavailability than the Ceritinib tablet. As the bioavailability is increased the dose of the drug given can be decreased, i.e., the administered dose reaches the systemic circulation in its active form, it may be possible to achieve the desired therapeutic effect with a lower dose. This phenomenon is primarily due to the fact that a higher proportion of the administered dose is effectively utilized by the body, reducing the need for a larger dose to achieve the same pharmacological effect.
CONCLUSION
The current study revealed the utilization of NSLCs to increase the oral bioavailability of Ceritinib, a BCS class IV anticancer medication. The drug excipient compatibility experiments were conducted using FTIR and DSC, and the findings revealed that there was no incompatibility between the pure drug and the excipients employed. The concentrations of Capmul (Factor A), egg lecithin (Factor B), and Poloxamer (Factor C) are the independent variables, whereas particle size (Y1) and entrapment efficiency (Y2) are the dependent factors. The NSLCs were generated using a modified homogenisation process followed by probe sonication and tested for the smallest particle size with the highest entrapment effectiveness. The observed response levels were compared to their predicted values. SEM investigations on the optimised batches revealed a spherical shape. The particle size, polydispersity index, and zeta potential were all significant, and the optimised formulation remained stable at three different temperatures for 6 months. In vivo studies on male Wistar rats revealed a considerable increase in C-NSLC bioavailability. Enhancing bioavailability through NSLCs offers promising avenues for optimizing drug therapy, allowing for reduced doses while maintaining therapeutic efficacy, thereby potentially minimizing adverse effects and improving patient compliance in pharmaceutical applications. This approach capitalizes on NLCs’ ability to enhance drug solubility, permeability, and stability, leading to improved absorption and bioavailability.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by Institutional Animal Ethic Committee of St. Pauls College of Pharmacy, Hyderabad, India (Approval no.: SPCP/2021-22/CEU/01).
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Chennuru R, Koya RT, Kommavarapu P, Narasayya SV, Muthudoss P, Vishweshwar P, et al. In situ metastable form: a route for the generation of hydrate and anhydrous forms of ceritinib. Cryst Growth Des .2017;17(12):6341–52. CrossRef
2. Awasthi A, Dheeraj HM, Birangal S, Pai A, Pai G, Sathyanarayana MB. Fabrication of ceritinib cocrystals with improved solubility: preparation, solid-state characterization, solubility studies, and molecular docking studies. Rasayan J Chem. 2022;14(02):905–13.
3. Fda.gov. Available from: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ceritinib-advanced-anaplastic-lymphoma-kinase-positive-lung-cancer.
4. Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:S131–55. CrossRef
5. Date AA, Nagarsenker MS. Design and evaluation of self-nanoemulsifying drug delivery systems (SNEDDS) for cefpodoxime proxetil. Int J Pharm. 2007;329(1–2):166–72. CrossRef
6. Pouton CW. Lipid formulations for oral administration of drugs: non-emulsifying, self-emulsifying and ‘self-microemulsifying’ drug delivery systems. Eur J Pharm Sci. 2000;11:S93–8. CrossRef
7. Attama AA, Müller-Goymann CC. Freeze-drying of drug-free and drug-loaded solid lipid nanoparticles (SLN). Int J Pharm. 2008;355(1–2):249–56.
8. Hu FQ, Yuan H. Zhang Y. A novel chitosan oligosaccharide-stearic acid micelles for gene delivery: properties and in vitro transfection studies. Int J Pharm. 2010;395(1–2):248–56.
9. Souto EB, Müller RH. Lipid nanoparticles: effect on bioavailability and pharmacokinetic changes. Handb Exp Pharmacol. 2010;(197):115–41. CrossRef
10. Lohan S, Sharma S, Murthy RR. Formulation and evaluation of solid lipid nanoparticles of quetiapine fumarate and quetiapine hemifumarate for brain delivery in rat model. Pharm Nanotechnol. 2013;1:239–47.
11. Rajendra C, Harish P, Jain AK, Himesh HH, Saraogi GK. Preparation and evaluation of the self emulsifying drug delivery system containing atorvastatin HMG-COA inhibiter. Int J Pharm Pharm Sci. 2011;3:147–52.
12. Nekkanti V, Wang Z, Betageri GV. Pharmacokinetic evaluation of improved oral bioavailability of valsartan: proliposomes versus self-nanoemulsifying drug delivery system. AAPS PharmSciTech. 2016;17(4):851–62. CrossRef
13. Ke Z, Hou X, Jia XB. Design and optimization of selfnanoemulsifying drug delivery systems for improved bioavailability of cyclovirobuxine D. Drug Des Devel Ther. 2016;10:2049–60.
14. Potu AR, Pujari N, Burra S, Veerareddy PR. Formulation and evaluation of buccoadhesive quetiapine fumarate tablets. Braz J Pharm Sci. 2012;48(2):335–45. CrossRef
15. Fang CL, Al-Suwayeh SA, Fang JY. Nanostructured lipid carriers (NLCs) for drug delivery and targeting. Recent Pat Nanotechnol. 2013;7(1):41–55. CrossRef
16. Shah P, Desai M, Shah D, Sarolia J, Gandhi J. Formulation and evaluation of ezetimibe loaded solid lipid nanoparticles. Indo Am J Pharm Res. 2017;7:661–73.
17. Mishra B, Mittal P, Vardhan H, Ajmal G, Bonde GV, Kapoor R, et al. Genistein-loaded nanostructured lipid carriers for intravenous administration: a quality by design-based approach. Int Res J Pharm. 2019;10(1):119–34.
18. Gadad AP, Tigadi SG, Dandagi PM, Mastiholimath VS, Bolmal UB. Rosuvastatin loaded nanostructured lipid carrier: for enhancement of oral bioavailability. Ind J Pharm Educ. 2016;50(4):605–11. CrossRef
19. Shah B, Khunt D, Misra M, Padh H. Non-invasive intranasal delivery of quetiapine fumarate loaded microemulsion for brain targeting: formulation, physicochemical and pharmacokinetic consideration. Eur J Pharm Sci. 2016;91:196–207. CrossRef
20. Müller RH, Keck CM. Challenges and solutions for the delivery of biotech drugs—a review of drug nanocrystal technology and lipid nanoparticles. J Biotechnol. 2004;113(1–3):151–70.