INTRODUCTION
Oryza sativa L., or rice, originated in Asia and is one of the human staple foods (Apak et al., 2016; Ito and Lacerda, 2019). In Thailand, numerous varieties of rice can be classified according to color into two groups, white and colored rice. The colors of colored rice, e.g., red, purple, and black, come from pigments in the pericarp of the bran layer of the rice grain. Among red rice, the Sangyod and Red Hom Mali varieties are popular with the domestic market demand in Thailand of approximately 10,000 tons/year for each type. Black nonglutinous rice, or Riceberry rice, has the highest domestic demand, which is approximately 20,000 tons/year. On the other hand, black glutinous rice, consumed as a dessert, has a small market of 5,000-6,000 tons/year, more or less (Orachos, 2020). Nowadays, the popularity of black rice, both glutinous and nonglutinous, is growing because of its health benefits. Black rice contains a high amount of nutrients, including carbohydrates, proteins, and lipids. There are approximately 75% of carbohydrates, mainly starch, and other polysaccharides, including simple sugars and insoluble dietary fibers. Black rice also has more essential amino acids than wheat and corn. The predominant lipids in black rice are triglycerides, principally oleic, linoleic, and palmitic acids. In addition, black rice has vitamin B-complex, vitamin A, vitamin E, potassium, iron, zinc, copper, magnesium, manganese, and phosphorus (Ito and Lacerda, 2019).
In addition to nutrients, black rice contains numerous phytochemicals that have beneficial health properties, including γ-oryzanols, tocopherols, tocotrienols, phytosterols, phytic acid, flavonoids, and phenolic compounds (Goufo and Trindade, 2014; Ito and Lacerda, 2019). Flavonoids and other phenolic compounds can be found ubiquitously in many plants and grains, including rice (Jung et al., 2017). Black rice is a rich source of anthocyanins, the red to purple water-soluble flavonoids. Anthocyanin content is varied by the variety of rice, leading to the diversity of the color shade of black rice (Chen et al., 2012; Ito and Lacerda, 2019; Orachos, 2020). Many anthocyanins that have been isolated from black rice include cyanidin-3,5-diglucoside, cyanidin-3-glucoside, cyanidin-3-rutinoside, peonidin-3-glucoside, malvidin, and petunidin-3-glucoside (Chen et al., 2012; Shao et al., 2018). Furthermore, black rice is also high in phenolic acids (Ito and Lacerda, 2019; Shao et al., 2018). Flavonoids and phenolic compounds are known as excellent sources of antioxidants by acting as a radical scavenger and/or metal ion chelating agent (Tungmunnithum et al., 2018), and the antioxidant capacity parallels the total phenolic and flavonoid contents (Tai et al., 2021; Tungmunnithum et al., 2018). In 2010, Sadabpod et al. (2018) evaluated the total polyphenol content and antioxidant activity of black nonglutinous rice (Hom Nil) and black glutinous rice and found that black glutinous rice exhibited more total polyphenol content and antioxidant activity than Hom Nil rice (Sadabpod et al., 2018).
Oxidative stress is known as a cause of cell damage and eventually cell death. There are many strategies to prevent photoaging, and one of those is using external sources of antioxidants (De Jager et al., 2017; Pillai et al., 2005; Tungmunnithum et al., 2018). The natural antioxidants from plants have been employed in cosmetics because the addition of a natural extract can improve consumer perception of cosmetic products (Kumar et al., 2021). Natural ingredients are also perceived as more sustainable and more environmentally friendly (Fonseca-Santos et al., 2015). Moreover, black rice bran extract had the highest antioxidant activities, followed by red and brown rice bran extracts (Ghasemzadeh et al., 2018). To the best of our knowledge, there is no black rice extract in skin preparations currently on the market. Utilizing black rice in skin care not only allows adding the uniqueness of the raw material to the product but also allows the antioxidant effects of the product to be increased, thus increasing the economic value of black rice. Therefore, we aimed to investigate the potential of the black rice extract in cosmetic use. Our first objective was to evaluate the total anthocyanin, total flavonoid, and total phenolic contents (TPCs) and antioxidant activity of the Thai black glutinous rice grain (RG) and black glutinous rice bran (RB) extracts. The second objective was to formulate skincare products from the extract that exhibits the best antioxidant activity.
MATERIALS AND METHODS
Materials
Folin-Ciocalteu’s phenol reagent, 2,2?-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), potassium persulfate (K2S2O8), potassium chloride (KCl), sodium acetate (CH3COONa), and acetone were purchased from Merck (Darmstadt, Germany). Quercetin and 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich (WA). Aluminum chloride-6-hydrate (AlCl3.6H2O), sodium acetate (CH3COONa), and ferrous sulfate-7-hydrate (FeSO4.7H2O) were purchased from KEMAUS (New South Wales, Australia). 2,2-Di(4-tert-octylphenyl)-1-picrylhydrazyl (DPPH), 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ), gallic acid, and L-ascorbic acid were purchased from TCI (Tokyo, Japan). All solvents were analytical grade. Hydrochloric and acetic acid were purchased from RCI Labscan (Bangkok, Thailand). Methanol and dimethyl sulfoxide (DMSO) were from Unitywewell Co., Ltd. (Bangkok, Thailand), and CARLO ERBA Reagents (Val-de-Reuil, France). Sodium bicarbonate was purchased from Ajax Finechem (New South Wales, Australia), and ferric chloride-6-hydrate (FeCl3.6H2O) was purchased from PanReac AppliChem (Barcelona, Spain). Polysorbate 80 and xanthan gum were obtained from P.C. Drug Center Co., Ltd. (Bangkok, Thailand). Potassium sorbate, sorbitol syrup, glyceryl monostearate, white petrolatum, PEG-40 hydrogenated castor oil, liquid paraffin, and stearyl alcohol were purchased from Chemipan (Bangkok, Thailand). Glycerin, triethanolamine, propylene glycol, and sorbitan monooleate were obtained from Chanjao Longevity Co., Ltd. (Bangkok, Thailand).
Extraction of rice extracts
RB from glutinous black rice was separated from RG by milling. RG (2 kg) was extracted with 75% of ethanol (2 l) on a shaker (N-Biotek, USA) for 24 hours (37°C and protected from light). The extractant was then filtrated. The marc, residues left after the first extraction, was repeatedly extracted. The filtrate from the repeated extraction was pooled and evaporated by a rotary evaporator (R100 Buchi, Switzerland) and was dried using a freeze-dryer (Labconco, USA) to obtain the RG crude extract. The RB crude extract was obtained by extracting 400 g of RB with 75% ethanol 400 ml using the aforementioned method used for RG extraction. Finally, the dried ethanolic crude extracts of RG and RB were weighed to calculate % the yield.
Determination of monomeric anthocyanin content
Monomeric anthocyanin was quantified using a pH differential method with a two-buffer system, which consisted of potassium chloride (KCl) buffer (pH 1.0, 0.025 M) and sodium acetate buffer (pH 4.5, 0.4 M), as described in Lee et al. (2005), and Yang et al. (2019). In brief, the rice extract was dissolved in 40% acetone, resulting in a 10% w/v sample solution. The dilution factor (DF) was determined by diluting the sample solution with a 0.025 M KCl buffer (pH 1.0) until the absorbance at 510 nm was in the range of 0.2–1.4 AU. Thereafter, the mixtures of sample solution and the buffers, KCl (pH 1) and acetate (pH 4.5), were prepared by adding the buffers with the same volume as obtained from DF to the sample and incubated for 15 minutes. The absorbance of the mixtures was measured by a UV-visible spectrophotometer (LabTech, USA) at 510 and 700 nm. The acetone was used as a blank. Anthocyanin content was calculated from the following equation and expressed as cyanidin-3-glucoside equivalent (CYE) in 100 g of dried powder extract (mg CYE/100 g dried powder extract):
Monomeric anthocyanin (mg/l) = A × MW × DF × 1,000 / ? × l, (1)
where A (absorbance) = (A510 nm-A700 nm)pH 1.0-(A510 nm-A700 nm)pH 4.5, MW (molecular weight of CYE) = 449.2 g/mol, DF = dilution factor, ? = molar extinction coefficient of CYE (26,900 l × mol−1 × cm−1), and l = pathlength in cm. The measurement of anthocyanin content was performed in triplicate.
Total phenolic contents (TPC) determination
The measurement method of TPC was carried out as described by Vongsak et al. (2020). Briefly, the gallic acid standard solution (0.52–33.33 μg/ml) and the sample stock solution (1 mg/ml) were diluted in DMSO. The sample or standard solution (20 μl) was added to a 96-well plate, to which 50 μl of 10% Folin-Ciocalteu’s reagent in distilled water and 80 μl of a 7.5% (w/v) sodium bicarbonate solution in distilled water were then added. The mixtures were stored in the dark at room temperature for 30 minutes. The absorbance was measured by a microplate reader (FLUOstar Omega, BMG Labtech, Germany) at 765 nm. The standard curve for the concentration of gallic acid was constructed and used for the calculation of gallic acid in the extract. The experiment was performed in triplicate, and the results were reported as mean ± standard deviation (SD) of mg gallic acid equivalent (GAE)/mg extract.
Total flavonoid contents (TFC) determination
The total flavonoid contents were determined as described by Charinrat et al. (2021). In brief, quercetin standard solution (1.95–125 μg/ml) and sample stock solutions of rice extracts (10 mg/ml) were diluted in DMSO. Eighty microliters of sample or quercetin standard solutions was mixed with 80 μl of a 2% (w/v) aluminum chloride solution in methanol in a 96-well plate. The mixtures were incubated for 10 minutes at room temperature. The absorbance was measured at 415 nm with the microplate reader. The results of TFC were calculated from the standard quercetin equation. The experiment was performed in triplicate and reported as mean ± SD of mg quercetin equivalent (QE)/mg extract.
Antioxidant assays
The antioxidant of the whole grain and the RB extracts was determined by the DPPH radical scavenging assay, ABTS radical scavenging assay, and ferric reducing antioxidant power (FRAP).
DPPH radical scavenging activity assay
The antioxidant determination by the DPPH radical scavenging assay was modified from a previous study (Jiangseubchatveera et al., 2021). The 0.5 mM DPPH reagent was prepared in methanol. The stock solutions of the rice extracts were prepared as a 5 mg/ml solution in DMSO and then diluted in DMSO to final concentrations in the range of 15–250 μg/ml. The 20 μl of sample solution was mixed with 150 μl of DPPH solution in a 96-well plate. The mixture was then incubated for 30 minutes in the dark at room temperature. The absorbance was measured at 520 nm using the microplate reader. The assay of each concentration of the sample was performed in triplicate, and the %scavenging activity of DPPH was calculated using the following equation:
%scavenging activity of DPPH = [(Abscontrol — Abssample)/Abscontrol] × 100 (2)
Abscontrol is the absorbance of the DPPH solution without sample and Abssample is the absorbance of the sample or standard solutions. The %scavenging activity of DPPH was reported as IC50 ± SD, and IC50 of the samples were compared with those of standards, Trolox, and L-ascorbic acid.
ABTS radical scavenging activity assay
The ABTS radical scavenging activity assay was modified from Vongsak et al. (2020), and Jiangseubchatveera et al. (2021). The blue-green ABTS cation free radical was prepared by mixing a 14 mM ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) aqueous solution and a 4.9 mM potassium persulfate aqueous solution at a 1:1 ratio. The blue-green ABTS cation free radical was then stored in the dark at room temperature for 16–20 hours before use. The sample stock solution was diluted in DMSO to the final concentrations in the range of 5–100 μg/ml. The prepared ABTS free radical solution was diluted with distilled water until the absorbance at 734 nm reached 700 ± 0.02. The 100 μl of the sample solution was mixed with 100 μl of the diluted ABTS solution in a 96-well plate. The absorbance was measured, after 6 minutes incubation, at 734 nm using the microplate reader. The experiment was performed in triplicate. The %scavenging activity of ABTS was calculated using Equation (2).
Ferric reducing activity power (FRAP) assay
The FRAP assay was modified from Vongsak et al. (2015). The FRAP reagent was freshly prepared by mixing 10 mM TPTZ in the 1:1:10 ratio of the mixture of 40 mM hydrochloric acid, 20 mM FeCl3.6H2O, and 300 mM acetate buffer pH 3.6. The sample stock solution was diluted in DMSO to the final concentration of 2.5 mg/ml. Twenty μl of samples or standard solutions was mixed with 80 μl FRAP reagent in a 96-well plate and then incubated at 37°C for 8 minutes. FeSO4 at the concentration of 40–720 μM was used as a reference standard. The assay was performed in triplicate. The absorbance was measured by the microplate reader at 593 nm. The linear standard curve between the absorbance and concentration of FeSO4 was generated. The reducing power of samples, Trolox, and L-ascorbic acid standards was expressed as μmol of FeSO4 equivalent/mg extract ± SD.
Preparation of water-based formulation containing rice extract
Black rice extract lotion was prepared by the beaker method modified from Jiangseubchatveera et al. (2021). The extract (50 mg) was dissolved in a mixture of propylene glycol (2 ml) and polysorbate 80 (1 ml) and heated to 50°C until the mixture was completely homogenous. The aqueous solution of potassium sorbate (50 ml of 2 mg/ml) used as a preservative was mixed with humectants (4 ml of 70% sorbitol solution and 1 ml of glycerin). The aqueous mixture was then added to the solubilized extract and adjusted with water to 100 ml, resulting in a lotion.
Preparation of lipid-based formulation containing rice extract
In this study, emulsion and cream were referred to as lipid-based formulations. Rice extract-loaded lipid-based formulations were prepared by the beaker method (Petchsomrit et al., 2020). To fabricate rice extract emulsions, rice extract (1%), sorbitan monooleate (6%), propylene glycol (10%), liquid paraffin (1%), and stearyl alcohol (1%) were mixed and used as the oil phase. Polysorbate 80 (6%), potassium sorbate (0.1 g), and water, to make 100 ml, were used in the water phase. Briefly, the oil and the water phase were heated to 75°C and 70°C, respectively. Then, the water phase was added to the oil phase while being constantly stirred. After that, the 2 ml of a 0.1% xanthan gum solution, which was used as a suspending agent, was mixed homogeneously. For the fabrication of rice extract cream, glyceryl monostearate (2%), stearyl alcohol (7%), white petrolatum (3%), sorbitan monooleate (3%), propylene glycol (5%), and rice extract (0.5%) were mixed and used as the oil phase. The water phase was composed of potassium sorbate (0.1 g), PEG-40 hydrogenated castor oil (5%), and water (adjusted to 100 ml).
Characterization of the water-based and lipid-based formulations containing rice extract
Type of emulsion
The type of emulsion of each lipid-based formulation was determined using three tests as described below.
Water solution test
A gram of cream was dispersed in distilled water or mineral oil in a test tube and shaken (Orbital Shaker NB-101M, Bangkok High Lab, Thailand) for 30 minutes. The solubility of the solution was observed and determined as shown in Table 1.
Dye test
A dye test was used to identify the type of emulsion using microscopy to observe the droplets that were not visible to the naked eye. Under a phase-contrast inverted microscope (Olympus CKX41 Inverted Microscope, Japan), the emulsion and cream were stained with brilliant blue and covered with a coverslip. The o/w type presents as scattered colorless globules on a blue backdrop and vice versa for w/o preparations.
Conductivity test
Because water is a good electrical conductor, o/w emulsions conduct electricity while w/o emulsions do not. An electric conductivity meter (VIVOSUN TSD & EC Meter, CA) was used for the measurement.
Stability study
To evaluate the stability of the products, a centrifugation assay (Charoenjittichai et al., 2016) and six cycles of an acceleration test were performed. The phase separation was observed after centrifugation (Centrifuge Legend X1R, Thermo Scientific, MA) at 3,500 rpm for 30 minutes at room temperature. For the heating-cooling cycle, samples were translocated between 4°C and 40°C and stored for 48 hours for six cycles. Physical appearances, color, phase separation, and pH values were determined after the preparation and at the end of these experiments. The pH values were determined using a pH meter (Mettler-Toledo, USA).
Statistical analysis
All results were expressed as the mean ± SD. Differences between two related parameters were assessed by Student?s t-test or one-way analysis of variance (GraphPad Prism 5.02, GraphPad Software Inc., USA). Differences were considered significant at p < 0.05.
 | Table 1. Interpretation of type of emulsion by water solution test. [Click here to view] |
RESULTS AND DISCUSSION
Extraction yield and total anthocyanin, phenolic, and flavonoids contents
The % yields of the RG and RB crude extracts were 0.68% and 19.33%, respectively. Total anthocyanin content, TPC, and TFC in both the RB and RG extracts are shown in Table 2. The RG extract had a higher total anthocyanin content than the RB extract (Table 3). The TPC and TFC of the RB extract were significantly higher than those of the RG extract. In this study, the high amounts of phenolic compounds were not correlated with high quantities of anthocyanin, which is a group of flavonoid phenolic compounds, suggesting that anthocyanins were not the main flavonoids and phenolics in the extract. This agrees with Rafi et al. (2018), who reported that the amount of flavonoid and anthocyanin in an extract did not always correspond to the number of total phenolics in the extract.
Furthermore, the amount of bran received from the milling process might be the reason why the anthocyanin content of the RB extract was less than the RG extract. The whole rice grain consists of four main components, including hull or husk, bran, germ or embryo, and endosperm. The hulling process separates the hull, the outermost part, from the rice grain to give brown rice. On the other hand, the milling process is a process that removes all the outer parts, not only the hull but also the bran and germ, from the rice grain. This process produces polish or white rice (Juliano and Tuano, 2019; Shafie and Norhaizan, 2017). However, the milling process did not remove all the bran layers from the black rice grain, but only a small amount of bran layer was removed. Therefore, most of the bran layer was still intact on the black rice grain, resulting in the level of anthocyanin of the black rice grain being higher than in the black RB (Ito and Lacerda, 2019).
Antioxidant properties
At least two methods are recommended for the determination of the antioxidant activity of plant extracts (?íž et al., 2010). The antioxidant activities of the rice extracts evaluated by the DPPH, ABTS, and FRAP assays are displayed in Table 3. The RB extract exhibited more potent activities than the RG extract in both antioxidant mechanisms, free radical scavenging (DPPH and ABTS assays) and reduction of the ferric-TPTZ complex to a ferrous complex (FRAP assay).
The results of the DPPH and ABTS assays revealed that the RB extract exhibited greater antioxidant capacity than the RG extract. The antioxidant activity of the RB extract was lower than the standard control (L-ascorbic acid and Trolox). The antioxidant of the rice extracts in the FRAP assay was determined by the formation of a blue Fe2+-TPTZ complex. The assay showed that reduction of the ferric-TPTZ complex to a ferrous one occurred with the RB extract significantly better than with the RG extract (p < 0.05). The antioxidant capacities of the RB extract were greater than those of the RG extract. This might be because of the presence of other antioxidants in the RB extract. According to previous research, tocopherols, tocotrienols, and γ-oryzanol, which are antioxidants, were found in the RB extract in a higher amount than in the RG extract (Goufo and Trindade, 2014).
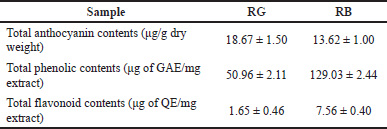 | Table 2. Total anthocyanin contents, total phenolic contents, and total flavonoid contents from rice extracts. [Click here to view] |
Antioxidant activity assays can be classified by the chemical reactions into three groups, including hydrogen atom transfer (HAT) and electron transfer- (ET-) based assays and mixed mode. The DPPH and ABTS methods have been classified as a mixed mode assay, having both HAT and ET reactions, whereas the FRAP assay is an ET-based method (Apak et al., 2016; Gupta, 2015). Phenolic compounds exhibited antioxidant activity in all the assays; thus, the phenolic compounds in the RB/RG extract might have acted as an antioxidant by donating both hydrogen atoms and electrons to the radicals (Ito and Lacerda, 2019; Saikia et al., 2012; Zhang et al., 2015).
Selection of the extract to incorporate in skin preparation
From these results, the %yield, TPC, TFC, and antioxidant capacities were parameters used for selecting an appropriate extraction as a candidate. The RB extract was selected to incorporate in water- and oil-based formulations as the RB extract provided the highest % yield, contained a high amount of phenolics and flavonoids, and exhibited potent antioxidant properties.
The characteristic of formulations containing RB extract
Type of emulsion
The type of emulsion in the lipid-based formulations was determined. The homogenous mixture occurred after mixing the emulsion or cream with water (Fig. 1). Bright-field microscope images from the dye test showed colorless oil droplets scattered on a blue backdrop (Fig. 2). Next, the conductivity test revealed that these formulations conducted electricity (128 ± 6.56 and 36 ± 3.61 μS/cm for emulsion and cream, respectively). Therefore, we concluded that the formulated emulsion and cream in this study were o/w emulsions that are nongreasy and easily removable from the skin surface (Viyoch et al., 2003).
Stability study
This study showed that the extract could be incorporated into both water- and oil-based formulations. After the stability test, the color of the rice extract in all types of preparations did not change (Fig. 3). The color of the product depended on the quantity of the rice extract and excipient color. Phase separation did not occur after the centrifugation test and six-cycle acceleration test. Additionally, the lotion was not aggregate. The pH values of the lotion, emulsion, and cream ranged between 5 and 5.5 (Table 4), which was the range that was suitable for skin application.
 | Table 3. The antioxidant activities of rice extracts. [Click here to view] |
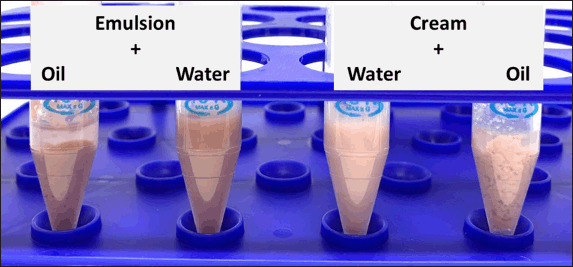 | Figure 1. Type of emulsion by water solution test. [Click here to view] |
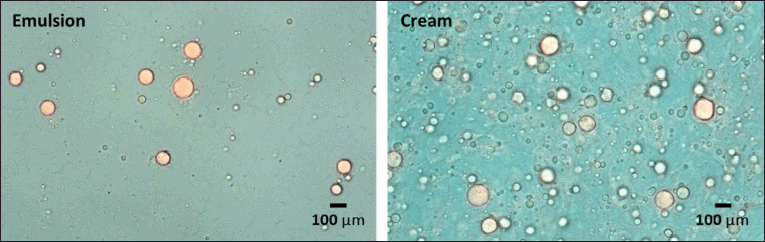 | Figure 2. Light microscope images (40×) from the dye test. [Click here to view] |
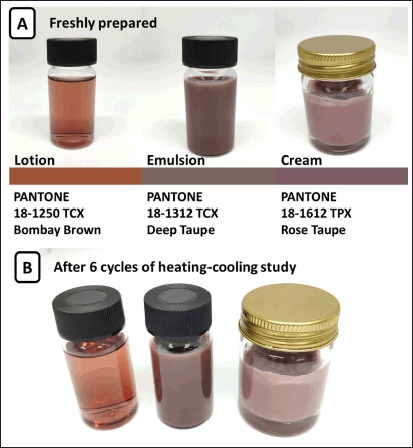 | Figure 3. The appearance of water- and oil-based formulations. (A) Freshly prepared and (B) After six cycles of heating-cooling study. [Click here to view] |
 | Table 4. The pH values of all formulations before and after six cycles of the heating-cooling cycle. [Click here to view] |
CONCLUSION
This research revealed that the RB extract could be a potential source of natural antioxidants. The RB extract provided higher TPC, TFC, and antioxidant capacities than the rice grain extract. After the incorporation of the RB extract into the water- and oil-based formulations, the rice-extract-loaded formulations were stable and yielded good physical appearances. We concluded that the RB extract has an excellent antioxidant capacity and is compatible with water- and lipid-based formulations. Moreover, the RB extract might be used as an active component in cosmeceutical products.
ACKNOWLEDGMENTS
This work was financially supported by the Research Unit in Synthetic Compounds and Synthetic Analogues from Natural Product for Drug Discovery (RSND), Burapha University, the Pharmaceutical Innovations of Natural Products Unit (PhInNat), Burapha University, and the Faculty of Pharmaceutical Sciences, Burapha University.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Apak R, Özyürek M, Güçlü K, Çapano?lu E. Antioxidant activity/capacity measurement. 1. classification, physicochemical principles, mechanisms, and electron transfer (ET)-based assays. J Agric Food Chem, 2016; 64(5):997–1027. CrossRef
Chen XQ, Nagao N, Itani T, Irifune K. Anti-oxidative analysis, and identification and quantification of anthocyanin pigments in different coloured rice. Food Chem, 2012; 135(4):2783–8. CrossRef
Charinrat S, Kaewsrichan J, Leelakanok N, Petchsomrit A. Antioxidant in cosmeceutical products containing Calophyllum inophyllum oil. OCL, 2021; 28(28). CrossRef
Charoenjittichai R, Charnvanich D, Panapisal V. Effects of surfactant mixture ratio and concentration on nanoemulsion physical stability. Thai J Pharm Sci, 2016; 40:45–8.
?íž M, ?ížová H, Denev P, Kratchanova M, Slavov A, Lojek A. Different methods for control and comparison of the antioxidant properties of vegetables. Food Control, 2010; 21(4):518–23. CrossRef
De Jager TL, Cockrell AE, Du Plessis SS. Ultraviolet light induced generation of reactive oxygen species. Adv Exp Med Biol, 2017; 996:15–23. CrossRef
Fonseca-Santos B, Corrêa MA, Chorilli M. Sustainability, natural and organic cosmetics: consumer, products, efficacy, toxicological and regulatory considerations. Braz J Pharm Sci, 2015; 51(1):17–26. CrossRef
Ghasemzadeh A, Karbalaii MT, Jaafar HZE, Rahmat A. Phytochemical constituents, antioxidant activity, and antiproliferative properties of black, red, and brown rice bran. Chem Cent J, 2018; 12(1):17. CrossRef
Goufo P, Trindade H. Rice antioxidants: phenolic acids, flavonoids, anthocyanins, proanthocyanidins, tocopherols, tocotrienols, gamma-oryzanol, and phytic acid. Food Sci Nutr, 2014; 2(2):75–104. CrossRef
Gupta D. Methods for determination of antioxidant capacity: a review. Int J Pharm Sci Res, 2015; 6(2):546–66.
Jiangseubchatveera N, Saechan C, Leelakanok N, Petchsomrit A. The evaluation of antioxidant and antityrosinase efficacy of Carissa carandas fruit extracts and the development of a preliminary skincare product. J App Pharm Sci, 2021; 11(07):153–7.
Juliano BO, Tuano AP. 2-Gross structure and composition of the rice grain. In: Bao J (ed.). Rice chemistry and technology, AACC International Press, Cambridge, UK, pp 31–53, 2019. CrossRef
Jung TD, Shin GH, Kim JM, Choi SI, Lee JH, Lee SJ, Park SJ, Woo KS, Oh SK, Lee OH. Comparative analysis of gamma-oryzanol, beta-glucan, total phenolic content and antioxidant activity in fermented rice bran of different varieties. Nutrients, 2017; 9(6):571. CrossRef
Ito VC, Lacerda LG. Black rice (Oryza sativa L.): a review of its historical aspects, chemical composition, nutritional and functional properties, and applications and processing technologies. Food Chem, 2019; 301:125304. CrossRef
Kumar S, Dhir A, Talwar S, Chakraborty D, Kaur P. What drives brand love for natural products? The moderating role of household size. J Retail Consum Serv, 2021; 58:102329. CrossRef
Lee J, Durst RW, Wrolstad RE, Eisele T, Giusti MM, Hach J, Hofsommer H, Koswig S, Krueger DA, Kupina S, Martin SK, Martinsen BK, Miller TC, Paquette F, Ryabkova A, Skrede G, Trenn U, Wightman JD. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: collaborative study. J AOAC Int, 2005; 88(5):1269–78. CrossRef
Orachos N. Thailand’s colored rice standard and markets: food and fertilizer technology center for the Asian and Pacific Region, 2020. Available via https://ap.fftc.org.tw/article/1756
Petchsomrit A, McDermott MI, Chanroj S, Choksawangkarn W. Watermelon seeds and peels: fatty acid composition and cosmeceutical potential. OCL, 2020; 27:54. CrossRef
Pillai S, Oresajo C, Hayward J. Ultraviolet radiation and skin aging: roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation - a review. Int J Cosmet Sci, 2005; 27(1):17–34. CrossRef
Rafi M, Febriany S, Wulandari P, Suparto IH, Ridwan T, Rahayu S, Siswoyo DM. Total phenolics, flavonoids, and anthocyanin contents of six vireya rhododendron from indonesia and evaluation of their antioxidant activities. J Appl Pharm Sci, 2018; 8(9):49–54. CrossRef
Sadabpod K, Kangsadalampai K, Tongyonk L. Antioxidant activity and antimutagenicity of hom nil rice and black glutinous rice. J Health Res, 2018; 24(2):49–54.
Saikia S, Dutta H, Saikia D, Mahanta CL. Quality characterisation and estimation of phytochemicals content and antioxidant capacity of aromatic pigmented and non-pigmented rice varieties. Food Res Int, 2012; 46(1):334–40. CrossRef
Shafie NH, Norhaizan M. The healing components of rice bran. In: In Ismail A, Azlan A (ed.). Functional foods: wonder of the world evidence-based functional foods in health & disease. 1st edition, Penerbit Universiti Putra Malaysia (UPM), Serdang, Malaysia, pp 341–68, 2017.
Shao Y, Hu Z, Yu Y, Mou R, Zhu Z, Beta T. Phenolic acids, anthocyanins, proanthocyanidins, antioxidant activity, minerals and their correlations in non-pigmented, red, and black rice. Food Chem, 2018; 239:733–41. CrossRef
Tai L, Huang S, Zhao Z, Huang G. Chemical composition analysis and antioxidant activity of black rice pigment. Chem Biol Drug Des, 2021; 97(3):711–20. CrossRef
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines (Basel), 2018; 5(3):93. CrossRef
Viyoch J, Klinthong N, Siripaisal W. Development of oil-in-water emulsion containing tamarind fruit pulp extract I. Physical characteristics and stability of emulsion. Nares Univ J, 2003; 11(3):29–49.
Vongsak B, Jaisamut S, Gonsap K, Parmontree P. Optimization of Maclura cochinchinensis extract as a cosmeceutical component for antioxidant and anti-tyrosinase activities. Key Eng Mater, 2020; 859:188–93. CrossRef
Vongsak B, Kongkiatpaiboon S, Jaisamut S, Machana S, Pattarapanich C. In vitro alpha glucosidase inhibition and free-radical scavenging activity of propolis from Thai stingless bees in mangosteen orchard. Rev Brasil Farmacog 2015; 25(5):445–50. CrossRef
Yang L, Rong-Rong C, Ji-Li F, Ke Y. Total anthocyanins and cyanidin-3-O-glucoside contents and antioxidant activities of purified extracts from eight different pigmented plants. Pharmacogn Mag, 2019; 15(60):124. CrossRef
Zhang H, Shao Y, Bao J, Beta T. Phenolic compounds and antioxidant properties of breeding lines between the white and black rice. Food Chem, 2015; 172:630–9.