INTRODUCTION
The inflammatory bowel disease, commonly called ulcerative colitis, is characterized by a broad inflammation of the colonic mucosa. It starts in the rectum and spreads proximally along the colon for a considerable length of time (Conrad et al., 2014). The disease can be categorized as left-sided colitis (inflammation up to the splenic flexure) or extensive colitis (inflammation beyond the splenic flexure) (Kugathasan et al., 2003; Vucelic, 2009). Most of the digestion and absorption occur in the small intestine. Any undigested material moves to the large intestine (Borgström et al., 1957; Carey et al., 1983; Hinsberger and Sandhu, 2004). Irritable bowel syndrome, inflammatory bowel disease (IBD), and colon cancer are relapsing–remitting disorders that affect a number of people all over the world, limiting their daily activities and affecting their quality of life (Orscheln, 2018). IBD is a term that refers to a group of chronic inflammatory disorders that affects both large and small intestines. IBD is characterized by persistent chronic inflammation of the intestine. India has over 12 lakh cases of IBD annually, but very few people are even aware of the seriousness and symptoms of the disease. IBD impacts over 1–2 million U.S. adults and many more worldwide. Currently, pharmaceutical medications for IBD are rarely curative and frequently have substantial side effects. Drugs including 5-aminosalicylic acid, corticosteroids, azathioprine, mercaptopurines, and cyclosporine are used to manage IBD. However, the use of these medications is restricted because of their toxicity. Alternative treatments that are more efficient, less harmful, and affordable are in high demand (Danese et al., 2004; Heyman et al., 2005; Nightingale et al., 2000; Pithadia and Jain, 2011; Shanahan, 2001).
Most natural product alternatives are being explored rather than synthetic small molecules. One such is curcumin, a low-molecular weight polyphenol extracted from the rhizomes of turmeric (Curcuma longa Linn.); it is a yellow color spice mostly used in foods as a coloring agent. For ages it has been utilized in Ayurvedic and Chinese medicines. Due to its wide range of favorable pharmacological actions, comprising antibacterial, antifungal, anti-inflammatory, antiviral, antidiabetic, antioxidant, anticarcinogenic, and Alzheimer’s disease, interest in this dietary polyphenol has developed in recent years. The primary health benefit of curcumin is its potent anti-inflammatory activity in the colon region. In addition, curcumin suppresses inflammation by inhibiting the enzymes cyclooxygenase-2, lipoxygenase, and nitric oxide synthase. TNF-α, interleukin -1, -2, -6, -8, and -12, monocyte chemoattractant protein, and migration inhibiting protein are among the inflammatory cytokines inhibited by it. It is also extremely photoreactive and has a solubility of less than 1 g/ml. Even though it has no negative effects when consumed up to 12 g per day, it has less absorption and metabolism, leading to reduced bioavailability. Therefore, to achieve maximum response to curcumin, novel strategies are required to enhance its bioavailability (Aggarwal et al., 2007; Anand et al., 2007; Hatcher et al., 2008; Orscheln, 2018; Sharma et al., 2005; Tønnesen and Karlsen, 1985; Zhou et al., 2011).
The emergence of nanotechnology leads to the era of efficient dosage forms. A viable method for producing individual functionalized particles known as “nanosponge” has been designed. The invention of nanosponges, a novel colloidal carrier, has the ability to solve these issues. Nanosponge technology is a new and developing technology. It can accurately adjust the release kinetics for targeted therapy. Nanosponges are small sponges around the size of a virus that can be packed with various medications. These little sponges may circulate in the body till they reach a particular target spot, where they attach to the surface and start releasing the drug in a regulated and consistent way (Jilsha and Viswanad, 2013; Pandey et al., 2018; Shivani and Poladi, 2015). The additional feature of such sponges is their aqueous solubility, enabling them to be used efficiently for medications with low solubility. Nanosponges, a controlled release nanoparticle system, show a promising targeted drug delivery system to the colon. Hence, curcumin nanosponges have been developed, as they often can improve the therapeutic potential of curcumin in colon targeting. Furthermore, CURNS could be able to solve the challenges of curcumin’s poor solubility and bioavailability. The major focus of this research is to enhance the solubility of curcumin to improve its anti-inflammatory properties. This research attempts to formulate the selected drug into nanosponges in order to increase the dosage form’s treatment efficacy and provide targeted drug delivery.
MATERIALS AND METHODS
Curcumin was given as a gift sample by the Himalayan Drug Company, Bangalore. All other chemicals used were present in the lab and were of analytical grade.
Fourier transform infrared spectroscopy (FTIR)
FTIR was designed to evaluate the drug’s compatibility with the excipients employed in the formulation using a Shimadzu FTIR spectrophotometer. The investigation was conducted using the potassium bromide (KBr) pellet technique. The samples were thoroughly blended with dry potassium bromide crystals. The mixture was compressed. The spectra were obtained between 400 and 4,000 cm−1 after placing the disk in the sample cell. The pure drug and the optimized formulation’s FTIR spectra were recorded and compared.
Preparation of nanosponges
The emulsion solvent diffusion technique was used to develop curcumin nanosponges. The internal phase contains polymer, i.e., eudragit-L-100 in 20 ml dichloromethane. With constant stirring at 600 rpm, the drug was added slowly to the polymer solution. The internal phase was then dropped into an aqueous exterior phase having polyvinyl alcohol (1% w/v) in a dropwise fashion. Continuous stirring resulted in the formation of nanosponges. The product was filtered, dried, and stored in an airtight container for further processing. The design is shown in Table 1.
Evaluation of nanosponges
Percentage yield
The percentage yield of nanosponges of all the formulations was determined by measuring the final weight of the product after drying in comparison to the total weight of the drug, polymer, and other excipients used for the preparation of nanosponges.
Micromeritic properties
The nanosponges were evaluated for their micromeritic properties, bulk density, tapped density, Carr’s index, Hausner’s ratio, compressibility index, and angle of repose.
where M is the mass of the powder and V0 is the unsettled apparent volume.
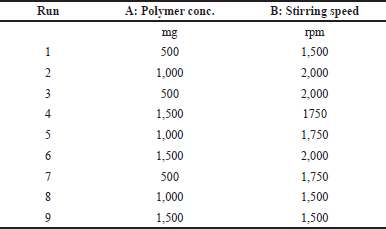 | Table 1. Formulation design for the preparation of curcumin nanosponges. [Click here to view] |
where Mf is the mass of the powder in the measuring cylinder.
where V0 is the apparent volume and Vf is the final tapped volume.
Compressibility index (%) = (1–Vf/V0) × 100
where V0 and Vf are the volumes of the sample before and after the standard tappings, respectively.
Angle of repose
It was determined by the reposograph instrument using a following equation:
tanθ = h/r
where h = height of the pile and r = radius of the base of the pile on the graph paper
Drug entrapment efficiency
Curcumin nanosponges containing 20 mg of the drug were fractured and isolated by vortexing and centrifuging at 2,000 rpm for 10 minutes with 10 ml methanol. Upon separating the insoluble remnant, the supernatant was spectrophotometrically examined at 423 nm after sufficient dilution. Then, with the help of the following formula, the encapsulation efficiency was determined:
In vitro drug release study
An accurately weighed amount of dried curcumin nanosponges, equivalent to 20 mg curcumin, is transferred to a glass cylinder having a length of 7 cm and diameter of 2.5 cm (disintegration tubes), where one side of the tube is completely covered with preactivated cellophane membrane. This cylinder was fitted to the paddle and was suspended in the dissolution flask, of which 200 ml of 0.1 N HCl and the apparatus was adjusted to a constant speed of 50 rpm and a temperature of 37°C. The samples were collected for 30, 60, 90 and 120 minutes. This was immediately assayed using UV spectroscopy for drug content at 432 nm. Then, the whole paddle system is uplifted and the beaker containing 0.1N HCl is replaced with a 7.4 pH phosphate buffer solution, and again the apparatus is set to the same conditions as previously mentioned and the samples are collected and analyzed at 3, 4, 6, 8, and 12 hours.
Optimization
The response variables were statistically analyzed using Design Expert software, version 11, with analysis of variance at the 0.05 level. To construct polynomial equations, F-tests were used to evaluate each parameter and the responses were exposed to multiple regression analysis.
Formulation design
Central Composite Design with a single central point was utilized for the optimization of the formulation. The independent variables chosen were polymer concentration and stirring speed. The factors and levels are mentioned in the Table 2. The lower and higher levels of independent factors were selected based on the reported literature and initial screening experiments conducted. The total runs were found to be 9. Experiments were carried out and the obtained data were used for optimization.
Curve-fitting analysis
Models such as Higuchi, first-order, zero-order, and Korsemayer–Peppas were used to explore the factors of curcumin release from nanosponges, and a regression (R2) value was derived.
Particle size analysis
Particle size distribution and polydispersity index were measured by Zetasizer (Malvern Instrument, Zetasizer ver. 7.10, serial number: MAL 1045544). Dried curcumin-loaded nanosponges of optimized formulation were reconstituted and analyzed with 10 ml of Milli-Q water and diluted 10-fold with the same water using a vortex mixer.
Zeta potential
The zeta potential of the optimized formulation was measured using a Horiba Zetasize analyzer. The nanosponges were diluted in the ratio of 1:10 with Millipore water; vortexed for a minute; and subjected to analysis.
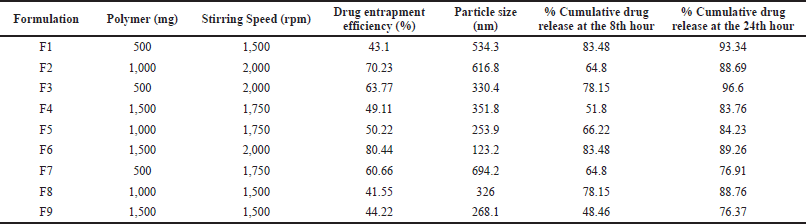 | Table 2. Factors and related responses for various formulations. [Click here to view] |
Scanning electron microscopy
The shape and surface properties of the prepared microparticles were determined by using a scanning electron microscope (SEM; TESCAN-VEGA3 LMU). The microparticles were sprinkled on both sides of the adhesive tape and adhered to a stub to make the SEM samples. A sputter coater was used to coat the stub in gold–palladium. The coated substances were then randomly scanned and photomicrographs were taken with an SEM.
In vivo pharmacokinetic study
In vivo studies were conducted in the Department of Pharmacology, Government College of Pharmacy, Bengaluru, which is dully licensed by the Committee for the Purpose of Control and Supervision of Experiments in Animals (CPCSEA). The institutional animal ethics committee for studies in experimental animals authorized the study protocols in accordance with current the CPCSEA standards (approval number: DCD/GCP/20/E.C/ADM/2018-2019, date: 04.06.2018). Rabbits used for this study were obtained from a drug testing laboratory in Bengaluru. They were kept in typical laboratory settings at room temperature with a 12-hour light/12-hour dark cycle and were fed a standard pellet diet (Venkateshwara Enterprises Pvt. Ltd. Bengaluru), with free access to water. A total of 10 rabbits (either sex) were selected for the present study.
Inclusion criteria
Adult animals aged between 3 and 9 months and those with normal behavioral parameters, healthy food consumption and excretory activities, healthy body weight, temperature, and heart rate were selected.
Exclusion criteria
Animals which did not exhibit the abovementioned inclusion criteria are omitted. Female animals assumed to be in the gestational period were excluded.
Preparation of the animals for the study
For a minimum of a week before the research, the rabbits were not given any vegetables or green leafy food. All the animals were given only a standard pellet diet from a week before the study till the end. All the animals were fasted for 18 hours before the study, with water ad libitum.
Mode of Administration
Curcumin and CURNS were administered as an oral suspension with a dose of curcumin at 15 mg/kg. Rabbits were restrained using a rabbit restrainer; an oral gag was used to keep the mouth open. Using an oral gavage, the rabbits were dosed with curcumin and CURNS that were suspended in water (suspension).
Method for blood sampling
Blood samples were taken from the marginal ear vein. A sharp 26-gauge needle was used to pierce the dilated marginal ear vein in the direction of venous blood flow. The blood samples (up to 1 ml each sample) were collected into Eppendorf tubes. The blood samples were centrifuged at 6,000 rpm for 10 minutes with a drop of heparin solution; the supernatant fluid (plasma) was decanted into a clean and dry Eppendorf tube. Samples were stored at −20°C until further analysis.
Experimental design
Healthy rabbits among both sexes weighing 1.25–2.25 kg were chosen. They were kept under normal laboratory conditions with a 12-hour light/12-hour dark cycle, fed with a standard pellet diet, with free access to water. The animals were fasted for 18 hours prior to the experiment. Animals were divided into two groups; each consisted of five animals. Blood samples were collected through the marginal ear vein at intervals of 0 minute (pre-dose), 1, 2, 4, 6, 8, 12, and 24 hours post oral dosing of curcumin and CURNS. To extract the plasma, the collected blood was centrifuged at 6,000 rpm for 10 minutes. Plasma drug concentration was analyzed by the HPLC method (Gao et al., 2010).
Stability studies
The optimized formulation packed in its primary pack 30 ml tubular vial with a 20 mm rubber stopper was subjected to accelerated stability studies using an environmental testing chamber (Remi Instrument Ltd) for 30 days. Storage conditions of the testing chamber are maintained at 40° ± 2°C and 75% ± 5% relative humidity (RH). In addition, the formulation was evaluated for appearance, drug entrapment efficiency, drug loading, and in vitro drug release.
RESULTS AND DISCUSSION
Compatibility studies
Compatibility of curcumin with different excipients was carried out using FTIR spectroscopy. The IR spectra of pure curcumin and curcumin with excipient are shown in Figure 1. The IR spectra showed that there were no significant changes in the characteristic peaks of pure drug and formulation. This confirms that there was no interaction between drug and excipients.
Formulation of nanosponges
Nanosponges were prepared as per the central composite design using the emulsion solvent diffusion method.
In vitro dissolution studies
The in vitro drug release of formulations F1–F9 is shown in Figure 2. F1 and F6 showed the maximum drug release of about 83%. This suggests that as polymer concentration increases, the drug release is sustained.
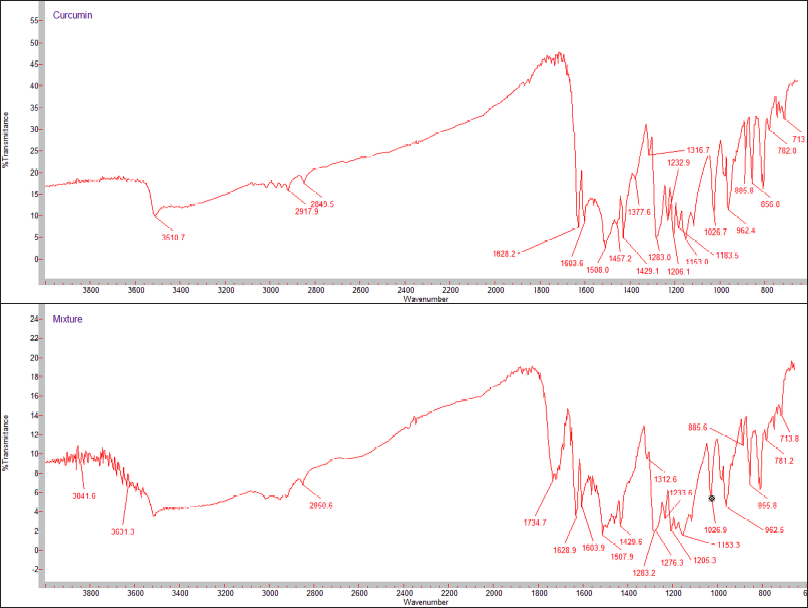 | Figure 1. FTIR Spectra of (a) curcumin and (b) physical mixture of curcumin and excipients. [Click here to view] |
Optimization
The prepared formulations were analyzed for drug entrapment and particle size (Table 2).
Entrapment efficiency
Drug entrapment efficiency = –45.98944 + 0.002080A + 0.057047B
The model’s F-value of 6.44 shows that the model is significantly relative to noise. p-values less than 0.05 indicate that the model terms are very significant. The actual and predicted values of %DEE were correlated. The equation proves that the increase in polymer concentration and stirring speed also enhanced the amount of drug entrapment in the nanosponges.
Particle size
Particle size= +388.74 − 135.97 A − 9.67 B
The model’s F-value of 2 indicates that it is not significant in comparison to the noise. Model terms with p-values greater than 0.05 are not significant. The actual and predicted values of particle size were correlated. The abovementioned equation indicated that stirring speed and polymer concentration had a negligible effect on the particle size of nanosponges.
% Cumulative drug release at the 8th hour
Equation of %CDR at the 8th hour = +136.325 − 0.062648 A − 0.022367 B + 0.000018AB
The model’s F-value of 208.36 indicates that the model is statistically significant in comparison to the noise. Model terms with p-values less than 0.05 are considered significant. The actual and predicted values of %CDR of 8 hours were correlated. The polymer concentration and stirring speed had a negative effect on release at the 8th hour, i.e., as their concentration increases, the release rate was found to decrease, which was desirable.
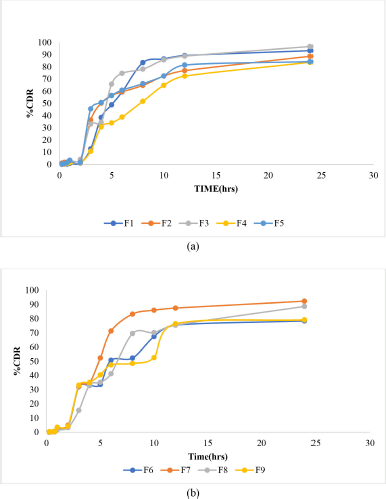 | Figure 2. In vitro drug release profile of formulations (a) F1–F5 and (b) F6–F9. [Click here to view] |
%Cumulative drug release at the 24th hour
Equation of %CDR at the 24th hour = +97.67722 − 0.013670A + 0.001833B
The model’s F-value of 21.45 indicates that the model is statistically significant in comparison to the noise. Because the p-values are less than 0.0500, the model terms are significant. The actual and predicted values of %CDR of 8 hours were correlated. The polymer concentration at the 24th hour had a negating effect, may be because of complete erosion or diffusion (Fig. 3).
Regression analysis
The desirability approach of numerical optimization was used to improve the formulations. For dependent and independent variables, optimal settings were determined. Except for particle size, all response parameters were subjected to regression analysis to obtain regression coefficients, and all dependent variables were determined to be significant (Table 3). The optimal formulation was created using the predicted model and the results were analyzed. The reports revealed that each of the independent variables played a significant influence in the creation of nanosponges.
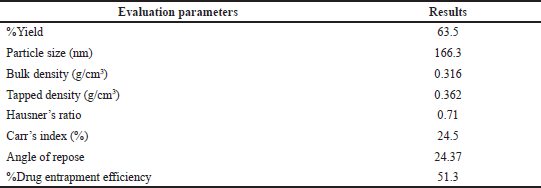
The solution for the optimized formula given by the software is shown in Table 4. The results for this optimized formula are shown in Table 5.
Evaluation of optimized formulation
The optimized formulation’s experimental (actual) and expected values were compared and found in close agreement with each other, indicating the appropriateness of the optimization process in the efficient development of nanosponge formulation.
Scanning electron microscopic study
The image of the optimized formulations is shown in Figure 4. The SEM results revealed that the nanosponges of curcumin were spherical in shape and spongy in nature.
Partsicle size and polydispersity index
The average particle size of the optimized formulation was found to be 166.3 nm (Fig. 5). The PDI of the optimized formulation was found to be 0.219 (Fig. 5), which was within the acceptable range (0–0.3), indicating homogenous particle size distribution.
Zeta-potential
The average zeta potential of optimized nanosponges was found to be −26.2 mV, which was sufficient to keep the nanosponges away from aggregation (Fig. 6).
In vitro drug release kinetics
The drug release mechanism was analyzed by fitting the release data into various models, such as zero-order, first-order, Higuchi, and Korsmeyer–Peppa. The results are compiled in Table 6. The percentage of drug release for the optimized formulation is shown in Figure 7. It was observed that the R2 value of the Higuchi model was close to 1; the drug release follows the Higuchi model as the major model of drug release from the formulation. Hence, diffusion may be the main mechanism for drug release.
Bioanalytical Method development and validation
The bioanalytical HPLC method was developed and validated for its linearity. Several mobile phase compositions, strength, various flow rates (0.7–1.2 ml/min) and detector wavelength of 420 nm were used in the study to obtain optimum chromatographic conditions. Best separation and resolution of peaks were observed using Cosmicsil® 100, C18 (250 × 4.6 mm, 5 μm) column as a stationary phase with a mobile phase of methanol: water (pH adjusted to 3.0 with orthophosphoric acid) in the ratio 70:30% v/v. The flow rate was maintained at 0.8 ml/min. When standard curcumin solutions (1 mg/ml) were injected, the retention time of curcumin was found to be 4.3 minutes (Table 7, Figs. 8 and 9).
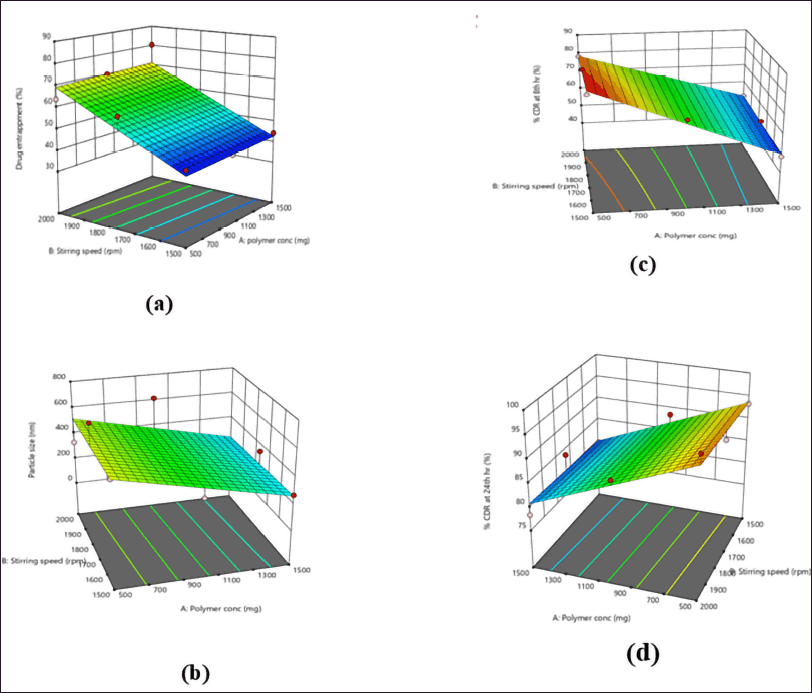 | Figure 3. 3D response surface curve for (a) %EE, (b) particle size in nm, (c) %CDR at the 8th hour, and (d) %CDR at the 24th hour. [Click here to view] |
 | Table 3. Regression analysis. [Click here to view] |
 | Table 4. Solution for optimized formulation given by Design Expert. [Click here to view] |
 | Table 5. Results of optimized formulation. [Click here to view] |
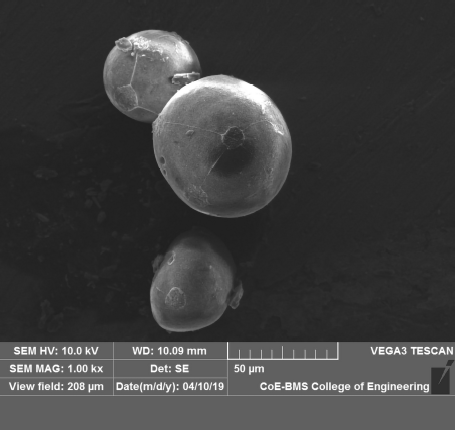 | Figure 4. SEM image of CURNS. [Click here to view] |
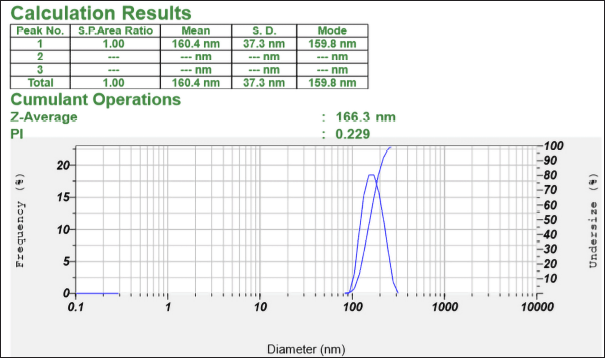 | Figure 5. Particle size analysis report of the optimized formulation. [Click here to view] |
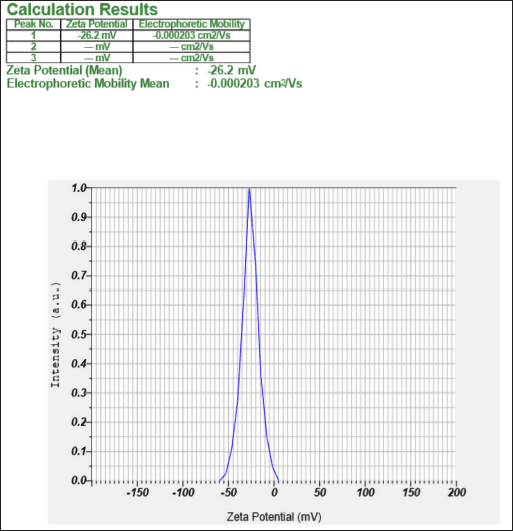 | Figure 6. Zeta potential report of the optimized CURNS formulation. [Click here to view] |
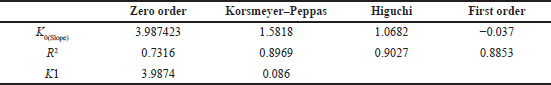 | Table 6. In vitro release kinetic models. [Click here to view] |
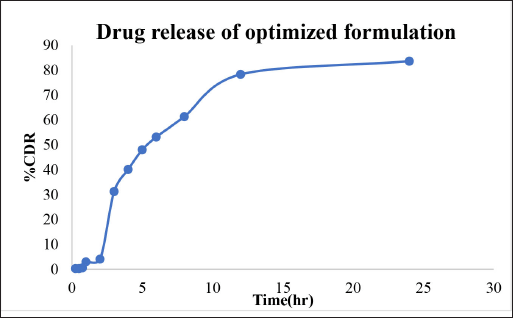 | Figure 7. In vitro drug release profile of the optimized formulation. [Click here to view] |
 | Table 7. Plasma linearity parameters of serum curcumin. [Click here to view] |
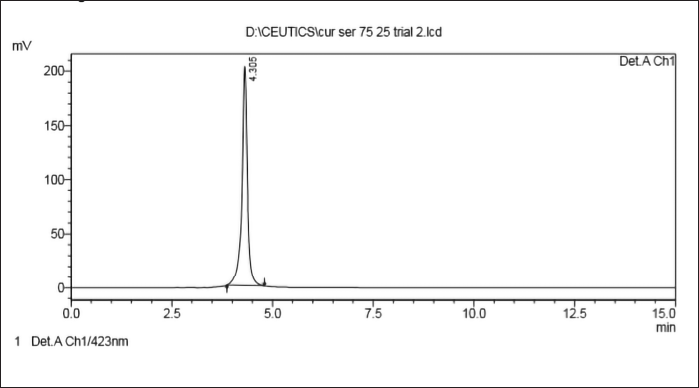 | Figure 8. HPLC chromatogram of curcumin [Click here to view] |
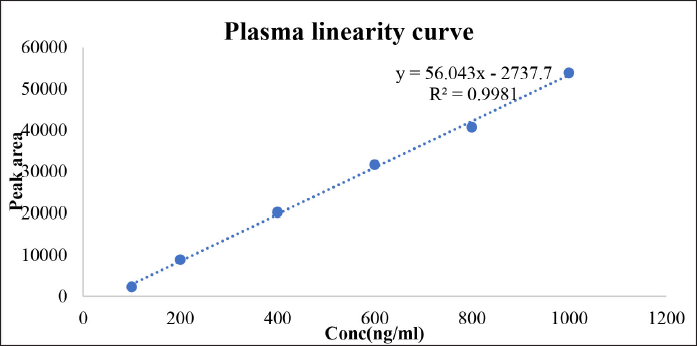 | Figure 9. Plasma linearity curve of pure curcumin. [Click here to view] |
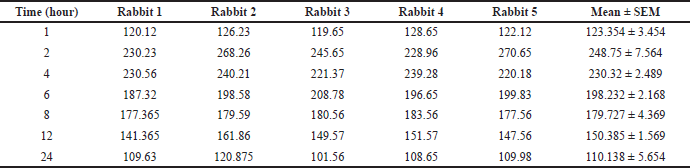 | Table 8. Pure curcumin plasma concentration (ng), n = 5. [Click here to view] |
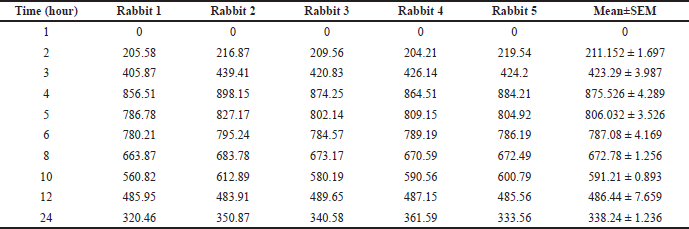 | Table 9. Optimized CURNS plasma concentration (ng), n = 5. [Click here to view] |
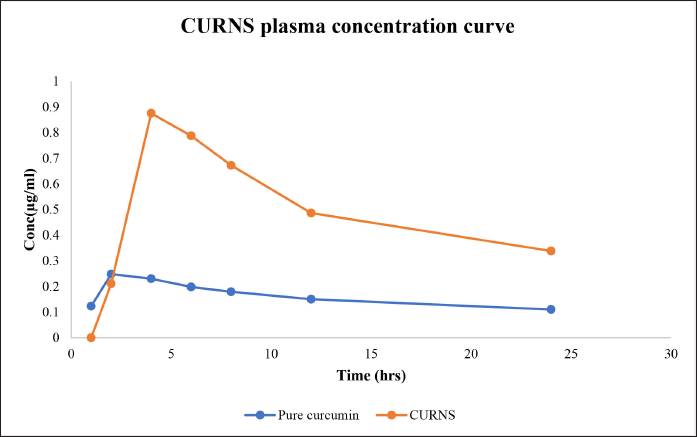 | Figure 10. Plasma concentration curve of the pure curcumin and the optimized CURNS formulation. [Click here to view] |
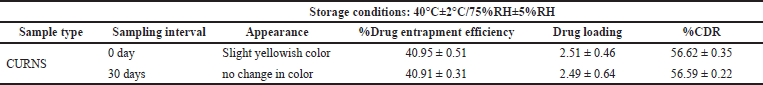 | Table 11. Stability studies. [Click here to view] |
In vivo pharmacokinetic studies
Peak plasma concentration of pure curcumin was found to be at the 2nd hour and Cmax was found to be 0.25 ± 0.012 μg/ml. The peak plasma concentration of curcumin nanosponges was found to be at the 4th hour and Cmax was found to be 0.875 ± 0.053 μg/ml. t1/2 was found to be 2.384 ± 0.343 and 4.869 ± 0.135 hours for pure curcumin and CURNS, respectively. Mean residence time (MRT) was found to be 23.921 ± 5.653 hours for curcumin and 34.0118 ± 4.563 for CURNS. The pharmacokinetic data are presented in Tables 8–10.
From the results obtained, it can be depicted that the AUC(0–t) is almost 3.07-fold increased in the case of CURNS when compared with pure curcumin (Fig. 10).
Stability studies
Accelerated stability studies showed that CURNS was stable, and the results are shown in Table 11.
CONCLUSION
In the present work, curcumin-loaded nanosponges were successfully prepared using eudragit-L-100 by the emulsion solvent diffusion technique. FTIR spectra and DSC thermograms of the pure drug and formulation indicated drug–excipient compatibility. SEM analysis of the nanosponges revealed that the prepared nanosponges were porous, smooth, spongy, and spherical particles. Zeta potential analysis suggested that the formulations were stable. Using optimal polymer concentration led to an increase in %yield and %entrapment efficiency. The in vitro drug release was shown to decrease as the polymer concentration was increased and there was no significant effect with stirring speed. The curve-fitting analysis showed that the drug release mechanism might be diffusion controlled. The independent variables, polymer concentration, and stirring speed had a negligible effect on particle size. The in vivo pharmacokinetic data revealed that the bioavailability of curcumin had increased 3.05-fold when formulated as nanosponges compared to pure curcumin. According to stability analyses, there was no substantial change in percentage drug content or percent in vitro drug release during the storage time, and the product was deemed to be stable at the conclusion of the storage time. The research work can be continued using various natural or synthetic polymers by different formulation methods for the formulation of nanosponges. The method for preparing nanosponge drug delivery can be utilized for other drugs with more adverse effects, low bioavailability, and low solubility—incorporation into a variety of delivery vehicles such as film-forming solutions.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
The IAEC approved the study protocols (approval number: DCD/GCP/20/E.C/ADM/2018-19, date: 04.06.2018).
DATA AVAILABILITY
All data generated and analyzed are included with in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Aggarwal BB, Surh Y-J, Shishodia S. The molecular targets and therapeutic uses of curcumin in health and disease, Vol. 595. Springer Science & Business Media, New York,NY 2007. CrossRef
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB. Bioavailability of curcumin: problems and promises. Mol Pharm, 2007; 4(6):807–18. CrossRef
Borgström B, Dahlqvist A, Lundh G, Sjövall J. Studies of intestinal digestion and absorption in the human. J Clin Investig, 1957; 36(10):1521–36. CrossRef
Carey MC, Small DM, Bliss CM. Lipid digestion and absorption. Annu Rev Physiol, 1983; 45(1):651–77. CrossRef
Conrad K, Roggenbuck D, Laass MW. Diagnosis and classification of ulcerative colitis. Autoimmun Rev, 2014; 13(4–5):463–6. CrossRef
Danese S, Sans M, Fiocchi C. Inflammatory bowel disease: the role of environmental factors. Autoimmun Rev, 2004; 3(5):394–400. CrossRef
Gao Y, Li Z, Sun M, Li H, Guo C, Cui J, Li A, Cao F, Xi Y, Lou H, Zhai G. Preparation, characterization, pharmacokinetics, and tissue distribution of curcumin nanosuspension with TPGS as stabilizer. Drug Dev Ind Pharm, 2010; 36(10):1225–34. CrossRef
Hatcher H, Planalp R, Cho J, Torti FM, Torti SV. Curcumin: from ancient medicine to current clinical trials. Cell Mol Life Sci, 2008; 65(11):1631–52. CrossRef
Heyman MB, Kirschner BS, Gold BD, Ferry G, Baldassano R, Cohen SA, Winter HS, Fain P, King C, Smith T, El-Serag HB . Children with early-onset inflammatory bowel disease (IBD): analysis of a pediatric IBD consortium registry. J Pediatr, 2005; 146(1):35–40. CrossRef
Hinsberger A, Sandhu BK. Digestion and absorption. Curr Paediatr, 2004; 14(7):605–11. CrossRef
Jilsha G, Viswanad V. Nanosponges: a novel approach of drug delivery system. Int J Pharm Sci Rev Res, 2013; 19(2):119–23.
Kugathasan S, Judd RH, Hoffmann RG, Heikenen J, Telega G, Khan F, et al. Epidemiologic and clinical characteristics of children with newly diagnosed inflammatory bowel disease in Wisconsin: a statewide population-based study. J pediatr, 2003; 143(4):525–31. CrossRef
Nightingale AJ, Middleton W, Middleton SJ, Hunter JO. Evaluation of the effectiveness of a specialist nurse in the management of inflammatory bowel disease (IBD). Eur J Gastroenterol Hepatol, 2000; 12(9):967–73. CrossRef
Orscheln S. Curcumin as an aid to improve symptoms of inflammatory or irritable bowel diseases, School of Physician Assistant Studies, Portland,OR, 2018.
Pandey P, Purohit D, Dureja H. Nanosponges–A promising novel drug delivery system. Recent Pat Nanotechnol, 2018; 12(3):180–91. CrossRef
Pithadia AB, Jain S. Treatment of inflammatory bowel disease (IBD). Pharmacol Rep, 2011; 63(3):629–42. CrossRef
Shanahan F. Inflammatory bowel disease: immunodiagnostics, immunotherapeutics, and ecotherapeutics. Gastroenterology. 2001; 120(3):622–35. CrossRef
Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer, 2005; 41(13):1955–68. CrossRef
Shivani S, Poladi KK. Nanosponges-novel emerging drug delivery system: a review. Int J Pharm Sci Res, 2015; 6(2):529.
Tønnesen HH, Karlsen J. Studies on curcumin and curcuminoids. Zeitschrift für Lebensmittel-Untersuchung und Forschung. 1985; 180(5):402–4. CrossRef
Vucelic B. Inflammatory bowel diseases: controversies in the use of diagnostic procedures. Digestive Diseases, 2009; 27(3):269–77. CrossRef
Zhou H, S Beevers C, Huang S. The targets of curcumin. Curr Drug Targets, 2011; 12(3):332–47. CrossRef