INTRODUCTION
Carcinoma of the breast is the second leading cause of cancer and is the fifth cause of death from cancer (Ferlay et al., 2015; Momenimovahed and Salehiniya, 2019). Breast cancer is a collective term used to describe different types of neoplastic diseases affecting the mammary gland (Sainsbury et al., 2000). Clinically relevant breast cancer typically derives from epithelial cells and thus termed as carcinoma (Makki, 2015).ER+ (estrogen receptor alpha positive) and PR+ (progesterone receptor alpha positive) breast cancer account for 60%–70% of human breast cancer. Less common cancer like human epidermal growth factor receptor 2 and triple-negative cancers are more aggressive and difficult to treat (Yersal and Barutca, 2014). Since many drugs used to treat cancer are obtained from plants, searching for new molecules which can battle cancer cells in a better way is a goal of researchers in the field
The root of Ophiorrhiza mungos is traditionally used for cancer treatment in Ayurveda (Warrier and Nambiar, 1994). The whole plant of O. mungos is a source of monoterpene indole alkaloid camptothecin having a potent anticancer activity (Gharpure et al., 2010). The presence of forty-seven species and nine varieties of the genus Ophiorrhiza was reported in the Indian subcontinent (Deb and Mondal, 2001). Out of this, 16 species and three varieties are reported from Southern Western Ghats (Sasidharan, 2004). Ophiorrhiza eriantha Wight is an erect subshrub belonging to the family Rubiaceae. high-performance thin layer chromatography densitometry-based quantification of camptothecin in methanol extract of the whole plant of O. eriantha Wight is done by Satheeshkumar et al. (2012). Jose and Satheesh Kumar (2004) developed a method for micro-propagation of O. eriantha Wight through leaf explant cultures. After an extensive literature review, it was found that no other research works were conducted on O. eriantha Wight.
Apoptosis is the major defense strategy of the cells from being cancerous (Kerr et al., 1972). Regulation of apoptosis is controlled by initiator caspases and executioner caspases (Olsson and Zhivotovsky, 2011). In this study, we selected two plants O. mungos L. and O. eriantha Wight, roots and aerial parts were separated, and sequentially extracted with petroleum ether, ethyl acetate, and ethanol. A preliminary anticancer screening study of all the 12 extracts was done on daltons lymphoma ascites (DLA) cell lines. Among them, the petroleum ether fraction of the aerial part of the O. eriantha Wight shows the maximum activity. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay of petroleum ether fraction on Michigan Cancer Foundation-7 (MCF-7) cell lines was conducted and shows significant activity. AO/EB staining is used to study nuclear changes and apoptotic body formations and the results show significant activity. Analysis of Bcl-2, Bax, Caspase-7, Caspase-9, and p53 was performed using the western blot method and the petroleum ether fraction of the aerial part shows significant activity. DNA fragmentation assay and Flow cytometry analysis show that petroleum ether fraction of aerial part of O. eriantha Wight has significant apoptotic activity against breast cancer cell lines.
MATERIALS AND METHODS
Plant materials
The whole plant of O. eriantha Wight (voucher specimen number PARC/2020/4207) and the whole plant of O. mungos L. (voucher specimen number PARC/2018/4018) were collected from Palode, Trivandrum district of Kerala, India and authenticated by Prof. Jayaraman, Director, Plant anatomy research center, West Tambaram, Chennai. The roots and aerial parts were separated, shade dried, and powdered by using an electric blender.
Extraction
40 grams of powdered roots and aerial part of O. eriantha Wight and O. mungos were separately packed in Soxhlet extractor and extracted sequentially with petroleum ether (60–80), Ethyl acetate, and Ethanol. Petroleum ether (60–80) and Ethyl acetate were purchased from Merck and absolute alcohol was purchased from Travancore sugars and Chemicals, Thiruvalla, Kerala (distilled to remove impurities). All the 12 extracts were evaporated to dryness under reduced pressure and stored at 0°C–4°C until for further use. All the 12 extracts were given the following codes. Ophiorrhiza eriantha root petroleum ether, O. eriantha root ethyl acetate, O. eriantha root ethyl alcohol. Ophiorrhiza O. eriantha aerial petroleum ether (OEAPE), O. eriantha aerial ethyl acetate, O. eriantha aerial ethyl alcohol, O. mungos root petroleum ether, O. mungos root ethyl acetate, O. mungos root ethylalcohol. O. mungos aerial petroleum ether, O. mungos aerial ethyl acetate, O. mungos aerial ethyl alcohol.
Short term in vitro cytotoxicity study using DLA cells
The Daltons Lymphoma Ascites cells were obtained from Amala Cancer Research Centre, Thrissur, Kerala, washed thrice with phosphate buffer solution. Trypan blue exclusion method is used for determining Cell viability (Kanagamani et al., 2017). Cells were treated with various concentrations of all the 12 extracts of O. eriantha Wight and O. mungos.
MTT assay
MCF-7 cell line (Passage number 30), was procured from National centre for Cell Sciences, Pune, India (Vijayarathna and Sasidharan, 2012). Stock cell was then cultured and maintained in Dulbecco’s modified eagle medium (DMEM) supplemented with 10% FBS, 100 μg/ml streptomycin, 100 U/ml penicillin, and incubated at 37°C with 5% Co2. MTT assay is used to estimate cytotoxicity. In a 48 well plate, approximately 1 ×105/ml cells were seeded, with complete growth media (DMEM) and allowed to attach. Added 5,10,25,50, and 100 μg/ml of OEAPE. After 48 hours of incubation, in fresh medium, 20 μl of 0.5% MTT were added, again incubated for 4 hours. By measuring absorbance at 570 nm, the percentage cell viability was calculated using the following formula
Acridine orange/ethidium bromide (AO/EB) double staining
AO/EB double staining is used to study characteristic features observed during apoptosis (Nath et al., 2014). MCF-7 cells were treated with 5, 10, and 20 μg/ml of OEAPE. Acridine orange stains all cells (live and dead). Ethidium bromide stains only dead cells. The viable cells appear uniformly green. Cells in the early stages of apoptosis are observed light green and late stages appears in orange to red color.
Western blotting, flow cytometry
MCF-7 cells were treated with 5, 10, and 20 μg of OEAPE for 48 hours. Cells solubilized with lysis buffer. The protein lysates were separated by 12% sodium dodecyl-sulfate polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Diaminobenzidine is used for developing color (Bai et al., 2020). Flow cytometry is a method for studying the cell cycle. Cellular DNA content can be measured and it gives the correct picture of cells in different phases of the cell cycle (Mousavi et al., 2009). MCF-7 cells were treated with 0, 5, 10, and 20 μg/ml of OEAPE for 48 hours. Cells were harvested and incubated. Cell death was quantified using a flow cytometer. Data were analyzed with CellQueste software.
DNA fragmentation assay
DNA fragmentation is a characteristic feature of programmed cell death. Cleavage pattern of DNA due to cytotoxic effect of OEAPE was analyzed using agarose gel electrophoresis (Fakai, 2019) MCF-7 Cell line obtained from National Centre for Cell Science, Pune, India. It is then treated with control, 5, 10 and 20 μg/ml of OEAPE and incubated for 24hours.
RESULT AND DISCUSSION
Figure 1 represents the results of short-term invitro cytotoxicity using DLA cells. Among all the 12 extracts of aerial and root part of O. eriantha Wight and O. mungos, petroleum ether fraction of aerial part of O. eriantha Wight is the most active fraction (IC50 21.954 μg/ml). Since O. mungos is a known anticancer plant and a source of camptothecin, better activity for O. eriantha Wight compared to O. mungos was surprising, and we selected OEAPE for further study. MTT assay of OEAPE were conducted on MCF-7 human breast cancer cell line and the activity compared with quercetin. Tables 1 and 2 shows the percentage cell death by OEAPE and Quercetin on MCF-7 cell line. Figure 2 represents significant anti breast cancer activity by OEAPE on MCF-7 cell line (IC50 23.92 μg/ml).
After confirming the anti-breast cancer activity, acridine orange/ethidium bromide assay, western blot analysis of Bcl-2, BAX, P53, Caspase 7, Caspase-9 were conducted for studying the apoptotic process. AO/EB staining is an excellent method for studying nuclear changes happening during apoptosis. Figure 3 represents the effect of control, 5, 10, and 20 μg/ml OEAPE on MCF-7 human breast cancer cells. Cells treated with 20 μg/ml of extract show the decreased percentage of live cells when compared to control cells.Figure 4 represents the percentage live cells against different concentration of OEAPE. Live cells and apoptotic cells can be easily distinguished by the percentage uptake of AO/EB. Live cells appear green in color due to permeation of acridine orange. Ethidium bromide permeates only when cells lose their membrane integrity, and hence dead cells appear red and the apoptotic nucleus stains orange.
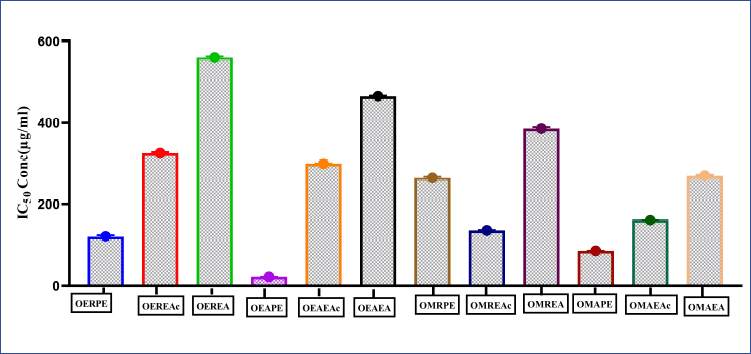 | Figure 1. Short term in vitro cytotoxicity study using daltons lymphoma ascites (DLA) cells, of various extracts of O. eriantha Wight and O. mungos L. Data analysis was performed using Graph pad prism 8, values were denoted by m ea n ± SD. Statistical difference was found by One way analysis of variance (ANOVA) followed by Dunnet’s Test among groups. [Click here to view] |
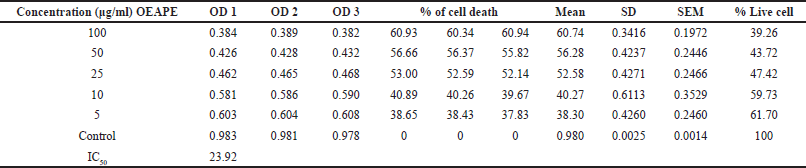 | Table 1. MTT assay on MCF-7 cell line. Percentage cell death by OEAPE. [Click here to view] |
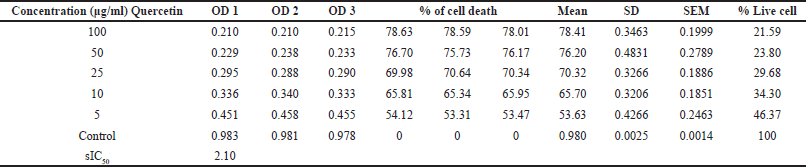 | Table 2. MTT assay on MCF-7 cell line. Percentage cell death by Quercetin. [Click here to view] |
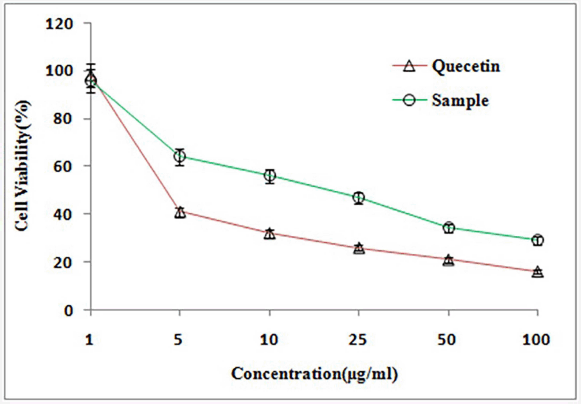 | Figure 2. MTT Assay on MCF-7 cell line. [Click here to view] |
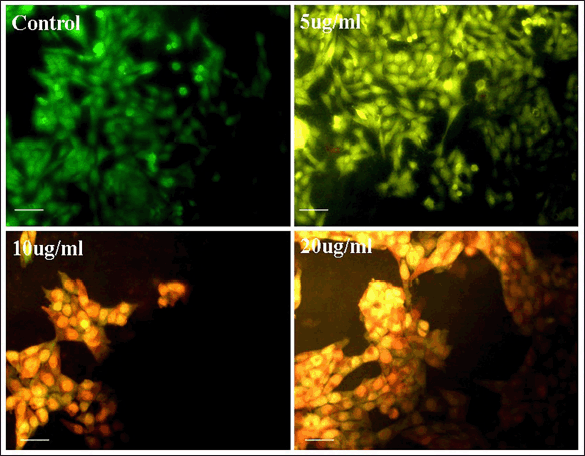 | Figure 3. Images of acridine/ethidium bromide staining. [Click here to view] |
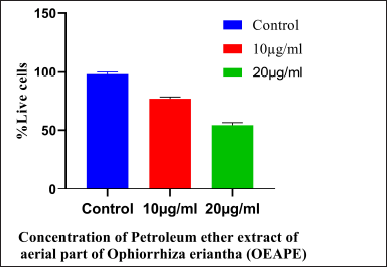 | Figure 4. Acridine orange/ethidium bromide staining. Concentration of OEAPE. Data analysis was performed using Graph pad prism 8, values were denoted by mean ± SD. Statistical difference was found by One way ANOVA followed by Dunnet’s Test among groups. The values were compared with control group (*p <0.05, **p <0.005, ***p < 0.001). As compared with control group 20 μg/ml of OEAPE showed decreased percentage of live cells (p < 0.001). [Click here to view] |
The intrinsic mechanism of apoptosis which occurs in mitochondria are controlled and regulated by proteins of Bcl-2 family (Cory and Adams, 2002). Bcl-2 proteins plays major role in regulation of permeability of the mitochondrial membrane. Bcl-2, Bcl-x, Bcl-XL, Bcl-XS, Bcl-w, and BAG are anti-apoptotic in nature and Bcl-10, Bax, Bak, Bid, Bad, Bim, Bik, and Blk are pro-apoptotic (Siddiqui et al., 2015). p53 has an important role in regulation of the Bcl-2 family of proteins. Bax proteins induce the release of cytochrome c from the mitochondrial intermembrane space into the cytosol which triggers caspase cascade (Gogvadze et al., 2006). Figure 5 represents results of western blot analysis. The Bax/Bcl2 ratio plays a major role in the regulation of the mitochondrial apoptotic pathway (Salakou et al., 2007). Increase in the ratio of Bax (pro-apoptotic protein) expression to Bcl-2 (antiapoptotic protein) expression indicates apoptosis. Western blot analysis of pro-apoptotic Bax protein shows significant elevation after treatment with 20 μg OEAPE (Fig. 6). Bcl-2 concentration is significantly reduced upon treatment with 20 μg OEAPE (Fig. 7). There was significant difference in Bax /Bcl-2 ratio between control and OEAPE-treated cells.
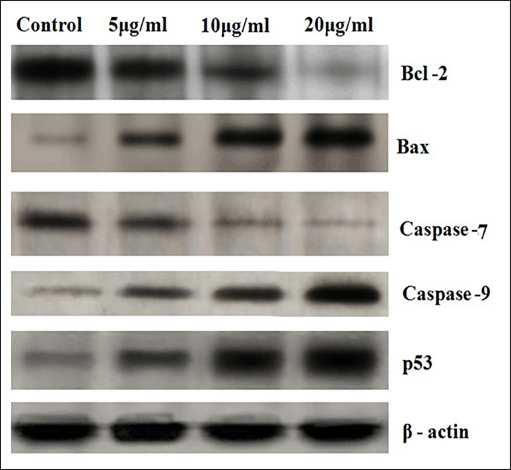 | Figure 5. Western Blot analysis. [Click here to view] |
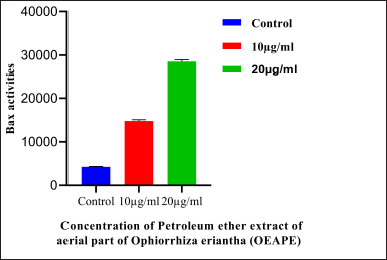 | Figure 6. Western blot analysis of Bax protein. Concentration of OEAPE. Data analysis was performed using Graph pad prism 8, values were denoted by mean ± SD. Statistical difference was found by one way ANOVA followed by Dunnet’s test among groups. The values were compared with control group (*p <0.05, **p <0.005, ***p <0.001). As compared with control group 20 μg/ ml of OEAPE showed significant increase in Bax Activity (p <0.001). [Click here to view] |
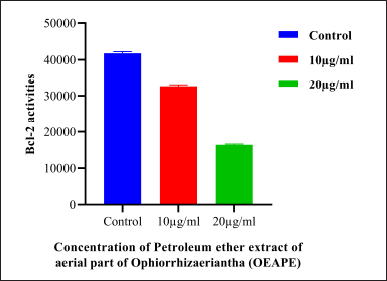 | Figure 7. Western Blot analysis of Bcl-2 protein. Concentration of OEAPE. Data analysis was performed using Graph pad prism 8, values were denoted by m ea n ± SD statistical difference was found by One way ANOVA followed by Dunnet’s Test among groups. The values were com pa red with control group (*p < 0.05, **p < 0.005, ***p < 0.001). As compared with control group 20 μg/ml of OEAPE showed significant reduction in Bcl-2 activity (p < 0.001). [Click here to view] |
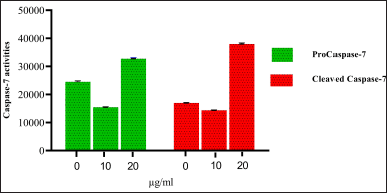 | Figure 8. Western Blot analysis of Caspase-7 activities petroleum ether extract of OEAPE. Concentration of petroleum either extract of OEAPE. Data analysis was performed using Graph pad prism 8, values were denoted by mean ± SD. Statistical difference was found by One way ANOVA followed by Dunnet’s Test among groups. The values were compared with control group (*p <0.05, **p <0.005, ***p <0.001). As compared with control group 20 μg/ml of OEAPE showed increased caspase-9 activity (p <0.001). [Click here to view] |
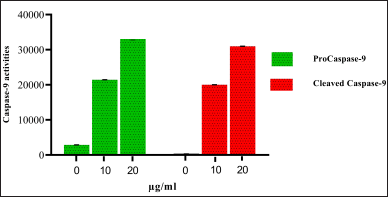 | Figure 9. Western Blot analysis of Caspase-9 activities OEAPE. Concentration of OEAPE. Data analysis was performed using Graph pad prism 8, values were denoted by mean ± SD. Statistical difference was found by One way ANOV A followed by Dunnet’s Test among groups. The values were compared with control group (*p <0.05, **p <0.005, ***p <0.001). As compared with control group 20 μg/ml of OEAPE showed increased caspase-9 activity (p <0.001). [Click here to view] |
Caspases are a family of cysteine aspartate proteases. On treatment with different concentrations of OEAPE, at 20 μg/ml of OEAPE shows a significant increase in caspase-7 and caspase-9 activities. Figures 8 and 9 shows the concentrations of pro caspase and cleaved caspase at control, 10 and 20 μg/ml OEAPE.
Flow cytometry is a method for studying the cell cycle. Cellular DNA content can be measured and it gives correct picture of cells in different phases of the cell cycle. Different stages of cell cycle are stoichiometrically stained with Propidium Iodide which allows differentiation cells in G1, S, and G2/M phase. MCF-7 Cells were incubated with 0, 5, 10, and 20 μg/ml of OEAPE. The flow cytometry analysis (Fig. 10) shows that the 10 and 20 μg/ml of OEAPE arrested the cell cycle in G2/M phase after 24 hours.
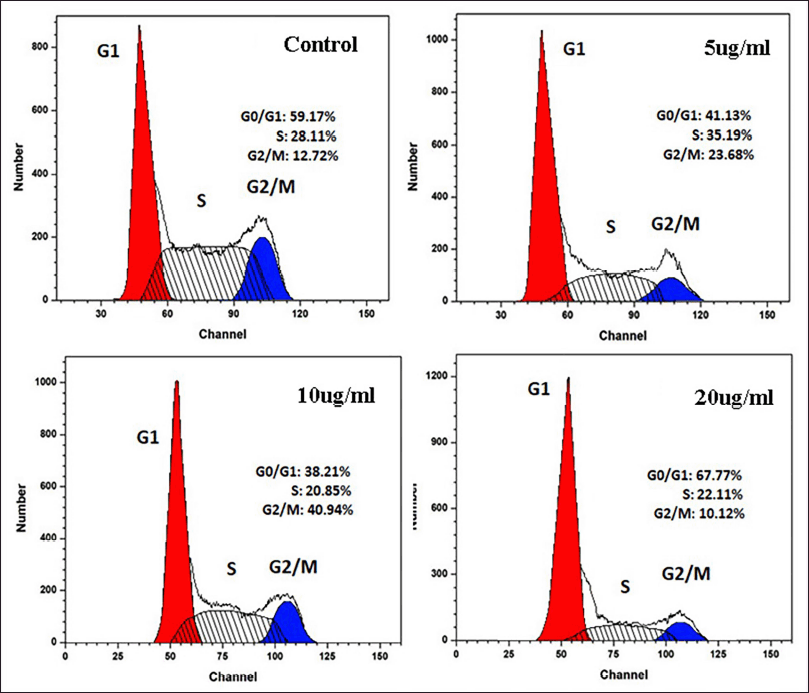 | Figure 10. Flow cytometry analysis. [Click here to view] |
Natural DNA fragmentation is a landmark for apoptosis. The DNA isolated from MCF-7 cells upon treatment with various concentrations of OEAPE for 24 hours, a typical ladder pattern of inter nucleosomal fragmentation was observed (Fig. 11). When MCF-7 cells were treated with 20 μg/l, OEAPE shows significant action on DNA. These results indicate that the OEAPE caused DNA fragmentation in MCF-7 cells.
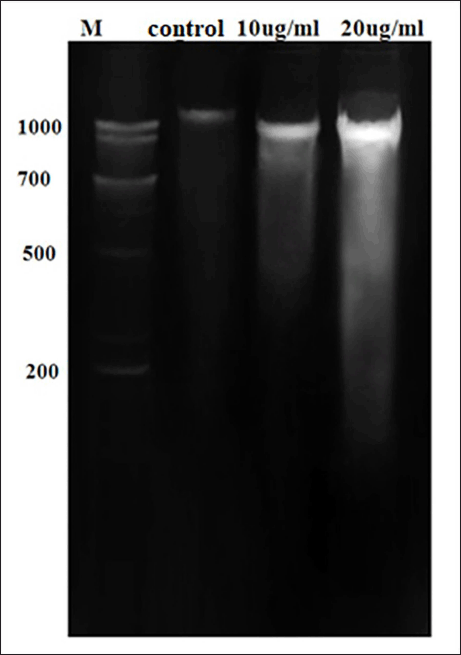 | Figure 11. DNA fragmentation analysis. [Click here to view] |
CONCLUSION
In the present study, we compared the anticancer activity of O. eriantha Wight with O. mungos. It was a surprise that the OEAPE showed better activity compared to the known anticancer plant O. mungos. The analysis of the results clearly indicates that the OEAPE induce apoptosis on breast cancer MCF-7 cell line.
ACKNOWLEDGMENT
The authors are very thankful to Dr. K. B. Ramesh Kumar, Dr. Druvan T, Dr. E. S. Santhosh kumar and other scientists, Jawaharlal Nehru Tropical Botanic Garden and Research Institute, Palode, Trivandrum for their help in selection and identification of the plant for study. The authors are also thankful to Prof P. Jayaraman, Director, Institute of Herbal Botany, Plant Anatomy Research Centre, Chennai.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included within this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
FUNDING
There is no funding to report.
REFERENCES
Bai HL, Kang CM, Sun ZQ, Li XH, Dai XY, Huang RY, Zhao JJ, Bei YR, Huang XZ, Lu ZF and Wu SG. TTDA inhibited apoptosis by regulating the p53-Bax/Bcl2 axis in glioma. Exp Neurol, 2020; 1(331):113380. CrossRef
Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer, 2002; 2(9):647–56. CrossRef
Deb ADB, Mondal DC. Taxonomic revision of the genus Ophiorrhiza L (Rubiaceae) in Indian subcontinent. Bull Bot Surv Ind, 2001; 39:1–148.
Fakai MI, Abd Malek SN and Karsani SA. Induction of apoptosis by chalepin through phosphatidylserine externalisations and DNA fragmentation in breast cancer cells (MCF-7). Life Sci, 2019; 1(220):186–93. CrossRef
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer, 2015; 136(5):E359–86. CrossRef
Gharpure G, Chavan B, Lele U, Hastak A, Malpure N and Patwardhan A. Camptothecin accumulation in Ophiorrhiza rugosa var prostrata from Northern Western Ghats. Curr Sci, 2010; 98(3):302–4.
Gogvadze V, Orrenius S and Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta Bioenerg, 2006; 1(1757)(5–6):639–47. CrossRef
Jose B, Satheesh kumar K. In vitro mass multiplication Ophiorrhiza mungos Linn. Indian J Exp Biol, 2004; 42(6):639–42.
Kanagamani K, Muthukrishnan P, Ilayaraja M, Kumar JV, Shankar K and Kathiresan A. Synthesis of Leucaena mediated silver nanoparticles: assessing their photocatalytic degradation of Cr (VI) and in vitro cytotoxicity against DLA cells. J Photochem Photobiol A, 2017; 1(346):470–8. CrossRef
Kerr J, Wyllie A and CurrieA. Apoptosis: a basic biological phenomenon with wide ranging implications in tissue kinetics. Br J Cancer, 1972; 26:239–257. CrossRef
Makki J. Diversity of breast carcinoma:histological subtypes and clinical relevance. Clin Med Insights Pathol, 2015; 8:CPath–S31563. CrossRef
Momenimovahed Z, Salehiniya H. Epidemiological characteristics of and risk factors for breast cancer in the world. Breast Cancer Targets Ther, 2019; 11:151-64 CrossRef
Mousavi SH, Tavakkol-Afshari J, Brook A and Jafari-Anarkooli I. Direct toxicity of rose bengal in MCF-7 cell line: role of apoptosis. Food Chem. Toxicol, 2009; 1(47)(4):855–9. CrossRef
Nath M, Vats M and Roy P. Design, spectral characterization, anti-tumor and anti-inflammatory activity of triorganotin (IV) hydroxycarboxylates, apoptosis inducers: in vitro assessment of induction of apoptosis by enzyme, DNA-fragmentation, acridine orange and comet assays. Inorg Chim Acta, 2014; 1(423):70–82. CrossRef
Olsson M, Zhivotovsky B. Caspases and cancer. Cell Death Differ, 2011; 18(9):1441–9. CrossRef
Sainsbury JR, Anderson TJ, Morgan DA. Breast cancer. Br Med J, 2000; 23(321)(7263):745–50. CrossRef
Salakou S, Kardamakis D, Tsamandas AC, Zolota V, Apostolakis E, Tzelepi V, Papathanasopoulos P, Bonikos DS, Papapetropoulos T, Petsas T and Dougenis D. Increased Bax/Bcl-2 ratio up-regulates caspase-3 and increases apoptosis in the thymus of patients with myasthenia gravis. In vivo, 2007; 21(1):123–32.
Sasidharan N. Biodiversity documentation for Kerala. Flowering plants. Kerala forest research institute, Peechi, India, 2004.
Satheeshkumar K, Baby S, Venkataraman R, Kurup R, Gopalakrishnan, and R. Rajan. Search for camptothecin-yielding ophiorrhiza species from southern Western Ghats in India: a HPTLC-densitometry study. Ind Crops Prod, 2012; 43:472–6. CrossRef
Siddiqui WA, Ahad A and Ahsan H. The mystery of BCL2 family: Bcl-2 proteins and apoptosis: an update. Arch Toxicol, 2015; 1(89)(3):289–317. CrossRef
Vijayarathna S, Sasidharan S. Cytotoxicity of methanol extracts of elaeis guineensis on MCF-7 and vero cell lines. Asian Pac J Trop Biomed, 2012; 1(2)(10):826–9. CrossRef
Warrier PK. Nambiar VPK. Indian medicinal plants: a compendium of 500 species, volume 4. 1st ed, Orient Longman, Hyderabad, India, pp 180–1, 1994.
Yersal O, Barutca S. Biological subtypes of breast cancer: prognostic and therapeutic implications. World J Clin Oncol, 2014; 5(3):412. CrossRef