INTRODUCTION
Free radicals, atoms, or molecules that are reactive due to the possession of unpaired electrons exist in the human body as by-products of adenosine triphosphate (ATP) production in the form of reactive oxygen species (ROS) and reactive nitrogen species (Liguori et al., 2018; Pham-Huy et al., 2008). The existence of the highly unstable reactive oxygen and nitrogen species (RONS) not only comes as a result of ATP production but also comes from external sources, such as water pollution, air pollution, alcohol, tobacco, food, and radiation (Liguori et al., 2018). It was well-known that a moderate amount of RONS is useful for a cellular response, such as reduction–oxidation regulation, for protein activation (DrÓ§ge, 2002; Kim et al., 2002); however, a high amount of RONS is known to cause oxidative stress. This oxidative stress could result in cellular damage by oxidizing lipid in the membrane, thus disrupting the cellular structure (Pham-Huy et al., 2008), as well as inducing abstraction and addition reaction to the DNA structure, which alters the gene expression (Dizdaroglu et al., 2002; Kumar et al., 2012).
Oxidative stress could cause oxidative modification which results in the damage of cellular macromolecules, such as DNA, proteins, lipids, and carbohydrates (Liguori et al., 2018). Prolongation of the damage could increase the risk of several chronic diseases, such as cancer, autoimmune diseases, cardiovascular diseases, neurodegenerative diseases, mental disorders, and skin aging (Pham-Huy et al., 2008). In order to minimize the damage, antioxidants are needed.
Antioxidants are compounds that are able to stabilize free radicals by a mechanism of hydrogen atoms donation, inhibition of low-density lipoprotein (LDL) oxidation, and chelation of metal ions. Stabilization of free radicals by antioxidants could prevent and repair DNA damage (Pham-Huy et al., 2008; Santos-Sánchez et al., 2019). In a state of oxidative stress, the body is incapable of producing an adequate amount of antioxidants to neutralize free radicals; therefore, exogenous antioxidants are needed to overcome oxidative stress. One of the sources for antioxidants is from the consumption of plants containing antioxidant compounds. Phenolic and flavonoids compounds that are easily found in vegetables, fruits, and legumes are some of the phytochemicals that are known for their antioxidant activity (Santos-Sánchez et al., 2019).
Mangosteen (Garcinia mangostana) is a tropical fruit whose biological activities, such as antimicrobial activity (Chomnawang et al., 2005), antidiabetic activity (Taher et al., 2016), antitumor activity (Nakagawa et al., 2007), anti-inflammatory activity (Chen et al., 2008), and antioxidant activity (Weecharangsan et al., 2006), have been extensively studied. Among these studies, its antioxidant activity is the one receiving prominent interest. Several studies have confirmed that the administration of the mangosteen extract could help in improving the condition of oxidant-related diseases, such as diabetes, hyperlipidemia, neurological disorders, skin aging, acne, and others (Huang et al., 2014; Im et al., 2017; Leontowicz et al., 2006; Nelli and Kilari, 2013). Due to these findings, patents and commercialization of several mangosteen-based products, such as Verve®, Vemma®, and Mastin®, have recently progressed. However, despite the commercialization, conflicting results of the study about their antioxidant effect in various disease models are still discovered. In addition, an extensive review that summarizes the antioxidant effect of mangosteen products toward oxidant-related diseases was not available. Due to these factors, a systematic review is needed to properly assess the effectiveness of mangosteen antioxidant activity in alleviating oxidant-related diseases in various clinical and in vivo study models. Hence, this study aimed to carry out a systematic review to evaluate scientific evidence regarding the antioxidant activity of mangosteen on animal models and clinical trials in relation to its role in improving the pathology of the related diseases.
MATERIALS AND METHODS
The protocol of this study was based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist (Moher et al., 2009).
Search strategies
Databases that were used were PubMed, PubMed Central (PMC), Cochrane Library, ScienceDirect, and Google Scholar up to 30th June 2020. Keywords that were used for searching the studies were “mangosteen”, “manggis”, “Garcinia mangostana”, “oxidative stress”, “antioxidant”, “oxidant-related disease”, “cardiovascular disease”, “atherosclerosis”, “diabetes”, “neurological disorder”, “cancer”, “acne”, and “skin aging”. The search was restricted to articles that were published in English and Indonesian.
Inclusion and exclusion criteria
Screening and selection of included studies were carried out by two investigators (BE and PH) independently based on the inclusion and exclusion criteria. Studies that were included in this review were clinical and animal studies published in a peer-reviewed journal and indexed in either SCOPUS or Web of Science and which evaluated the mangosteen fruit antioxidant activity with an outcome related to the antioxidant power, including but not limited to the changes in the level of total antioxidant capacity, catalase (CAT), superoxide dismutase (SOD), and others. In vitro studies and review papers were excluded from this study but manual screening of related review papers references was carried out for additional studies. The titles and abstracts of the studies were screened first before screening the full articles. Any disagreements were resolved through discussion between the reviewers.
Data extraction
Extracted information from the included studies was the author’s name, year of publication, type of study, subjects or disease model, sample size, intervention and comparator, treatment duration, intervention and comparator dose, route of administration, and outcomes of the study.
Quality assessment
Quality of the clinical trial studies was assessed using Downs and Black’s (1998) checklist with evaluated parameters including reporting, external validity, internal validity (bias), internal validity (confounding), and power. For in vivo studies, the quality assessment was carried out using ToxRTool with evaluated parameters including test intervention identification, test organism characterization, study design description, study results documentation, and plausibility of study design and results (Schneider et al., 2009). Quality assessment was carried out independently by two investigators (BE and PH). Any disagreements were resolved through discussion between the reviewers.
RESULTS AND DISCUSSION
Study selection
The total amount of related articles that was obtained from PubMed, PMC, Cochrane Library, ScienceDirect, and Google Scholar searches was 251 articles, 968 articles, 25 articles, 1,786 articles, and 5,982 articles, respectively. The checking of article duplications was conducted using Mendeley, while the abstract and full-text screening based on the inclusion and exclusion criteria was carried out by both the authors individually and manually. The final screening of articles resulted in a total of 47 included articles (41 articles for in vivo studies and 6 articles for clinical trials). The process of study screening and selection is detailed in Figure 1.
Quality assessment
Clinical trial studies’ quality was assessed using Downs and Black’s checklist (Supplementary Data Table 1). Two of them showed poor quality (Suthammarak et al., 2016; Kondo et al., 2009), while two others showed fair quality (Xie et al., 2015a, 2015b), and the rest showed good quality (Baroroh et al., 2018; Sutono, 2013). The poor quality score of the studies was due to insufficient information regarding the characteristics of patients who dropped out of the trials and lack of external validity of the subject population. The qualities of in vivo studies were assessed using ToxRTool and all studies showed reliable quality without restriction needed (Supplementary Data Table 2).
 | Figure 1. Flowchart of studies’ screening and selection. [Click here to view] |
Clinical trials
A total of six studies were conducted using human subjects to evaluate the antioxidant activity of mangosteen through the oral route (Table 1). Among the six studies, two studies used mangosteen pericarp extract as the intervention (Baroroh et al., 2018; Suthammarak et al., 2016), while the other four studies used commercial mangosteen supplement as the intervention (Kondo et al., 2009; Sutono, 2013; Xie et al., 2015a; 2015b). The result of the intervention showed that antioxidant capacity in plasma and red blood cells (RBC) increased after the administration of mangosteen extract or products (Kondo et al., 2009; Suthammarak et al., 2016; Xie et al., 2015a; 2015b). However, unfortunately, two of the studies did not carry out adequate statistical analysis, wherein the result from the treated group was not statistically compared to the placebo group (Kondo et al., 2009; Xie et al., 2015a).
Malondialdehyde (MDA) is one of the products of lipid peroxidation and also one of the markers of oxidative stress. A high amount of MDA indicates a high level of reaction between oxygen and unsaturated lipids in the body (Ayala et al., 2014). Among the six studies, two studies evaluated the MDA level of the subjects after the intervention (Sutono, 2013; Baroroh et al., 2018). Both of them showed a decrease in MDA level; however, it was not statistically significant when compared to the control group. These results showed that either the mangosteen extract or products could not reduce the MDA level or the dose administered was not enough to show a significant effect on the MDA level.
Mangosteen products that were used in the studies were Verve®, Vemma®, and mangosteen rind extract capsule from PT and Sido Muncul (Kondo et al., 2009; Sutono, 2013; Xie et al., 2015a, 2015b). All products showed antioxidant activity in human subjects by increasing plasma antioxidant capacity and reducing the MDA level. However, ingredients contained in the products, such as vitamin C, vitamin E, and green tea extracts, might also contribute to the antioxidant activity, implying that the antioxidant activity did not solely come from mangosteen.
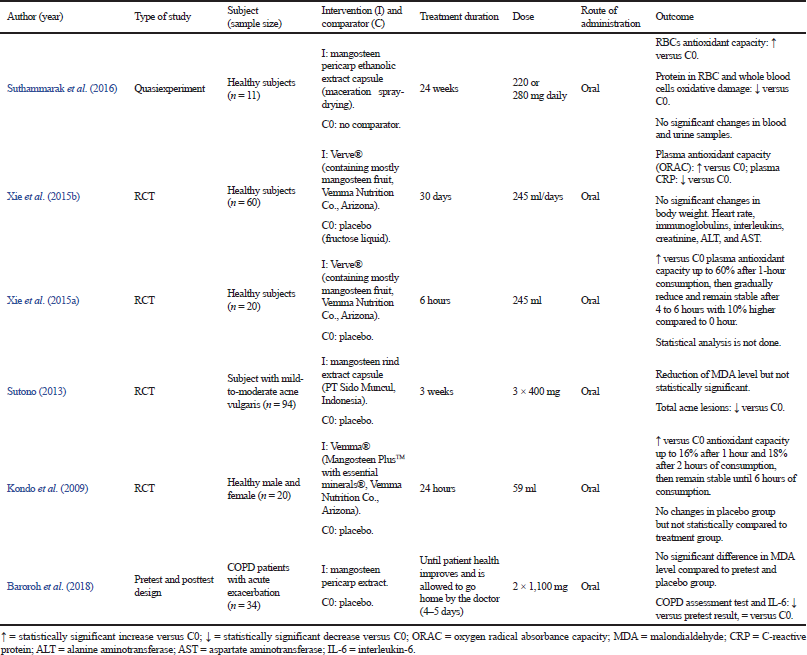 | Table 1. Data extraction of clinical studies. [Click here to view] |
In vivo animal studies
A total of 41 articles studied the antioxidant activity of mangosteen in in vivo animal models (listed in Table 2). Most of the studies used mangosteen pericarp as the intervention where 15 used the extracts, two used the dried and ground pericarp, and 17 used the isolated compounds, such as xanthone, ɑ-mangostin (AM), or ɣ-mangostin. In addition to mangosteen pericarp, five studies used mangosteen flesh as the intervention and two studies used commercial products from Lord Duke Biotechnology Company.
Nearly all the 22 studies that evaluated the MDA level as one of the oxidative stress markers showed a significant decrease after the subjects were treated with mangosteen extract or products. Out of the 22 studies, only three of them showed no significant decrease in MDA level (Herrera-Aco et al., 2019; Oberholzer et al., 2018; Subani, 2014). The negative results could be due to either different routes of administration used (Subani, 2014) or insufficient dose of mangosteen extract (Herrera-Aco et al., 2019; Oberholzer et al., 2018).
SOD is an antioxidant enzyme that is known as one of the first-line defenses in our body against oxidative stress along with CAT and glutathione (GSH) peroxidase (GPx) (Ighodaro and Akinloye, 2018). It was explained that SOD worked by dismutating the superoxide radicals into hydrogen peroxide and an oxygen molecule. The excess hydrogen peroxide was then broken down further by CAT and GPx into water and oxygen molecules in the peroxisome and mitochondria, respectively. Additionally, GPx is also responsible for converting lipid peroxides into their corresponding alcohol forms (Ighodaro and Akinloye, 2018), and it also acts as the catalyst of GSH reaction with radicals to form oxidized glutathione or glutathione disulfide before being excreted from the cells (Lushchak, 2012).
 | Table 2. Data extraction of in vivo animal studies. [Click here to view] |
Among 20 studies that evaluated SOD level, three of them showed no significant difference in SOD level (Adyab et al., 2019; Herrera-Aco et al., 2019; Zhang et al., 2016), one of them showed a significant decrease in SOD level (Chang et al., 2020), and the rest showed a significant increase in SOD level signifying increased antioxidant activity. Many studies have evaluated the antioxidant activity of different parts of the mangosteen fruit and showed that mangosteen pericarp exhibits the highest antioxidant capacity compared to mangosteen flesh and seed (Lim et al. 2013), thus explaining its low SOD level (Adyab et al., 2019; Zhang et al., 2016). A significant increase in SOD levels showed that mangosteen extract or products promote the activity of SOD enzyme in neutralizing oxidative stress. A significant decrease in the SOD level could be due to the direct activity of mangosteen extract or products in neutralizing ROS which reduces the need for an antioxidant enzyme such as SOD (Ismail et al., 2018). All studies with evaluated CAT (n = 13) and GPx (n = 10) levels showed a significant increase in both enzyme levels after the subjects were treated with mangosteen extract or product. A total of 12 studies evaluated the GSH level after administration of mangosteen extract or product and one of the studies showed no significant difference in the GSH level (Parkhe et al., 2020), while the rest showed a significant increase in GSH level.
Mangosteen antioxidant activity in type II diabetes model
Out of 41 in vivo studies, seven of them studied mangosteen antioxidant activity in the type II diabetes model obtained by either inducing the subjects (mice or rats) with streptozotocin (STZ) or STZ accompanied with a high glucose diet. Among the seven studies, five of them showed a positive correlation between the increase in mangosteen antioxidant activity and the decrease in blood glucose level (Jariyapongskul et al., 2015; Karim et al., 2018, 2019a; Kumar et al., 2016; Nelli and Kilari, 2013), one study showed a positive correlation with the increase in plasma insulin level (Karim et al., 2019b), and the other study showed improvement in pancreatic histology (Wahjuni et al., 2017).
Based on the result, the administration of mangosteen extracts showed an increase in antioxidant enzyme (SOD, CAT, or GPx) or a decrease in oxidative marker (MDA), which also correlates with a reduction in the blood glucose level, an increase in insulin level, and improvement of pancreatic histology. Induction of STZ and high glucose diet is known to cause oxidative stress and hepatotoxicity (Karim et al., 2018). Prolongation of these could damage the DNA, proteins, lipids, and other macromolecules of beta cells which then cause a reduction in insulin production, thus resulting in type II diabetes (Oberley, 1988). Phytochemicals, such as xanthone, that are contained in mangosteen are known for their antioxidant and free-radical scavenging activities (Gondokesumo et al., 2019; Sinaga and Siregar, 2016). They are capable of donating their hydrogen atoms to stabilize the free radicals and inhibit lipid peroxidation which minimizes the beta cells injury and improves their functions in regulating blood glucose level. Therefore, administration of mangosteen extracts that possess antioxidant activity could improve type II diabetes subjects’ condition.
Mangosteen antioxidant activity and cholesterol
A total of five studies evaluated mangosteen antioxidant activity and correlated them with total cholesterol levels. Two of them were tested in a type II diabetic model induced with STZ and a high-fat diet (Husen et al., 2017a, 2017b), and the rest of them were tested in an animal models fed with nonoxidized cholesterol (Haruenkit et al., 2007; Leontowicz et al., 2006, 2007). Cholesterol is often divided into high-density lipoprotein (HDL) and LDL (Elshourbagy et al., 2014). It was explained that HDL’s function is to deliver free cholesterol from peripheral cells to the liver for it to be removed, while LDL’s function is to transport cholesterol from the liver to the peripheral tissue. Around 60%–70% of the total cholesterol consists of LDL, and a high level of LDL is susceptible to oxidation and is associated with cardiovascular disease through the formation of atherosclerotic plaque (Elshourbagy et al., 2014). Administration of the mangosteen extract was able to decrease the cholesterol level by increasing antioxidant capacity which inhibits LDL oxidation, thus resulting in a low level of MDA (Husen et al., 2017a, 2017b; Leontowicz et al., 2006, 2007). However, one study showed that the administration of lyophilized mangosteen flesh did not cause a significant increase in antioxidant capacity; hence, it also did not show a significant effect on total cholesterol level (Haruenkit et al., 2007).
Mangosteen antioxidant activity in cardiovascular disease
A total of four studies evaluated mangosteen antioxidant activity in cardiovascular disease models which were myocardial infarction model (Devi Sampath and Vijayaraghavan, 2007; Sampath and Kannan, 2009), atherosclerosis model (Hafisalevi et al., 2012), and hypertension model (Boonprom et al., 2017). The results showed that the administration of AM from the mangosteen pericarp extract improved the antioxidant level [SOD, CAT, GPx, glutathione s-transferase (GST), and GSH]. It also improved the condition of myocardial infarction subjects by decreasing the lipid peroxidation level and marker enzymes [such as lactate dehydrogenase (LDH), creatine phosphokinase (CPK), glutamate pyruvate transaminase (GPT), and glutamate oxaloacetate transaminase (GOT)] and increasing respiratory chain enzyme, tricarboxylic acid cycle enzymes, and mitochondrial cytochromes (Devi Sampath and Vijayaraghavan, 2007; Sampath and Kannan, 2009).
In the atherosclerosis model, oxidation of LDL could cause atherosclerosis plaque which is one of the primary causes of cardiovascular diseases (Cervantes Gracia et al., 2017). Based on the results in Hafisalevi et al.’s (2012) study, the administration of mangosteen pericarp extract showed a significant increase in SOD antioxidants which then reduced the lipid peroxidation level (showed as MDA level), thus alleviating the atherosclerosis subjects’ condition.
For the hypertension model, subjects were induced with L-NAME that is capable of causing hypertension and cardiovascular remodeling through oxidative stress and inflammation (Boonprom et al., 2017). L-NAME is a known synthase inhibitor of nitric oxide (NO). It was explained that the inhibition of NO will induce inflammation, oxidative stress, and high blood pressure (Boonprom et al., 2017). Results showed that the administration of mangosteen pericarp aqueous extract was able to counteract the L-NAME effects by decreasing the production of superoxide radicals and plasma MDA level in L-NAME-induced mice. These decreases contributed to reducing systolic blood pressure, increasing tumor necrosis factor alpha (TNF-É‘) level, improving hemodynamic status, increasing nitrate or nitrite production, and improving cardiovascular morphology of the subjects (Boonprom et al., 2017).
Mangosteen antioxidant activity in neurological disorder
A total of eight studies tested mangosteen antioxidant activity in an animal model related to a neurological disorder. Among these, two studies used Alzheimer’s model (Avinash et al., 2016; Huang et al., 2014), while others used Parkinson’s model (Parkhe et al., 2020), depression model (Oberholzer et al., 2018), memory impairment model (Sattayasai et al., 2013), cognitive impairment model (Phyu and Tangpong, 2014), schizophrenia immune-inflammatory model (Lotter et al., 2020), and closed head injury model (Indharty et al., 2019). There are two neurological disorders that commonly occur in elderly patients, i.e., Alzheimer’s and Parkinson’s. Alzheimer’s is a condition wherein the brain structure degenerates severely enough, thus resulting in memory and cognitive impairment that interfere with daily life (Avinash et al., 2016). The hallmark of this disease is the accumulation of β-sheet aggregated amyloid peptides (Aβ) plaque in the brain parenchyma (Huang et al., 2014). Based on the results, it was shown that the administration of mangosteen extract or supplement helps in increasing antioxidant level (SOD, GSH, CAT, and GPx) and improving several Alzheimer’s parameters, such as increasing spatial learning ability, short-term memory, habituation memory, cognitive function, neurotransmission antibody [5-hydroxytryptamine (5-HT), calbindin, choline acetyltransferase (ChAT), NeuN, and tyrosine hydroxylase (TH)], and decreasing amyloid-β antibody and acetylcholinesterase (AChE) level (Avinash et al., 2016; Huang et al., 2014). Furthermore, other studies have shown that treatment with mangosteen reduced brain ROS level, as well as MDA level in brain tissue and RBC, thus improving the condition of the memory impairment model, increasing AChE level, and inhibiting neurobehavioral defects in the cognitive impairment model (Phyu and Tangpong, 2014; Sattayasai et al., 2013).
The difference between Parkinson’s and Alzheimer’s is that Parkinson’s mostly affects memory and behavioral problems, such as movement, tremor, and balance disturbance, while Alzheimer’s mostly affects memory, language, and cognitive function, such as problem-solving function and speed of thinking (Han et al., 2018). The hallmark of Parkinson’s disease is the loss of dopaminergic neurons related to the inhibition of TH activity and the presence of Lewy bodies that are made of phosphorylated a-synuclein aggregates (Parkhe et al., 2020). It was shown that treatment with AM resulted in an increase in GSH antioxidant enzyme and decrease in MDA level, which helps improve locomotor activity, memory deficiency, and TH activity and decreases phosphorylated É‘-synuclein.
Depression is a psychiatric disorder that is difficult to treat due to its complex pathophysiologies, such as dysregulated levels of monoamine, inflammation, and oxidative stress (Oberholzer et al., 2018). It was stated that treatment with mangosteen pericarp powder showed a decrease in lipid peroxidation or MDA level, as well as improvement of hippocampal tissue damage, memory recognition, and serotonergic effects. Other than depression, schizophrenia is also another type of psychiatric disorder that causes hallucination and cognitive impairment with symptoms such as low working memory, attention, and altered information processing (Lotter et al., 2020). It was explained that the pathophysiology of schizophrenia is still unclear. However, the hypothesis suggests that the interaction between genetic predisposition and stress in early life such as malnutrition and trauma could cause schizophrenia. Trauma induces inflammation and oxidative stress which causes a delay in the brain and cognitive development, thus increasing the risk of schizophrenia. Based on these, the study by Lotter et al. (2020) showed that the administration of dried ground mangosteen pericarp (GML) and AM improved parameters related to schizophrenia. The result showed no reversal observed in sensorimotor gating (psychosis-like behavior). However, it was shown that GML and AM alone or in combination with haloperidol as the standard treatment were capable of inhibiting depressive-like behavior. Also, GML and AM were shown to decrease proinflammatory cytokines [TNF-É‘ and interleukin-6 (IL-6)], while AM alone was able to decrease the MDA level, thus exhibiting their anti-inflammatory and antioxidant capabilities.
In a closed head injury model, the injury was carried out using Feeney’s weight-drop procedure by dropping a metal mass on the opened scalp on the right frontal area of a rat (Indharty et al., 2019). It was explained that traumatic brain injury causes two kinds of damage, which are the initial damage due to the physical force and the secondary damage that occurs hours or days after the initial damage due to neuroinflammatory response and oxidative stress. Results showed that mangosteen pericarp ethanolic extract improved traumatic brain injury subjects by significantly increasing the SOD antioxidant level and reducing neuronal apoptosis based on the downregulation of apoptosis-inducing factor (AIF), caspase-8, caspase-9, and MDA level (Indharty et al., 2019).
Mangosteen antioxidant activity toward liver and kidney
A total of four studies tested the mangosteen antioxidant activity toward liver in the acute liver injury model (Wang et al., 2018; Yan et al., 2018), chronic liver injury model (Wang et al., 2019), and hepatic steatosis model (Tsai et al., 2016). The acute and chronic liver injury could occur when the liver is exposed to chemical stresses, such as alcohol and drug consumption, and the difference between the two is the duration of stress exposure (Wang et al., 2018). In acute liver injury, the liver is exposed to the stress in a shorter duration compared to chronic injury. Similar to other injuries, acute and chronic liver injury induces inflammation and oxidative stress (Cichoż-Lach and Michalak, 2014). In acute liver injury, the administration of ɣ-mangostin showed an increase in SOD and GSH antioxidant levels. It also showed an improved liver condition through increasing nuclear factor erythroid 2-related factor-2 (NRF2) and sirtuin 1 (SIRT1) level (Wang et al., 2018). Meanwhile, the administration of AM increased GSH antioxidant level and inhibited inflammation, apoptosis, and autophagy (Yan et al., 2018). In chronic liver injury, the administration of ɣ-mangostin showed an increase in SOD and GSH antioxidant levels, as well as an improved liver condition through an increase in sirtuin 3 (SIRT3) level and a decrease in high mobility group box 1 (HMGB1) level, reducing the accumulation of collagen I and ɑ-smooth muscle actin (ɑ-SMA) in a chronic liver injury model (Wang et al., 2019).
Hepatic steatosis is a condition wherein there is an accumulation of triglyceride in the hepatocytes which usually occur due to disrupted lipid metabolism by hepatocytes that cause lipid peroxidation and inflammation. It is also known as a hallmark for non-alcohol fatty liver disease. The administration of mangosteen pericarp showed that it was able to reverse hepatic steatosis by increasing the level of antioxidants (GSH, GPx, SOD, and CAT) and decreasing lipid peroxidation levels, plasma-free fatty acids, and hepatic triglyceride levels (Tsai et al., 2016).
A total of two studies evaluated mangosteen antioxidant activity toward the kidneys in the chronic kidney disease model induced with lead acetate (Rana et al., 2020) and nephrotoxicity model induced with cisplatin (Pérez-Rojas et al., 2009). In the chronic kidney disease model, the administration of xanthones from mangosteen pericarp showed an increase in SOD antioxidant levels and inhibited inflammation and apoptosis (Rana et al., 2020). In the nephrotoxicity model, the administration of AM increased GPx, GSH, and CAT antioxidant levels which were known to inhibit oxidative stress, nitrosative stress, inflammation, and fibrotic pathway (Pérez-Rojas et al., 2009).
Mangosteen antioxidant activity toward a stress-induced model
A total of five studies evaluated mangosteen antioxidant activity in animal models that were exposed to stress inducers, which include organophosphate (Wihastuti et al., 2015), alcohol (Zhang et al., 2016), exercise (Chang et al., 2020), ultraviolet B (UVB), radiation (Im et al., 2017), and oxidized palm oil as an unhealthy diet model (Febriane et al., 2015). Organophosphate is a common pesticide used for crops and accumulation of organophosphate could cause toxicity through inflammation, oxidative stress, and increase in lipid peroxidation. Additionally, organophosphate toxicity is also capable of inhibiting AChE activity which causes headache, nausea, and muscle spasm, and therefore could lead to paralysis (Wihastuti et al., 2015). In the study, the administration of mangosteen pericarp ethanolic extract showed an increased in the level of AChE and a decreased level of oxidized LDL.
High consumption of alcohol is known to cause liver injury because most of the alcohol is metabolized in the liver with several responsible enzymes, such as alcohol dehydrogenase (ADH), which convert alcohol into acetaldehyde and aldehyde dehydrogenase (ALDH) that in turn converts acetaldehyde into acetate (Zhang et al., 2016). It was explained that alcohol and acetaldehyde itself could result in a decrease in antioxidant activity with symptoms such as nausea, vomiting, rapid pulse, and lightheadedness. Administration of mangosteen flesh juice did not show any significant difference in alcohol, acetaldehyde, ADH, ADLH, and SOD antioxidant concentration; however, it did show a significant decrease in MDA level (Zhang et al., 2016). The ineffective result of this study might be due to the antioxidant amount in the mangosteen flesh that is not high enough to show a significant result compared to the other parts of the fruit, such as the pericarp (Lim et al. 2013).
Extensive exercise for a long duration could also significant muscle damage, oxidative stress, and fatigue (Chang et al., 2020). It was stated that the administration of mangosteen supplements showed the ability to alleviate muscle fatigue by decreasing the MDA level, increasing antioxidant level, and lactate clearance. UVB radiation is common stress especially for people who enjoy outdoor activity. Exposure to UVB radiation is known to cause oxidative stress and inflammation which increases skin degeneration (Im et al., 2017). It was observed that the administration of AM could inhibit UVB-induced skin wrinkles, increase the antioxidant level, and decrease proinflammatory cytokines.
Meanwhile, in the unhealthy diet model, the animals were fed with oxidized palm oil and treated with mangosteen pericarp methanolic extract, with or without microencapsulation to mask the extract taste (Febriane et al., 2015). The result showed that the administration of mangosteen extract significantly reduced the MDA level, with higher reduction observed in the group treated with microencapsulated mangosteen extract.
Mangosteen antioxidant activity toward other disease models
Several studies have also evaluated mangosteen antioxidant activity in a malarial model (Tjahjani et al., 2019), prostatic hyperplasia model (Tsai et al., 2020), infertility model (Subani, 2014), arthritis model (Herrera-Aco et al., 2019), obese model (Adyab et al., 2019), and as a supplement in healthy subjects (Samuagam et al., 2015). In the malarial model, mice were inoculated with Plasmodium berghei and treated with ethyl acetate fraction of mangosteen pericarp (Tjahjani et al., 2019). Results showed that mangosteen fraction significantly decreases the parasitemia level and increases the total antioxidant level. In the prostatic hyperplasia model, rats were induced with 3,2′-dimethyl-4-aminobiphenyl (DMAB) and a high-fat diet that was supplemented with dried mangosteen pericarp for the treatment group (Tsai et al., 2020). Results showed that mangosteen supplement could alleviate prostatic hyperplasia development by increasing GPx antioxidant and decreasing serum testosterone, dihydrotestosterone (DHT), lipid peroxidation level, prostate weight, and lipid profile. In the infertility model, mice were induced with 2-methoxyethanol (2-ME) and treated with mangosteen pericarp ethanolic extract (Subani, 2014). Results showed that mangosteen extract was able to increase spermatozoa motility, viability, normal morphology, and membrane integrity. However, it was also discovered that the treatment did not result in a significant decrease in MDA level. In the arthritis model, mice were induced with a collagen solution and treated with AM (Herrera-Aco et al., 2019). Results showed that treatment with AM reduced arthritic score in the first 18 days of the treatment, MDA level, and proinflammatory cytokines and increased GSH antioxidant. In the obese model, rats were induced with STZ and fed a high-fat diet that was supplemented with dried mangosteen flesh for the treatment group (Adyab et al., 2019). Results showed that supplementation of mangosteen flesh reduced subject body weight, total cholesterol, and proinflammatory cytokines and increased total antioxidant capacity.
CONCLUSION
Mangosteen extracts, products, and isolated compounds were shown to increase antioxidant levels through in vivo studies by either increasing antioxidant enzymes (such as SOD, CAT, GPx, and GSH) or by decreasing oxidative stress markers (such as MDA level). Mangosteen showed a positive effect in alleviating disease-related parameters in type II diabetes models, cardiovascular models, neurological disorder models, stress-induced models, and liver and kidney injury models. These results signified that mangosteen could be a promising adjuvant drug or supplement to oxidant-related diseases. However, in clinical trials, although mangosteen intervention significantly increased plasma antioxidant capacity, it did not show any significant effect toward other parameters, such as the MDA level. Moreover, interventions in clinical trials mostly used commercial products that contain other ingredients with antioxidant activity, such as vitamin C and green tea extract. Therefore, more clinical trial results measuring the antioxidant parameter (such as level of SOD, CAT, GPx, and GSH antioxidant enzymes) are needed to conclude whether mangosteen extract or their isolated compounds possess significant antioxidant activity capable of alleviating oxidant-related diseases.
ACKNOWLEDGMENT
The authors would like to express gratitude to Agnes Anania Triavika Sahamastuti, and Audrey Amira Crystalia who are involved in the proofreading process.
CONFLICT OF INTEREST
All the authors declare that they have no conflicts of interest for this work.
FUNDING
None.
REFERENCES
Adyab NSM, Rahmat A, Abdul Kadir NAA, Jaafar H, Shukri R, Ramli NS. Mangosteen (Garcinia mangostana) flesh supplementation attenuates biochemical and morphological changes in the liver and kidney of high fat diet-induced obese rats. BMC Complement Altern Med, 2019; 19(1):44; https://doi.org/10.1186/s12906-019-2764-5
Avinash P, Reddy A, Begum N, Bakshi V. Neuroprotective effect of Garcinia mangostana on streptozotocin induced sporadic type Alzheimer’s disease in mice. Int J Appl Pharm Sci Res, 2016; 1(1):8–15; https://doi.org/10.21477/ijapsr.v1i1.9603
Ayala A, Munoz MF, Arguelles S. Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev, 2014; 2014:1–31; https://doi.org/10.1155/2014/360438
Baroroh K, Suradi S, Rima A. Effects of mangosteen pericarp extract to clinical improvements, the plasma level of IL-6 and malondialdehyde in acute exacerbation of COPD patients. J Respir Indones, 2018; 38(3):164–72; https://doi.org/10.36497/jri.v38i3.6
Boonprom P, Boonla O, Chayaburakul K, Welbat JU, Pannangpetch P, Kukongviriyapan U, Kukongviriyapan V, Pakdeechote P, Prachaney P. Garcinia mangostana pericarp extract protects against oxidative stress and cardiovascular remodeling via suppression of p47(phox) and iNOS in nitric oxide deficient rats. Ann Anat, 2017; 212:27–36; doi:10.1016/j.aanat.2017.03.007
Cervantes Gracia K, Llanas-Cornejo D, Husi H. CVD and oxidative stress. J Clin Med, 2017; 6(2):22; https://doi.org/10.3390/jcm6020022.
Chang CC, Chen CW, Owaga E, Lee WT, Liu TN, Hsieh RH. Mangosteen concentrate drink supplementation promotes antioxidant status and lactate clearance in rats after exercise. Nutrients, 2020; 12(5):1447; https://doi.org/10.3390/nu12051447
Chen LG, Yang LL, Wang CC. Anti-inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol, 2008; 46(2):688–93; https://doi.org/10.1016/j.fct.2007.09.096
Chomnawang MT, Surassmo S, Nukoolkarn VS, Gritasanapan W. Antimicrobial effects of thai medicinal plants against acne-inducing bacteria. J Ethnopharmacol, 2005; 101(1–3):330–3; https://doi.org/10.1016/j.jep.2005.04.038
Cichoż-Lach H, Michalak A. Oxidative stress as a crucial factor in liver diseases. World J Gastroenterol, 2014; 20(25):8082–91; https://doi.org/10.3748/wjg.v20.i25.8082.
Devi Sampath P, Vijayaraghavan, K. Cardioprotective effect of alpha-mangostin, a xanthone derivative from mangosteen on tissue defense system against isoproterenol-induced myocardial infarction in rats. J Biochem Mol Toxicol, 2007; 21(6):336–9; https://doi.org/10.1002/jbt.20199
Dizdaroglu M, Jaruga P, Birincioglu M, Rodiguez H. Free radical-induced damage to DNA: mechanisms and measurement. Free Radical Bio Med, 2002; 32(11):1102–15; https://doi.org/10.1016/s0891-5849(02)00826-2
Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomized studies of health care interventions. J Epidemiol Community Health, 1998; 52:377–84; https://doi.org/10.1136/jech.52.6.377
DrÓ§ge W. Free radicals in the physiological control of cell function. Physiol Rev, 2002; 82:47–95; https://doi.org/10.1152/physrev.00018.2001
Elshourbagy NA, Meyers HV, Abdel-Meguid SS. Cholesterol: the good, the bad, and the ugly - therapeutic targets for the treatment of dyslipidemia. Med Princ Pract, 2014; 23(2):99–111; https://doi.org/10.1159/000356856
Febriane NN, Giriwono PE, Koswara S, Prangdimurti E. Suplementasi Mikroenkapsulat Ekstrak Kulit Buah Manggis (Kbm) Menurunkan Kadar Malonaldehida Hati Tikus. Penelitian Gizi Dan Makanan (J Nutr Food Res), 2015; 38(1):61–70; https://doi.org/10.22435/pgm.v38i1.4423.61-70
Gondokesumo ME, Pardjianto B, Sumitro SB, Widowati W, Handono K. Xanthones analysis and antioxidant activity analysis (applying ESR) of six different maturity levels of mangosteen rind extract (Garcinia mangostana Linn.). Pharmacogn J, 2019; 11(2):369–73; https://doi.org/10.5530/pj.2019.11.56
Hafisalevi MD, Setiawan M, Sargowo D. Effect of extract from pericarp of mangosteen (Garcinia Mangostana Linn) as antioxidant in rats models of atherosclerosis. J Kardiol Indones, 2012; 33(2):75–80; https://doi.org/10.30701/ijc.v33i2.55
Han Z, Tian R, Ren P, Zhou W, Wang P, Luo M, Jin S, Jiang Q. Parkinson's disease and Alzheimer's disease: a Mendelian randomization study. BMC Med Genet, 2018; 19(Suppl 1):215; https://doi.org/10.1186/s12881-018-0721-7
Haruenkit R, Poovarodom S, Leontowicz H, Leontowicz M, Sajewicz M, Kowalska T, Delgado-Licon E, Rocha-Guzmán NE, Gallegos-Infante JA, Trakhtenberg S, Gorinstein S. Comparative study of health properties and nutritional value of durian, mangosteen, and snake fruit: experiments in vitro and in vivo. J Agric Food Chem, 2007; 55(14):5842–9; https://doi.org/10.1021/jf070475a
Herrera-Aco DR, Medina-Campos ON, Pedraza-Chaverri J, Sciutto-Conde E, Rosas-Salgado G, Fragoso-González G. Alpha-mangostin: anti-inflammatory and antioxidant effects on established collagen-induced arthritis in DBA/1J mice. Food Chem Toxicol, 2019; 124:300–15; https://doi.org/10.1016/j.fct.2018.12.018
Huang HJJ, Chen WLL, Hsieh RHH, Hsieh-Li, HM. Multifunctional effects of mangosteen pericarp on cognition in C57BL/6J and triple transgenic alzheimer’s mice. Evid Based Complement Alternat med, 2014; 2014:1–18; https://doi.org/10.1155/2014/813672
Husen SA, Winarni D, Khaleyla F, Kalqutny SH. Activity test of various mangosteen (Garcinia mangostana) pericarp extract fractions to decrease fasting blood cholesterol levels and lipid peroxidation activity in diabetic mice. J Biol Res (Berkala Penelitian hayati), 2017a; 22(1):13–17; https://doi.org/10.23869/bphjbr.22.1.20163
Husen SA, Winarni D, Khaleyla F, Kalqutny SH, Ansori ANM. Activity assay of mangosteen (Garcinia mangostana L.) pericarp extract for decreasing fasting blood cholesterol level and lipid peroxidation in type-2 diabetic mice. AIP Conf Proc, 2017b; 1888(1):20026; https://doi.org/10.1063/1.5004303
Ighodaro OM, Akinloye OA. First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT), and glutahione peroxidase (GPX): their fundamental role in the entire antioxidant defece grid. Alex J Med, 2018; 54(4):287–93; https://doi.org/10.1016/j.ajme.2017.09.001
Im ARR, Kim YMM, Chin YWW, Chae S. Protective effects of compounds from Garcinia mangostana L. (mangosteen) against UVB damage in HaCaT cells and hairless mice. Int J Mol Med, 2017; 40(6):1941–9; https://doi.org/10.3892/ijmm.2017.3188
Indharty RRS, Japardi I, Siahaan AMP, Tandean S. Mangosteen extract reduce apoptosis via inhibition of oxidative process in rat model of traumatic brain injury. Bali Med J, 2019; 8(1):227–32; https://doi.org/10.15562/bmj.v8i1.1153
Ismail SM, Hui CK, Aminuddin A, Ugusman A. Piper sarmentosum as an antioxidant: a systematic review. Sains Malays, 2018; 47(10):2359–68; https://doi.org/10.17576/jsm-2018-4710-12
Jariyapongskul A, Areebambud C, Suksamrarn S, Mekseepralard C. Alpha-mangostin attenuation of hyperglycemia-induced ocular hypoperfusion and blood retinal barrier leakage in the early stage of type 2 diabetes rats. Biomed Res Int, 2015; 2015:785826: https://doi.org/10.1155/2015/785826
Karim N, Jeenduang N, Tangpong J. Anti-glycemic and anti-hepatotoxic effects of mangosteen vinegar rind from Garcinia mangostana against HFD/STZ-induced type II diabetes in mice. Pol J Food Nutr Sci, 2018; 68(2):163–9; https://doi.org/10.1515/pjfns-2017-0018
Karim N, Rahman A, Chanudom L, Thongsom M, Tangpong J. Mangosteen vinegar rind from Garcinia mangostana prevents high-fat diet and streptozotocin-induced type II diabetes nephropathy and apoptosis. J Food Sci, 2019a; 84(5):1208–15; https://doi.org/10.1111/1750-3841.14511
Karim N, Rahman MA, Changlek S, Tangpong J. Short-time administration of xanthone from Garcinia mangostana fruit pericarp attenuates the hepatotoxicity and renotoxicity of type II diabetes mice. J Am Coll Nutr, 2019b; 39(6):501–10; https://doi.org/10.1080/07315724.2019.1696251
Kim SO, Merchant K, Nudelman R, Beyer Jr WF, Keng T, DeAngelo J, Hausladen A, Stamler JS. OxyR A molecular code for redox-related signaling. Cell, 2002; 109(3):383–96; https://doi.org/10.1016/s0092-8674(02)00723-7
Kondo M, Zhang L, Ji H, Kou Y, Ou B. Bioavailability and antioxidant effects of a xanthone-rich Mangosteen (Garcinia mangostana) product in humans. J Agr Food Chem, 2009; 57(19):8788–92; https://doi.org/10.1021/jf901012f
Kumar H, Lim HW, More SV, Kim BW, Koppula S, Kim IS, Choi DK. The role of free radicals in the aging brain and parkinson’s disease: convergence and parallelism. Int J Mol Sci, 2012; 13:10478–504; https://doi.org/10.3390/ijms130810478
Kumar V, Bhatt PC, Kaithwas G, Rashid M, Al-abbasi FA, Khan JAJ, Anwar F, Verma A. α-mangostin mediated pharmacological modulation of hepatic carbohydrate metabolism in diabetes induced wistar rat. Beni Suef Univ J Basic Appl Sci, 2016; 5(3):255–76; https://doi.org/10.1016/j.bjbas.2016.07.001
Leontowicz H, Leontowicz M, Drzewiecki J, Haruenkit R, Poovarodom S, Park YS, Jung ST, Kang SG, Trakhtenberg S, Gorinstein S. Bioactive properties of Snake fruit (Salacca edulis Reinw) and Mangosteen (Garcinia mangostana) and their influence on plasma lipid profile and antioxidant activity in rats fed cholesterol. Eur Food Res Technol, 2006; 223(5):697–703; https://doi.org/10.1007/s00217-006-0255-7
Leontowicz M, Leontowicz H, Drzewiecki J, Jastrzebski Z, Haruenkit R, Poovarodom S, Park YS, Jung ST, Kang SG, Trakhtenberg S, Gorinstein S. Two exotic fruits positively affect rat’s plasma composition. Food Chem, 2007; 102(1):192–200; https://doi.org/10.1016/j.foodchem.2006.05.046
Liguori I, Russo G, Curcio F, Bulli G, Aran L, Della-Morte D, Gargiulo G, Testa G, Cacciatore F, Bonaduce D, Abete P. Oxidative stress, aging, and diseases. Clin Interv Aging, 2018; 13:757–72; https://doi.org/10.2147/CIA.S158513
Lim YS, Lee SSH, Tan BC. Antioxidant capacity and antibacterial activity of different parts of mangosteen (Garcinia mangostana Linn.) extracts. Fruits, 2013; 68(6):483–9; https://doi.org/10.1051/fruits/2013088
Lotter J, Möller M, Dean O, Berk M, Harvey BH. (2020). Studies on haloperidol and adjunctive α-mangostin or raw Garcinia mangostana linn pericarp on bio-behavioral markers in an immune-inflammatory model of schizophrenia in male rats. Front Psychiatry, 2020; 11:121; https://doi.org/10.3389/fpsyt.2020.00121
Lushchak VI. Glutahione homeostatis and functions: potential targets for medical interventions. J Amino Acids, 2012; 2012:1–26; https://doi.org/10.1155/2012/736837
Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med, 2009; 6(7):e1000097; https://doi.org/10.1136/bmj.b2535
Nakagawa Y, Iinuma M, Naoe T, Nazowa Y, Akao Y. Characterized mechanism of alpha-mangostin-induced cell death: caspase-independent apoptosis with release of endonuclease-G from mitochondria and increased miR-143 expression in human colorectal cancer DLD-1 cells. Bioorg Med Chem, 2007; 15(16):5650–8; https://doi.org/10.1016/j.bmc.2007.04.071
Nelli GB, Solomon KA, Kilari EK. Antidiabetic effect of α-mangostin and its protective role in sexual dysfunction of streptozotocin induced diabetic male rats. Syst Biol Reprod Med, 2013; 59(6):319–28; https://doi.org/10.3109/19396368.2013.820369
Oberholzer I, Möller M, Holland B, Dean OM, Berk M, Harvey BH. Garcinia mangostana Linn displays antidepressant-like and pro-cognitive effects in a genetic animal model of depression: a bio-behavioral study in the Flinders Sensitive Line rat. Metab Brain Dis, 2018; 33(2):467–80; https://doi.org/10.1007/s11011-017-0144-8
Oberley LW. Free radicals and diabetes. Free Radic Bio Med, 1988; 5(2):113–24; https://doi.org/10.1016/0891-5849(88)90036-6
Parkhe A, Parekh P, Nalla LV, Sharma N, Sharma M, Gadepalli A, Kate A, Khairnar A, Khairnar A, Parkhe A, Parekh P, Nalla LV, Sharma N, Sharma M, others. Protective effect of alpha mangostin on rotenone induced toxicity in rat model of Parkinson’s disease. Neurosci Lett, 2019; 716:134652; https://doi.org/10.1016/j.neulet.2019.134652
Pérez-Rojas JM, Cruz C, García-López P, Sánchez-González DJ, Martínez-Martínez CM, Ceballos G, Espinosa M, Meléndez-Zajgla J, Pedraza-Chaverri J. Renoprotection by alpha-Mangostin is related to the attenuation in renal oxidative/nitrosative stress induced by cisplatin nephrotoxicity. Free Radic Res, 2009; 43(11):1122–32; https://doi.org/10.1080/10715760903214447
Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci, 2008; 4(2):89–96.
Phyu MP, Tangpong J. Neuroprotective effects of xanthone derivative of Garcinia mangostana against lead-induced acetylcholinesterase dysfunction and cognitive impairment. Food Chem Toxicol, 2014; 70:151–6; https://doi.org/10.1016/j.fct.2014.04.035
Rana MN, Tangpong J, Rahman MA. Xanthones protects lead-induced chronic kidney disease (CKD) via activating Nrf-2 and modulating NF-kB, MAPK pathway. Biochem Biophys Rep, 2020; 21:100718; https://doi.org/10.1016/j.bbrep.2019.100718
Sampath PD, Kannan V. Mitigation of mitochondrial dysfunction and regulation of eNOS expression during experimental myocardial necrosis by alpha-mangostin, a xanthonic derivative from Garcinia mangostana. Drug Chem Toxicol, 2009; 32(4):344–52; https://doi.org/10.1080/01480540903159210
Samuagam L, Sia CM, Akowuah GA, Okechukwu PN, Yim HS. In vivo antioxidant potentials of rambutan, mangosteen, and langsat peel extracts and effects on liver enzymes in experimental rats. Food Sci Biotechnol, 2015; 24(1):191–8; https://doi.org/10.1007/s10068-015-0026-y
Santos-Sánchez NF, Salas-Coronado R, Villanueva-Cañongo C, Hernández-Carlos B. Antioxidant compounds and their antioxidant mechanism. In: Shalaby E (ed.). Antioxidant. IntechOpen, London, UK, 2019; https://doi.org/10.5772/intechopen.85270. Available via https://www.intechopen.com/books/antioxidants/antioxidant-compounds-and-their-antioxidant-mechanism
Sattayasai J, Chaonapan P, Arkaravichie T, Soi-Ampornkul R, Junnu S, Charoensilp P, Samer J, Jantaravinid J, Masaratana P, Suktitipat B, Manissorn J, Thongboonkerd V, Neungton N, Moongkarndi P, others. Protective effects of mangosteen extract on H2O2-induced cytotoxicity in SK-N-SH cells and scopolamine-induced memory impairment in mice. PloS One, 2013; 8(12):e85053; https://doi.org/10.1371/journal.pone.0085053
Schneider K, Schwarz M, Burkholder I, Kopp-Schneider A, Edler L, Kinsner-Ovaskainen A, Hartung T, Hoffmann S. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicol Lett, 2009; 189(2):138–44; https://doi.org/10.1016/j.toxlet.2009.05.013
Sinaga RN, Siregar NS. Phytochemical screening and test of antioxidant activity in the extract of mangosteen rind. Int Conf Public Health, 2016; 124; https://doi.org/10.26911/theicph.2016.057
Subani ND. Effect of skin extract mangosteen (Garcinia mangostana L.) against sperm quality and malondialdehyde levels of mice (Mus musculus) exposured with 2-methoxyethanol. J Info Kesehatan, 2014; 12(1):670–83; https://doi.org/10.31965/infokes.v12i1.50
Suthammarak W, Numpraphrut P, Charoensakdi R, Neungton N, Tunrungruangtavee V, Jaisupa N, Charoensak S, Moongkarndi P, Muangpaisan W. Antioxidant-enhancing property of the polar fraction of mangosteen pericarp extract and evaluation of its safety in humans. Oxid Med Cell Longev, 2016; 2016:1293036; https://doi.org/10.1155/2016/1293036
Sutono T. Efficacy of Garcinia mangostana L. (mangosteen rind extract) to reduce acne severity. Med J Indones, 2013; 22(3):167–72; https://doi.org/10.13181/mji.v22i3.586
Taher M, Tg Zakaria TMFS, Susanti D, Zakaria ZA. Hypoglycemic activity of ethanolic extract of Garcinia mangostana Linn. in normoglycemic and streptozotocin-induced diabetic rats. BMC Complement Altern Med, 2016; 16:135; https://doi.org/10.1186/s12906-016-1118-9
Tjahjani S, Biantoro Y, Tjokropranoto R. Ethyl acetate fraction of Garcinia mangostana L rind study as antimalarial and antioxidant in Plasmodium berghei inoculated mice. Open Access Maced J Med Sci, 2019; 7(12):1935; https://doi.org/10.3889/oamjms.2019.480
Tsai HH, Chen CW, Yu PL, Lin YL, Hsieh RH, Hui-Hsuan T, Chia-Wen C, Pei-Ling Y, Yu-Ling L, Rong-Hong H, Tsai HH, Chen CW, Yu PL, Lin YL, Hsieh RH. Mangosteen pericarp components alleviate progression of prostatic hyperplasia and mitochondrial dysfunction in rats. Sci Rep, 2020; 10(1):1–9; https://doi.org/10.1038/s41598-019-56970-2
Tsai SYY, Chung PCC, Owaga EE, Tsai IJJ, Wang PYY, Tsai JII, Yeh TSS, Hsieh RHH. Alpha-mangostin from mangosteen (Garcinia mangostana Linn.) pericarp extract reduces high fat-diet induced hepatic steatosis in rats by regulating mitochondria function and apoptosis. Nutr Metab, 2016; 13(1):88; https://doi.org/10.1186/s12986-016-0148-0
Wahjuni S, Laksmiwati AAIAM, Puspawati NM. Intake pericarp of Garcinia mangostana L. extract inhibited oxidative stress on wistar rat hyperglycemic through the increased of superoxide dismutase and. KnE Life Sci, 2017; 3(5):202–7; https://doi.org/10.18502/kls.v3i5.994
Wang A, Li D, Wang S, Zhou F, Li P, Wang Y, Lin L. γ-Mangostin, a xanthone from mangosteen, attenuates oxidative injury in liver via NRF2 and SIRT1 induction. J Funct Foods, 2018; 40:544–53; https://doi.org/10.1016/j.jff.2017.11.047
Wang A, Zhou F, Li D, Lu JJ, Wang Y, Lin L. γ-Mangostin alleviates liver fibrosis through Sirtuin 3-superoxide-high mobility group box 1 signaling axis. Toxicol Appl Pharmacol, 2019; 363:142–53; https://doi.org/10.1016/j.taap.2018.11.011
Weecharangsan W, Opanosopit P, Sukma M, Ngawhirunpat T, Sotanaphun U, Siripong P. Antioxidative and neuroprotective activities of extracts from the fruit hull of mangosteen (Garcinia mangostana Linn.). Med Princ Pract, 2006; 15(4):281–7; https://doi.org/10.1159/000092991
Wihastuti TA, Sargowo D, Heriansyah T, Rahmawati G, Sulfia YH. Modulation of paraoxonase activity (PON)-1 by xanthone in sub chronic exposure of organophosphate: antioxidant in dichorvos intoxicity. Toxicol Environ Health Sci, 2015; 7(2):136–42; https://doi.org/10.1007/s13530-015-0232-2
Xie Z, Sintara M, Chang T, Ou B. Daily consumption of a mangosteen-based drink improves in vivo antioxidant and anti-inflammatory biomarkers in healthy adults: a randomized, double-blind, placebo-controlled clinical trial. Food Sci Nutr, 2015b; 3(4):342–8; https://doi.org/10.1002/fsn3.225
Xie Z, Sintara M, Chang T, Ou B. Functional beverage of Garcinia mangostana (mangosteen) enhances plasma antioxidant capacity in healthy adults. Food Sci Nutr, 2015a; 3(1):32–8; https://doi.org/10.1002/fsn3.187
Yan X, Sun YS, Ren S, Zhao LC, Liu WC, Chen C, Wang Z, Li W. Dietary α-mangostin provides protective effects against acetaminophen-induced hepatotoxicity in mice via Akt/mTOR-mediated inhibition of autophagy and apoptosis. Int J Mol Sci, 2018; 19(5):1335; https://doi.org/10.3390/ijms19051335
Zhang YJ, Wang F, Zhou Y, Li Y, Zhou T, Zheng J, Zhang JJ, Li S, Xu DP, Li HB. Effects of 20 selected fruits on ethanol metabolism: potential health benefits and harmful impacts. Int J Environ Res Public Health, 2016; 13(4):399; https://doi.org/10.3390/ijerph13040399
SUPPLEMENTARY TABLES
 | Supplement Table 1. Included clinical trials quality assessment using Downs and Black’s checklist. [Click here to view] |
 | Supplement Table 2. Included in vivo studies quality assessment using ToxRtool. [Click here to view] |