INTRODUCTION
The use of antibacterial agent or antibiotic is one of the solutions to treat diseases caused by bacterial infections, but synthetic antibacterial drugs that are used clinically have weaknesses such as high toxicity, and their use often leads to the emergence of resistant bacteria (Pradhan et al., 2014). Staphylococcus epidermidis bacteria had generally been resistant to penicillin and methicillin antibiotics (Bartlett et al., 2000). One of the efforts in preventing and controlling the resistance is the use of natural compound, which has the potential as an antibacterial in dosage forms that are applicable and proven effective in diabetic foot ulcer wounds with bacterial infections. Regarding these problems, efforts emerged to develop and search for antibacterial alternatives. One of the commodities that have high potential in developing herbal medicine is mangosteen peels considered as waste that has many benefits to be developed into a drug that has a therapeutic effect. Mangosteen peel had hypoglycemic activity (Taher et al., 2016), antimalarial activity (Tjahjani, 2017), and strong antioxidant activity (Tjahjani et al., 2014). Mangosteen peels also had antibacterial activity (Phitaktim et al., 2016). Xanthone is a polyphenol compound, which has semi-polar properties. The most xanthone derivative with the best biological activity on mangosteen peel was α-mangostin (Parveen et al., 1988), so using ethyl acetate fraction (EAF) as solvent was expected to attract α-mangostin compounds. α-mangostin had anti-cancer activity (Fei et al., 2014; Zhang et al., 2017), antibacterial activity (Sundaram et al., 1983), arthritis (Zuo et al., 2018), Alzheimer's disease (Yao et al., 2016), and anti-inflammation (Chen et al., 2008).
In an effort to maintain the stability of α-mangostin and increase a comfort in use, it needs to be made into a preparation. One of the pharmaceutical preparations is self-nanoemulsifying drug delivery systems (SNEDDSs). This could improve bioavailability and was possible to make large-scale manufacturing, which can be done easily, and which process is economical so that it is industrially attractive, thermodynamically stable, and easier to store (Amrutkar et al., 2014). This research uses SNEDDS for antimicrobial activity because SNEDDS has a wide surface for absorption and good dispersion due to its small particle size, thus allowing even distribution of the skin and the ability to penetrate into the skin layer (Shah et al., 2010). SNEDDS can penetrate secondary metabolites compound of ethyl acetate fraction of mangosteen peels into bacterial cell membranes. SNEDDS was made using an optimal formula using the simplex lattice design method with variations in the concentration of oil, surfactants, and co-surfactants. The purposes of this study were to determine the comparison of Tween 80, PEG 400, and VCO to produce the optimal SNEDDS of the EAF and to analyze the effectiveness of the SNEDDS of the EAF against S. epidermidis bacteria. This research needs to be developed continuously to obtain an effective in vitro SNEDDS against S. epidermidis bacteria. The success of the research has an impact on the further development of the potential of developing a pharmaceutical preparation.
A novelty in this research is the unique EAF-loaded SNEDDS, which was formulated to increase the stability of EAF and utilization of waste mangosteen peels in ethyl acetate fraction. The optimization of SNEDDS uses Design-Expert Software with the simplex lattice design route topical as something new. Besides, it found the effectiveness of optimal SNEDDS against S. epidermidis.
MATERIALS AND METHODS
Materials
The equipment used in this study are a Digital Analysis Balance (Ohaus PA214, USA), glassware (Pyrex), stopwatch, vortex mixer (Thermolyne), ultrasonicator (J.P. Selecta), spektrofotometer UV-Vis (Shimadzu type 2450), quartz cuvette with a size of 1 cm, magnetic stirrer (Stuart CB162), pH meter (HANNA), filter paper, particle size analysis (Beckman Coulter), micropipette Socorex® (0.5–10, 5–50, 50–200, and 200–1,000 ¼l), Eppendorf tube, rotary evaporator (Heidolph Type Hei-VAP), oven (Memmert), water bath (Memmert Type WNB14), sieve size 120 mesh, aluminum foil, thermometer, mangosteen peels (Kaligesing, Purworejo, Jawa Tengah), ethanol 70% (Dwicentra), n-hexane (Merck), ethyl acetate (Merck), α-mangostin (Sigma-Aldrich 98%), methanol (Merck), Virgin Coconut Oil (VCO) (Bagoes), Tween 80 (Bratachem), and aquadest (Dwicentra).
EAF of Mangosteen peels
A thick 70% ethanol extract (EE) obtained from the previous study (Pratiwi et al., 2017a) is dissolved with n-hexane so that a soluble fraction of n-hexane and residue were obtained. Then, the ethyl acetate residue was added to obtain the ethyl acetate fraction and residue. Furthermore, the soluble n-hexane fraction, ethyl acetate fraction, and residue were collected and concentrated with a rotary evaporator and water bath at 40°C until a thick fraction was obtained (Rahimah et al., 2013).
Orientation of surfactant, co-surfactant, and oil composition formulations with simplex lattice design
Surfactants, co-surfactants, and oils selected in previous study (Pratiwi et al., 2017b) were subsequently obtained 14 runs in various mixture compositions for the three components to be optimized, namely, Tween 80, PEG 400, and VCO with a ratio of 3.67:1.67:1.67, 3:1:3, 3:3:1, 1:1:5, 5:1:1, 1:5:1, 1.67:1.67:3.67, 2.33:2.33:2.33, 3:3:1, 1:1:5, 1:5:1, 1:3:3, 5:1:1, and 1.67:3.67:1.67. The fraction of ethyl acetate mangosteen peel used was based on the results of the drug loading test (Pratiwi et al., 2017b).
Preparation of SNEDDS
SNEDDS was made with a combination of Tween 80, PEG 400, and VCO. Then, EAF was added. The mixture was conditioned in a water bath at 40°C for 10 minutes. The process of homogenizing the fraction in a carrier was maximized with a 1,000-rpm vortex for 15 minutes. The physical data from 14 SNEDDS runs can be used to determine the optimal formula. The determination of the optimal formula was performed by the simplex lattice design method using Design-Expert ® version 7.0.0 software. The characteristics of the physical properties used in determining the optimal formula were transmittance, emulsification time, and pH.
Transmittance
The preconcentration formula was obtained by adding 100.0 ¼l of distilled water to a final volume of 5 ml. The homogenization of the mixture was carried out with the help of a vortex for 30 seconds. The emulsion obtained was measured for absorption at a wavelength of 650 nm with a blank of distilled water to determine the level of clarity (Patel et al., 2011).
pH
Emulsion pH measurement was performed by dipping the electrode of the pH meter into the emulsion. About 100 ¼l of SNEDDS was added up to 5 ml with distilled water. The mixture was homogenized by flipping for 1 minute. The reading on the pH meter was noted after 5 minutes to make sure that the figure is stable and does not move anymore.
Measurement of emulsification time
About 500 ml of distilled water was conditioned on a magnetic stirrer with a speed of 120 rpm. A total of 1 ml of SNEDDS of the ethyl acetate fraction of mangosteen peel was quickly dripped into the media (Patel et al., 2011).
Determination of optimal SNEDDS
The optimal SNEDDS was obtained by assigning the values ​​and weights to responses, namely, transmittance, pH, and emulsification time to obtain optimal desirability and contour plot formula values. Furthermore, the verification between the optimal SNEDDS prediction software with the optimal SNEDDS was carried out the research results.
Observation of zeta size and potential
A total of 1 ml of SNEDDS was mixed with distilled water up to 5 ml and was homogenized by flipping for 1 minute. After that, 3 ml of it was taken and put into a cuvette for analysis. Particle size data obtained as an output on a computer were the average particle size, particle size distribution, and the deviation from the mean.
Preparation of nutrient agar (NA) Media
As much as 23 g of nutrient agar was dissolved in sterile aquadest as much as 1000 ml, it was heated until all got dissolved in a hot state, and the solution was then put into an Erlenmeyer, followed by checking the pH of the media around 6.8 ± 0.2. The media were then sterilized in 121°C with autoclave for 15 minutes (Difco, 1977).
Preparation of Mueller–Hinton Agar (MHA) media
MHA media were prepared by 38 g of media and dissolved with 1 l of distilled water while heating, and then, they were sterilized by autoclaving at 121°C for 15 minutes (Difco, 1977).
Bacterial rejuvenation
Pure bacterial culture test from NA media was aseptically with an Ose needle on oblique NA media. Scratching was done by zig-zag on the surface of the media. Then, it was incubated at 37°C for 24 hours.
Preparation of S. epidermidis bacterial suspensions
The culture of each bacterial suspension was inoculated on rejuvenation media for 24 hours, taken using an Ose needle, and suspended into a tube containing 5 ml of 0.9% sterile NaCl solution. The turbidity obtained was then compared to 0.5 McFarland standard that is equivalent to the number of growth of 1 × 108 bacterial cells/ml, and after the equivalence, this suspension was used as a test bacterium (Radji, 2010).
RESULTS AND DISCUSSION
Ethyl acetate fraction
The ethyl acetate fraction was used as a sample in this study because of its semi-polar nature, making it easier to compound into the oil phase of SNEDDS. The easier the active compound enters into the oil phase, the better the SNEDDS preparation is obtained. Fractionation is a separation procedure that aims to separate the main groups (Harborne, 1973).
Transmittance, emulsification time, and pH response
Results of transmittance, emulsification time, and pH response can be seen in the Figures 1–3. Based on the analysis of variance (ANOVA) test, the normal plot of residual curve analysis, and a lack of fit analysis, the special cubic model in the equation was a valid and appropriate model for transmitting. The ANOVA test results showed that the p-value is at a significance level of 95%, meaning that the special cubic model is the right model to explain the effect of components and their interactions on transmittance. This was also strengthened by the results of the lack of fit analysis, which showed that p-value > 0.05 is at the 95% significance level. These showed that there is no significant difference between the experimental data and the predicted data from the proposed model. The special cubic p-value model < 0.05 showed a significant difference in clarity from the use of different compositions of oil phases, surfactants, and co-surfactants. The simplex lattice design equation obtained can be seen in Equation 1.
Y = 45.35 (A) + 33.51 (B) + 31.14 (C) – 28.77 (A)(B) – 32.88 (A)(C) – 26.99 (B)(C) + 23.36 (A)(B)(C) (1)
Information:
Y = Transmittance
A = Tween 80
B = PEG 400
C = VCO
The results of the lack of fit analysis showed that p-value > 0.05 is at the significance level of 95%. This showed that there is no significant difference between the experimental data and the predicted data from the proposed model. The quadratic model had a p-value < 0.05, which indicates a significant pH difference from the use of different compositions of oil phases, surfactants, and co-surfactants. Based on Figure 2, the blue color contour plot showed the smallest pH value followed by green and red. The red color indicated the greatest pH. This red area was affected by an increase in PEG 400. This showed that PEG 400 has a role in increasing the pH value in SNEDDS formulation. The simplex lattice design equation for the pH response can be seen in Equation 2.
Y = 1.24 (A) + 1.51 (B) + 0.27 (C) – 0.33 (A)(B) + 0.02 (A)(C) + 0.06 (B)(C) (2)
The linear model had a p-value < 0.05, which indicates a significant difference in emulsification time from the use of different compositions of oil phases, surfactants, and co-surfactants. This result was reinforced by the value of lack of fit > 0.05, which indicates that there is no significant difference between the observations with the predicted data from the model made. This showed that the linear model is adequately good to explain the effect of material interactions on emulsification time. Figure 3 shows the response contour plot of emulsification time.
 | Figure 1. Contour plot of transmittance response (Design Expert Ver. 7.0.0.). [Click here to view] |
 | Figure 2. Contour plot of pH response (Design Expert Ver. 7.0.0.). [Click here to view] |
 | Figure 3. Contour plot response of emulsification time (Design Expert Ver. 7.0.0.). [Click here to view] |
Optimal SNEDDS
The optimal formulation results obtained from the simplex lattice design were formulas with a ratio of Tween 80:PEG 400:VCO (4.98:1.02:1) with a desirability value of 0.931. A desirability value close to one indicated that the response variable chosen for formula optimization can reach the optimal point in accordance with the desired target. The results of determining the compositions of Tween 80, PEG 400, and VCO obtained showed the composition of Tween 80 as a surfactant was only able to form a homogeneous mixture if the composition ratio was greater than PEG 400 as a co-surfactant. The combination of surfactant and co-surfactant interactions occurred due to the presence of hydrophilic parts (hydroxy groups) in these compounds. The higher the amount of surfactant in the comparison, the better the achieved interaction balance. Conversely, if the amount of co-surfactant increased, the interaction balance would not be achieved so that a non-homogeneous (separating) phase was formed.
Verification of optimal formula
Verification was done by comparing the optimal SNEDDS of the experimental results with software predictions. Based on the probability value of each response, the obtained p-value > 0.05, and it was concluded that there is no significant difference between the results of the prediction of simplex lattice design on the Design-Expert software with the results of experimental observations.
Transmittance evaluation
Transmittance test results are shown in Table 1. Based on observations, 3 of the 14 runs showed a clear display that is at run 5, 8, and 13 using VCO:Tween 80:PEG 400 with a ratio 5:1:1 and 2.33:2.33:2.33. Meanwhile, the ratio of oil, surfactants, and other co-surfactants showed a murky appearance. Transmittance illustrated the clarity, which is one of the characteristics of the emulsion that needs to be measured because it affects the particle size. The size of the dispersed phase greatly affected the emulsion visually. If the emulsion system has a very small globule size through which light is passed, the beam of light will be continued so that the color of the solution looks transparent and the resulting transmittance is even greater. Based on the contour plot of transmittance data and equation, it revealed that the transmittance value was not only determined by each optimized component but also influenced by the interaction between the components. The interactions between Tween 80 and PEG 400, Tween 80 and VCO, and Tween 80 and VCO had negative values. This negative interaction showed that the interaction reduced the transmittance value. The interaction of the three components caused an increase in transmittance. Tween 80 gave the greatest effect, indicated by the magnitude of the coefficients in the equation. PEG 400 had an influence on the value of transmittance that is greater than VCO. The interaction of the three components formed SNEDDS with a clear and stable display, which could ultimately increase the SNEDDS transmitting value.
 | Table 1. Fractionation results. [Click here to view] |
 | Table 2. Transmittance value, pH, and emulsification time (Design Expert Ver. 7.0.0.). [Click here to view] |
 | Table 3. ANOVA test on transmittance response (Design Expert Ver. 7.0.0.). [Click here to view] |
 | Table 4. ANOVA test for pH response (Design Expert Ver. 7.0.0.). [Click here to view] |
 | Table 5. ANOVA Test for emulsification time response (Design Expert Ver. 7.0.0.). [Click here to view] |
The clearer the emulsion gives the smaller particle size and the more turbid the emulsion gives larger the particle size. The surfactants and co-surfactants in SNEDDS formula must be able to reduce the interface tension between the oil and the dispersing medium. A large amount of surfactant can reduce the interface tension and reduce the size of the droplet. The size of the droplet can be predicted from the level of clarity of the emulsion (transmittance value). The clearer the formed emulsion gives the smaller the achieved droplet size. Based on Figure 2, the contour plot in blue showed the smallest transmittance, followed by green and yellow. Yellow indicated the largest transmittance. The yellow area was in the middle of the Tween 80, PEG 400, and VCO mixture. This revealed that the three components have a good role in increasing and maintaining the transmittance value in SNEDDS preparations.
pH evaluation
The pH test results are shown in Table 1. In the test results, almost all runs had a pH range that is allowed to be applied on the skin. The test of pH aimed to determine the safety of the preparation, especially when applied on the skin. When the pH value is too low, it leads to irritation when applied, whereas when it is too high, it results in scaly skin. The pH range of topical preparations was 4.5–6.5 (Tranggono and Fatma, 2007). Tween 80 and PEG 400 coefficient values were also positive between PEG 400 and VCO, meaning that the combination of components could increase the pH response. ween 80 and PEG 400 coefficient values, also between PEG 400 and VCO were positive, meaning that the combination of components could increase the pH response. The PEG 400 coefficient was greater than the Tween 80 and the Tween 80 coefficient was greater than the VCO coefficient. These increased the pH value. The interaction of Tween 80 and PEG 400 lowers the pH value, whereas the interaction of Tween 80 with VCO increases the pH value, however the effect is not greater than the interaction of PEG 400 with VCO. PEG 400 without the addition of other components, like Tween 80 and VCO, greatly increased the pH value. PEG 400 without the addition of other components, like Tween 80 and VCO, greatly increased the pH value.
Emulsification time evaluation
Emulsification time test results are shown in Table 1. The measurement of emulsification time at 14-run SNEDDS was able to form nanoemulsion on aquades media at various times. PEG 400 as a co-surfactant had a major influence on the time of emulsification. Based on research from Vilas et al. (2014), the dispersion capability of PEG 400 fell into category A, which can produce nanoemulsions quickly which takes about 1 minute, with a clear nanoemulsion display. This study was in accordance with the results obtained in the current study, except for runs 4 and 10, which fell into category B. The spontaneous formation of nanoemulsion is one of the important parameters in the SNEDDS formulation. Nanoemulsion is expected to form quickly when in water. The determination of emulsification time was done to obtain a picture of the ease, in which SNEDDS formed the emulsion. Based on the emulsification time, contour plot data, and equation, it showed that the value of the emulsification time was determined by each optimized component. The smaller the emulsification time value, the shorter the time needed for SNEDSS to form nanoemulsion. The Tween 80 component had the smallest coefficient compared to PEG 400, and VCO had the greatest coefficient. VCO was very influential in increasing the time of emulsification time, which causes the longer SNEDDS time to form nanoemulsion.
The short emulsification time was mediated by the action of surfactants and co-surfactants which can immediately form the oil interface layer. The co-surfactants played a role in emulsification time and not in reducing the size of the droplet. The co-surfactants were tucked and formed an empty space between surfactants and increase fluidity, so they were able to form nanoemulsions faster. This showed that PEG 400 as a co-surfactant has a role in accelerating the time of emulsification. The ability to increase emulsification in co-surfactants was determined at the length of the hydrophobic alkyl chain. The longer the chain, the better the emulsification ability (Parmar et al., 2011). The oil component could increase the emulsification time of nanoemulsion. Zhao et al. (2010) stated that the addition of ethyl oleate to SNEDDS can increase the emulsification time even though the particle size becomes smaller with increasing surfactant concentration. An increase in oil concentration could slow down the emulsification time because the surfactant and co-surfactant concentrations became smaller, so they could not form an emulsion in a short time (Beg et al., 2013; Eid et al., 2012). Short emulsification time was affected by small oil concentrations and high co-surfactant concentrations so that the viscosity became smaller (Basalious et al., 2010). Based on Figure 3 in the contour plot, blue indicated the smallest emulsification time value, followed by green, yellow, and red. The red color indicated the greatest emulsification time. This red area was affected by an increase in oil. The blue area was affected by surfactants and co-surfactants. The lower the value of emulsification time, the better and faster nanoemulsion formulation in aquades media.
 | Figure 4. Contour plot of desirability (Design Expert Ver. 7.0.0.). [Click here to view] |
 | Figure 5. Contour plot from optimal formulation (Design Expert Ver. 7.0.0.). [Click here to view] |
Optimization formulation
Based on the optimal formula produced by simplex lattice design in Design-Expert software version 7.0.0, it was predicted that the formula would produce a transmittance of 72.3768, emulsification time of 5.06, and pH of 6.50. Based on Figure 2, a superimposition generated a contour plot of the transmittance response, pH, and emulsification time. The resulted superimposition provided the yellow areas that had an optimal response. The area gave an optimal formula prediction with the desirability of 0.931. The optimal formula composition based on the obtained analysis revealed a comparison of Tween 80:PEG 400:VCO with a composition of 4.98:1.02:1.00 parts.
According to Shafiq-un Nabi et al. (2007), the solubility of drugs in oil in nanoemulsion was the most important component because it was related to the ability of nanoemulsion to maintain drugs in dissolved form which is strongly influenced by drug solubility in the oil phase. The amount of oil used in this optimization was one part of the total surfactant–co-surfactant–oil compositions. If the oil composition is increased, the interaction balance is not reached so that a nonhomogeneous (separating) phase is formed. The bond between VCO and Tween 80 occurred due to oleic acid contained in Tween 80. Oleic acid has an Xlog P of 6.5 so that oleic acid easily binds to other lipophilic compounds. Compounds that have log P > 4 were included in the category of high lipophilic compound (Pyka, 2009). This causes Tween 80 which contains 58% oleic acid to bind to VCO. The results of the SNEDDS observations were compared with the results of the predictive responses produced by the optimal formula in the simplex lattice design. Verification was then performed using the t-test from one-sample t-test on OpenStat software. The data analysis with SPSS used one-sample t-test. In the transmittance test parameters, the obtained p-value = 0.610 > 0.05, so there was no difference between the predictions of the Design-Expert software with the simplex lattice design method and the experimental results on the optimal SNEDDS formula. In the pH test, the obtained p-value = 0.321 > 0.05, indicating no difference between the prediction of the software and the experimental results. Furthermore, the emulsification time test showed the obtained p-value = 0.550 > 0.05, meaning that there was no significant difference between the prediction of the software and the experimental results.
 | Table 6. Optimal SNEDDS evaluation results (Design Expert Ver. 7.0.0.). [Click here to view] |
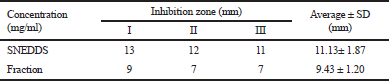 | Table 7. Result of the inhibition zone of ethyl acetate fraction against S. epidermidis. [Click here to view] |
Observation of the size and particle size distribution of nanoemulsion droplets of ethyl acetate fraction from mangosteen peel
The observation of the nanoemulsion droplet size was carried out to ensure that the nanoemulsion droplet size was less than 200 nm. Based on the test results, the average size of the nanoemulsion was less than 100 nm, which is 77.3 nm. This proved that SNEDDS preparations enable the production of nanoemulsions. Drop size decreased due to increased surfactant concentration (Kassem et al., 2016). The higher the ratio of surfactants compared to co-surfactants, the smaller the size of the obtained nanoemulsion (Xi et al., 2009). The surfactants could cause interface film to decrease and stabilize, resulting in small droplet diameters, whereas the addition of co-surfactants was possible to result in wider interface films (Fahmy et al., 2015; Hosny and Banjar, 2013). The relative proportions of surfactants and co-surfactants caused variations in droplet size (Singh et al., 2010).
The particle size in nanoemulsion was also influenced by oil composition (Singh et al., 2010). Oil was able to increase the ability of SNEDDS to carry drugs but made the size of nanoemulsion even greater so that the ratio of oil used was always smaller than surfactants (Fernandez et al., 2004). Nanoemulsion droplet size could regulate an effective drug release (Badran et al., 2014; Larsen et al., 2013; Parmar et al., 2011; Singh et al., 2010). In another study, an average obtained the droplet size of five formulations was in the range of 26.45–85.94 nm. This was related to the relative increase in the proportion of surfactants in the stabilization of oil droplets as a result of localization of surfactant molecules at the oil–water interface (Dixit et al., 2010; Parmar et al., 2011). The small size of the SNEDDS formula drops was due to the reduction in surface tension due to the presence of surfactants and co-surfactants (Yoo et al., 2010).
The value of polydispersity index (PDI) expresses the homogeneity of nanoemulsion particles. The PI value obtained from testing with distilled water was 0.232. PI values ​​varied from 0.0 to 1.0, and the closer they were to 0, the more homogeneous the particles (Patel et al., 2010). PDI of less than 0.5 indicated a uniform globule size distribution (Balakumar et al., 2013; Shakeel et al., 2014), so it can be concluded that the uniform distribution of nanoemulsion particle sizes and the methods of making nanoemulsion have good reliability.
Potential zeta nanoemulsion of ethyl acetate fraction from mangosteen peels
Based on the research, the zeta potential value was −8.29 mV. Nanoemulsion droplets generated from this study had zeta potential according to the requirements. The zeta potential value in the range of ± 30 mV was the limit value that is able to maintain the stability of the emulsion because the almost neutral value reduced the possibility of particles to form aggregates (Honary and Zahir, 2013). At the zeta potential, the droplet surface charge will affect the stability of the SNEDDS formulation because of the electrostatic repulsion force between the droplets which prevents the incorporation of nanoemulsion. The negative value obtained in the study was due to the presence of surfactants and co-surfactants in the emulsion (Yoo et al., 2010). Kaseem et al. (2016) showed that the optimized formulation was negatively charged, with values ranging from −15.3 to −23.9 mV, indicating a stable system and well-separated emulsion bubbles (Agrawal et al., 2015). Electrostatic repulsion between negatively charged droplets avoided the formation of coalescence in nanoemulsions (Badran et al., 2014). Nonionic surfactants produced a negatively charged interface at neutral pH due to the differential adsorptions of hydroxyl ions (OH~) and oxonium hydrated ions (H3O+) (Choi et al., 2014).
 | Figure 6. The effectiveness of SNEDDS FEA and EAF mangosteen peels against S. epidermidis (A) SNEDDS FEA and (B) EAF mangosteen peels. [Click here to view] |
Optimal SNEDDS FEA and FEA antibacterial activity
Antibacterial activity test of mangosteen peel that has been done showed that there is an inhibition of S. epidermidis growth characterized by the formation of inhibitory zones around the well that have been filled with test solutions, indicating that there is no bacterial growth. Based on Figure 6, FEA SNEDDS had a greater inhibition zone than FEA without preparations did. The antibacterial activity test results of SNEDDS ethyl acetate fraction resulted in an inhibition zone diameter of 11.13 mm ± 1.87 mm, whereas FEA without the preparation resulted in inhibition zone diameter of 9.43 mm ± 1.20 mm.
The antibacterial activities of SNEDDS FEA and FEA in inhibiting the growth of S. epidermidis were thought to be the influence of the content of several secondary metabolite compounds contained in the fraction. Active ingredients that are used as antibacterial could interfere with physiological processes and inhibit the formation of bacterial cell components such as cell wall synthesis, cytoplasmic membrane protein synthesis, and nucleic acid synthesis (Subandrio, 1995). The active ingredient, which has a high solubility in polar solvents, would penetrate the phospholipid layer of the cell membrane more easily so that it would disrupt the physiological function of bacteria more quickly, and eventually, cells would experience death (Kneblock et al., 1989).
Xanthone was a form of flavonoids contained in mangosteen peel which can denaturate proteins that cause cell metabolic activity to stop (Trease and Evan, 1978). Besides, the mechanism of xanthone antimicrobial activity was thought to be due to the reaction of the carbonyl group in xanthone with amino acid residues in cell membrane proteins, extracellular enzymes, and cell wall proteins, which cause proteins to lose their function (Nengah and Putera, 2010). Saponins, tannins, and flavonoids were compounds in plants that have antibacterial activity (Poeloengan and Praptiwi, 2010). Furthermore, tannins were thought to be able to shrink the cell walls of bacteria so that they could interfere with cell permeability. The disruption of bacterial cell permeability made these cells unable to perform living activities so that growth was stunted or dies (Ajizah, 2004).
In this study, SNEDDS was formulated from Tween 80 as a surfactant, PEG 400 as a co-surfactant, and VCO as an oil phase. Tween 80 can be used as a penetration enhancer because Tween 80 is a surfactant that works by dissolving lipophilic compounds and dissolving the lipid layer on the stratum corneum. Ionic surfactants tend to cause damage to human skin and increase a water loss in the skin because it can be retained on stratum corneum layer (Aiache, 1982). Nonionic surfactants were safer to use because they did not cause skin damage, were more stable, and were practically not absorbed in the stratum corneum layer. In fatty acids, the longer chain of fatty acids will increase the penetration. The fatty acids found in VCO are lauric acids. It can increase the penetration of hydrophilic or lipophilic compounds. The mechanism was done by interactions with lipids on the stratum corneum (Swarbrick and Boylan, 1995; William and Barry, 2004).
In other research, Sugita et al. (2017) used mangosteen pericarp nanosized extract, and the fractionation of extract provided six fractions. Fractions 5 and 6 had the highest antibacterial activity over Bacillus cereus, Staphylococcus aureus, and Shigella flexneri. This research indicated that the mangosteen peels have antimicrobial activity. Sari et al. (2016) tested the effectiveness of SNEDDS mangosteen peel extract against Proteus mirabilis and S. epidermidis bacteria in diabetic ulcers, and the results show that administration of extracts and SNEDDS provides a better inhibitory zone effect than the negative control group. The results of the analysis in the SNEDDS group and extracts on P. mirabilis showed 0.002, which means that there were significant differences in the two groups. This research shows that SNEDDS provides a better inhibitory zone effect than the negative control group. Romas et al. (2015) evaluated that the ethanol extract of mangosteen rind (with concentration 5%, 10%, 20%, 40%, 60%, and 80% w/v) can inhibit the growth S. aureus with a mean inhibition zone diameter of 10.6 mm, 12.6 mm, 14.8 mm, 15 mm, 15.8 mm, and 16.8 mm. These results are not significantly different from the results in this study despite using different bacteria.
CONCLUSION
The obtained SNEDDS of the optimal ethyl acetate fraction was a combination of Tween 80:PEG 400:VCO with a composition ratio of 4.98:1.02:1. Based on the evaluation, the transmittance value was 72.74% ± 1.08%, pH was 6.48 ± 0.03, the emulsification time was 4.83 ± 0.95 sec, the average droplet size was 77.3 nm, and the zeta potential value was −8.29 mV. SNEDDS FEA had antibacterial activity against S. epidermidis with the inhibition zones of 11.13 mm ± 1.87 mm, greater than of EAF without preparations with the inhibition zones of 9.43 mm ± 1.20 mm.
ACKNOWLEDGMENT
The authors would like to thank RISTEKDIKTI and Faculty of Medicine Tanjungpura University, Prof. Dr. Hadari Nawawi Street, West Kalimantan, Indonesia.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
Agrawal M, Agrawal Y, Itankar P, Patil A, Vyas J, Kelkar A. Phytochemical and HPTLC studies of various extracts of Annona squamosa (Annonaceae). Int J PharmTech Res, 2012; 4:364–8.
Aiache JM. Pharmacetics 2: biopharmaceuticals. Airlangga University Press, Surabaya, Indonesia, 1982.
Ajizah A. Salmonella thypimurium sensitivity against Psidium guajava leaf extract L. J Biosci, 2004; 1(1):31–8.
Amrutkar C, Salunkhe K, Chaudhari S. Review on self nanoemulsifying drug delivery system. Am J PharmTech Res, 2014; 4:2249–3387.
Badran MM, Taha EI, Tayel MM, Al-Suwayeh SA. Ultra-fine self nanoemulsifying drug delivery system for transdermal delivery of meloxicam: dependency on the type of surfactants. J Mol Liquids, 2014; 190:16–22. CrossRef
Balakumar K, Raghavan CV, Selvan NT, Prasad RH, Abdu S. Self nanoemulsifying drug delivery system (SNEDDS) of Rosuvastatin calcium: design, formulation, bioavailability and pharmacokinetic evaluation. Colloid Surf B Biointerfaces, 2013; 112:337–43. CrossRef
Bartlett J, Dowell S, Mandell L, File T, Musher D, Fine M. Practice guidelines for the management of community-acquired pneumonia in adults. Infectious Diseases Society of America. Clin Infect Dis, 2000; 31:347–82. CrossRef
Basalious EB, Shawky N, Badr-Eldin SM. SNEDDS containing bioenhancers for improvement of dissolution and oral absorption of lacidipine: development and optimization. Int J Pharm, 2010; 391:203–1. CrossRef
Beg S, Jena SS, Patra CN, Rizean M, Swain S, Sruti J, Rao ME, Singh B. Development of solid self-nanoemulsifying granules (SSNEGs) of Ondancentron hydrochloride with enhanced bioavailability potential. Colloid Surface B Biointerfaces, 2013; 101:414–23. CrossRef
Chen LG, Yang LL, Wang CC. Anti inflammatory activity of mangostins from Garcinia mangostana. Food Chem Toxicol, 2008; 46:688–93. CrossRef
Choi KO, Aditya NP, Ko S. Effect of aqueous pH and electrolyte concentration on structure, stability and flow behavior of non-ionic surfactant based solid lipid nanoparticles. Food Chem, 2014; 147:239–44. CrossRef
Difco. Manual of dehydrated culture media and reagents for microbiology and clinical laboratory procedures. 9th edition, Difco Laboratories, Detroit, MI, 1977.
Dixit AR, Rajput SJ, Patel SG. Preparation and bioavailability assessment of SMEDDS containing valsartan. Am Assoc Pharm Sci, 2010; 11:314–21. CrossRef
Eid AM, Baie SH, Arafat OM. The effect of surfactant blends on the production of a novel switeria macrophylla oil self-nanoemulsifying system. Int J Pharm Pharm Sci, 2012; 4:481–6.
Fahmy UA, Ahmed OA, Hosny KM. Development and evaluation of avanafil selfnanoemulsifying drug delivery system with rapid onset of action and enhanced bioavailability. Am Assoc Pharm Sci, 2015; 16:53–8. CrossRef
Fei X, Jo M, Lee B, Han SB, Lee K, Jung J-K, Seo S-Y, Kwak Y-S. Synthesis of xanthone derivates based on α-mangostin and their biological evaluation for anti-cancer agents. Bioorg Med Chem Lett, 2014; 24:2062–5. CrossRef
Fernandez P, Andre V, Rieger J, Kuhnle A. Nanoemulsion formation by emulsion phase inversion. Colloid Surf A Physichochem Eng Aspects, 2004; 251:53–8. CrossRef
Harborne J. Phyto chemical method. Command and Hall, London, UK, 1973.
Honary S and Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems—a review (Part 2). Trop J Pharm Res, 2013; 12:265–73. CrossRef
Hosny KM, Banjar ZM. The formulation of a nasal nanoemulsion zaleplon in situ gel for the treatment of insomnia. Expert Opin Drug Deliv, 2013; 10:1033–41. CrossRef
Kassem A, Mohsen AM, Ahmed RS, Essam TM. Self-nanoemulsifying drug delivery sytem (SNEDDS) with enhanced solubilization of nystatin for treatment of oral candidiasis: Design, optimization, in vitro and in vivo evaluation. J Mol Liquids, 2016; 218: 219–232. CrossRef
Kneblock KA, Pauli A, Iberl B, Weigland H, Weis N. Antibacterial and antifungal properties of essential oil components. J. Essensial Oil Res, 1989. CrossRef
Larsen AT, Åkesson P, Juréus A, Saaby L, Abu-Rmaileh R, Abrahamsson B, Østergaard J, Müllertz A. Bioavailability of cinnarizine in dogs: effect of SNEDDS loading level and correlation with cinnarizine solubilization during in vitro lipolysis. Pharm Res, 2013; 30:3101–13. CrossRef
Nengah I. Antibacterial activity of mangosteen (Garcinia mangostana L.) skin extract and compound content. Active J Technol Food Industry, 2010; 21(1):1–5.
Parmar N, Singla N, Amin S, Kohli K. Study of cosurfactant effect on nanoemulsifying area and development of lercanidipine loaded (SNEDDS) self nanoemulsifying drug delivery system. Colloid Surf Biointerfaces, 2011; 86: 327–338. CrossRef
Parveen N, Khan N. Two xanthones from Garcinia mangostana. Phytochemistry, 1988; 27:3694–6. CrossRef
Patel MJ, Patel N, Patel M. A self-microemulsifying drug delivery system (SNEDDS). Int J Pharm Pharm Sci, 2010; 4:29–33. CrossRef
Patel J, Kevin G, Patel A, Raval M, Sheth N. Design and development of a self-nanoemulsifying drug delivery system for telmisartan for oral drug delivery. Int J Pharm Investig, 2011; 1:112–8. CrossRef
Poeloengan M, Praptiwi P. Antibacterial activity test for mangosteen skin extract (Garcinia mangostana Linn). Media Litbang Kesehatan, 2010; 10(2):65–9.
Pratiwi L, Sari R, Apridamayanti P. Self-nanoemulsifying drug delivery system (SNEDDS) with enhanched solubilization of ethanol extract from mangosteen peels (Garcinia mangostana L.,) for treatment of topical gangrene foot: design and optimization. Int J Drug Deliv Technol, 2017a; 7(4):314–9. CrossRef
Pratiwi L, Fudholi A, Martien R, Pramono S. Self-nanoemulsifying drug delivery system (SNEDDS) for topical delivery of mangosteen peels (Garcinia mangostana L.,): formulation design and in vitro studies. J Young Pharm, 2017b; 9(3):341–6. CrossRef
Pradhan A, Pinheiro J, Seena S, Pascoal C, Cássioa P. Polyhydroxyfullerene binds cadmium ions and alleviates metalInduced oxidative stress in Saccharomyces cerevisiae. Appl Environ Microbiol, 2014; 80(18):5874–81. CrossRef
Pyka A. Evaluation of the lipophilicity of fat-soluble vitamins. J Planar Chromatogr Modern TLC, 2009; 22:211–5. CrossRef
Phitaktim S, Chomnawang M, Sirichaiwetchakoon K, Dunkhunthod B, Hobbs G, Eumkeb G. Synergism and the mechanism of action of the combination of α-mangostin isolated from Garcinia mangostana L. and oxacillin against an oxacillin-resistant Staphylococcus saprophyticus. BMC Microbiol, 2016; 16:195. CrossRef
Radji M. Buku Ajar Mikrobiologi: guide pharmacy and medical students. Medical Book Publisher EGC, Jakarta, Indonesia, 2010.
Rahimah, Sayekti E, Jayuska A. Characterization of flavonoid compounds as isolate result from Matoa leaf ethyl acetate (Pomentia pinnata J.R.Forst & G.Forst) fraction. J Equatorial Chem, 2013; 2:84–9.
Romas A, Rosyidah DU, Aziz MA. The antibacterial activity of ethanol extract of mangosteen rind (Garcinia mangostana l) against Escherichia coli ATCC 11229 and Staphylococcus aureus ATCC 6538 in vitro. University Research Colloquium, 2015, 127–32; ISSN 2407-918.
Sari R, Pratiwi L, Apridamayanti P. The effectiveness of SNEDDS mangosteen peel extract against P. mirabilis and S. epidermidis bacteria found in diabetic ulcers. Pharm Sci Res, 2016; 3:130–9. CrossRef
Shafiq-un-Nabi S, Shakeel F, Talegaonkar S, Ali J, Baboota S, Ahuja A, Khar RK, Ali M. Formulation development and optimization using nanoemulsion technique: a technical note. Am Assoc Pharm Sci, 2007; 8:12–7. CrossRef
Shakeel F, Haq N, Alanazi FK, Alsarra IA. Polymeric solid self-nanoemulsifying drug delivery system of glibenclamide using coffee husk as a low cost biosorbent. Powder Technol, 2014; 256:352–60. CrossRef
Shah P, Bhalodia D, Shelat P. Nanoemulsion: a pharmaceutical review. Syst Rev Pharm, 2010; 1:24. CrossRef
Singh SK, Verma PR, Razdan B. Glibenclamide-loaded self-nanoemulsifying drug delivery system: development and characterization. Drug Dev Ind Pharm, 2010; 36: 933–45. CrossRef
Subandrio WK. Antimicrobial chemotherapy, antibiotics. Faculty of Mathematics and Natural Sciences, Jakarta, Indonesia, 1995.
Sugita P, Arya S, Ilmiawati A, Arifin B. Characterization, antibacterial and antioxidant activity of mangosteen (Garcinia mangostana L.) pericarp nanosized extract. RASAYAN J. Chem, 2013; 10:707–15.
Sundaram BM, Gopalakrishnan C, Subramanian S, Shankaranarayanan D, Kameswaran L. Antimicrobial activities of Garcinia mangostana. Planta Med, 1983; 48:59–60. CrossRef
Swarbrick J and Boylan JC. Encyclopedia of pharmaceutical technology. Marcel Dekker, New York, 1995.
Taher M, Zakaria T, Susanti D, Zakaria Z. Hypoglycaemic activity of ethanolic extract of Garcinia mangostan Linn in nomoglycaemic and streptozotocin-induced diabetic rats. Complement Altern Med, 2016; 16:135. CrossRef
Tjahjani S. Antimalarial activity of Garcinia mangostana L rind and its synergistic effect with artemisinin in vitro. BMC Complement Altern Med, 2017; 17(131):2–5. CrossRef
Tjahjani S, Widowati W, Khiong K, Suhendra A, Tjokropranoto R. Antioxidant Properties of Garcinia Mangostana L (Mangosteen) Rind. Procedia Chem, 2014; 13:198–203. CrossRef
Tranggono R, Fatma L. Cosmetic science handbook. Gramedia Pustaka Utama, Jakarta, Indonesia, 2007.
Trease GE, Evans WC. A textbook of pharmacognosy. 11th edition, Bailleire Tindal, London, UK, 1978.
Vilas P, Gujarathi, N, Bhushan R. Preparation and in vitro evaluation of self-nanoemulsifying drug delivery system (SNEDDS) containing clopidogrel. Int J Pharm Sci Rev Res, 2014; 25:10–5.
Williams AC, Barry BW. Penetration enhancers. Adv Drug Deliv Rev, 2004; 56:603–18. CrossRef
Xi J, Chang Q, Chan CK, Meng ZY, Wang GN, Sun JB, Wang YT, Tong HH, Zheng Y. Formulation development and bioavailability evaluation of a self-nanoemulsified drug delivery system of oleanolic acid. Am Assoc Pharm Sci, 2009; 10:172–82. CrossRef
Yao L, Gu X, Song Q, Wang X, Huang M, Hu M, Hou L, Kang T, Chen J, Chen H, Gao X. Nanoformulated alpha-mangostin ameliorates alzheimer's disease neuropathology by elevating LDLR expression and accelerating amyloid-beta clearance. J Control Release, 2016; 226: 1-14. CrossRef
Yoo JH, Shanmugam S, Thapa P, Lee ES, Balakrishnan P, Baskaran R, Yoon SK, Choi HG, Yong CS, Yoo BK, Han K. Novel self-nanoemulsifying drug delivery system for enhanced solubility and dissolution of lutein. Arch Pharm Res, 2010; 33:417–26. CrossRef
Zhao Y, Wang C, Chow AH, Ren K, Gong T, Zhang Z, Zheng Y. Self-nanoemulsifying drug delivery system (SNEDDS) for oral delivery of Zedoary essential oil: Formulation and bioavailability studies. Int J Pharm, 2010; 383:170–7. CrossRef
Zhang C, Yu G, Shen Y. The naturally occurring xanthone α-mangostin induces ROS-mediated cytotoxicity in non-small scale lung cancer cells. Saudi J Biol Sci, 2017; 25:1090–5. CrossRef
Zuo J, Yin Q, Wang Y-W, Li Y, Lu L-M, Xiao Z-G, Wang G-D, Luan J-J. Inhibition of Nf-κb pathway in fibroblast-like synoviocytes by α-mangostin implicated in protective effects on joints in rats suffering from adjuvant-induced arthritis. Int Immunopharmacol. 2018; 56:78–89.