Pharmacological and Pharmaceutical Profile of Valsartan: A Review
Nadeem Siddiqui, Asif Husain, Lakshita Chaudhry, M Shamsher Alam, Moloy Mitra, Parminder S. Bhasin
Pages: 12-19

Quantitative analysis of valsartan in tablets formulations by High Performance Thin-Layer Chromatography
Della Grace Thomas Parambi, Molly Mathew, V.Ganesan
Pages: 76-78

A validated stability indicating HPLC method for the determination of Valsartan in tablet dosage forms
Della Grace Thomas Parambi, Molly Mathew, V.Ganesan
Pages: 97-99

Effect of Recrystallization on the Pharmaceutical Properties of Valsartan for Improved Therapeutic Efficacy
Buchi N. Nalluri, Ramya Krishna M, Rao TP, Peter A. Crooks
DOI: 10.7324/JAPS.2012.21025Pages: 126-132

Spectrophotometric Determination of Valsartan using p-Chloranilic Acid as \p-Acceptor in Pure and in Dosage Forms
S. M. Mallegowda, H. N. Deepakumari and H. D. Revanasiddappa
DOI: 10.7324/JAPS.2013.30122Pages: 113-116

Controlled drug release studies of valsartan using differently sulfonated methacryloxyacetophenone and methyl methacrylate co-polymer resins as drug carriers
K. Doraswamy, V. Nagaraju, R. Srinivasulu and P. Venkata Ramana
DOI: 10.7324/JAPS.2013.31120Pages: 110-116

Design, Development and Characterization of Valsartan Microsponges by Quasi Emulsion Technique and the Impact of Stirring Rate on Microsponge Formation
Madhuri Desavathu, Raghuveer Pathuri, Mounika Chunduru
DOI: 10.7324/JAPS.2017.70128Pages: 193-198

Validated Eco-Friendly Chromatographic Methods for Simultaneous Determination of Sacubitril and Valsartan in Spiked Human Plasma and in Pharmaceutical Formulation
Amal Mahmoud Abou Al Alamein
DOI: 10.7324/JAPS.2018.8202Pages: 011-017

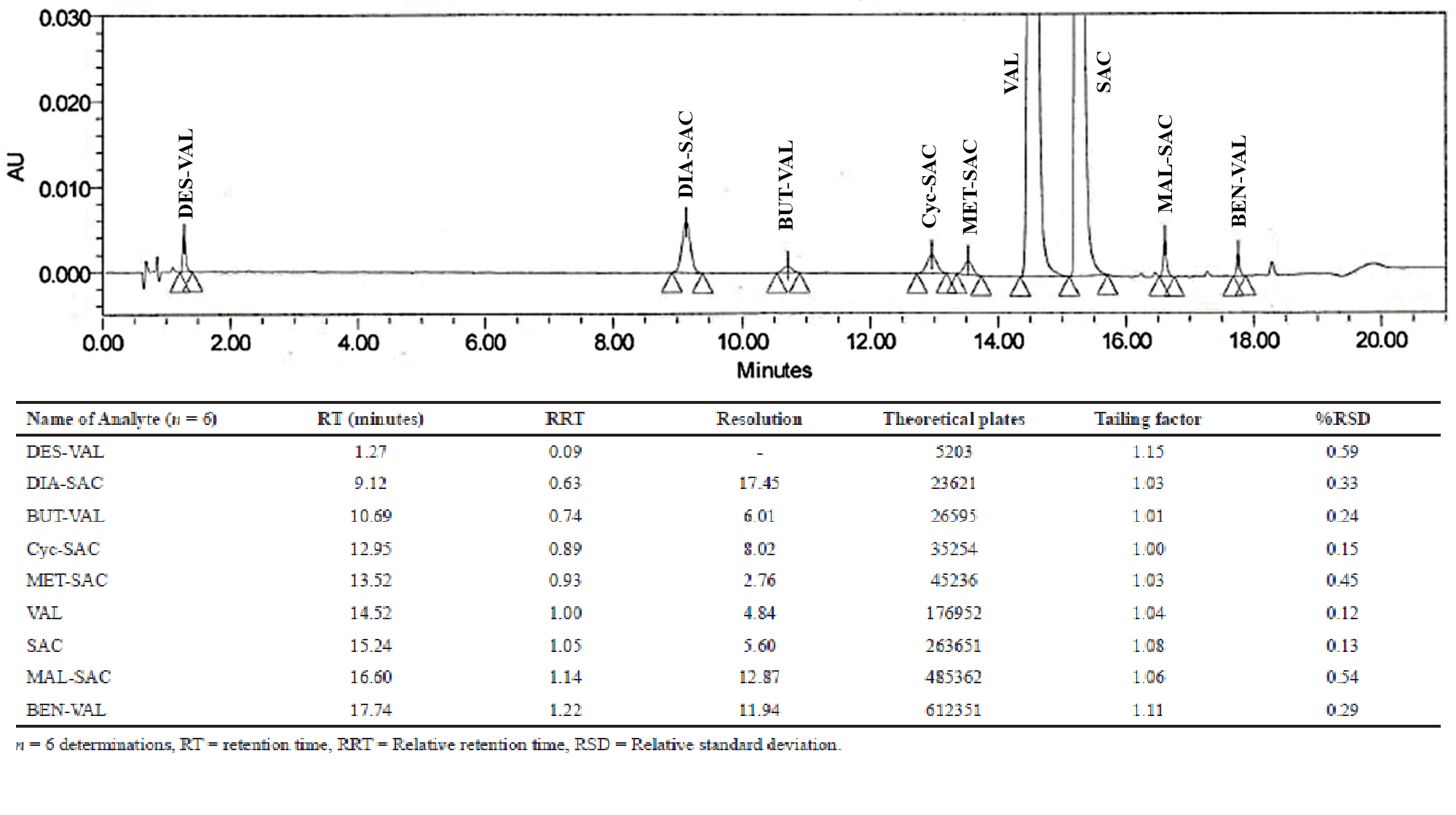
Development and validation of a stability indicating UHPLC method for Sacubitril/Valsartan complex in the presence of impurities and degradation products
Pintu Prajapati, Dhara Bhayani, Priti Mehta
DOI: 10.7324/JAPS.2020.102015Pages: 097-107