Development and Validation of UV-Spectroscopic Method for Estimation of Niacin in Bulk and Pharmaceutical Dosage Form
Indranil Chanda, Ripunjoy Bordoloi, Debarupa D. Chakraborty, Prithviraj Chakraborty, Smriti Rekha Chanda Das
DOI: 10.7324/JAPS.2017.70911Pages: 081-084

Estimation of Bosentan Monohydrate in Male Rabbit Plasma by using RP-HPLC Method
Revathi Mannam, Indira Muzib Yallamalli
DOI: 10.7324/JAPS.2017.71116Pages: 106-109

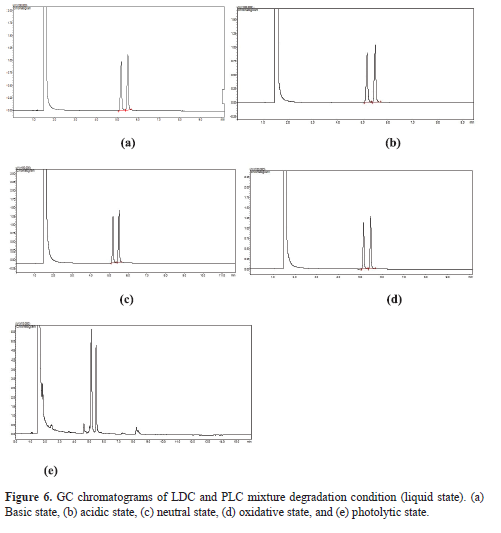
Simultaneous estimation of lidocaine and prilocaine in topical cream by green gas chromatography
Hashim Chekku Marakkarakath, Gurupadayya Bannimath, Prachi Pramesh Raikar
DOI: 10.7324/JAPS.2019.90310Pages: 066-072
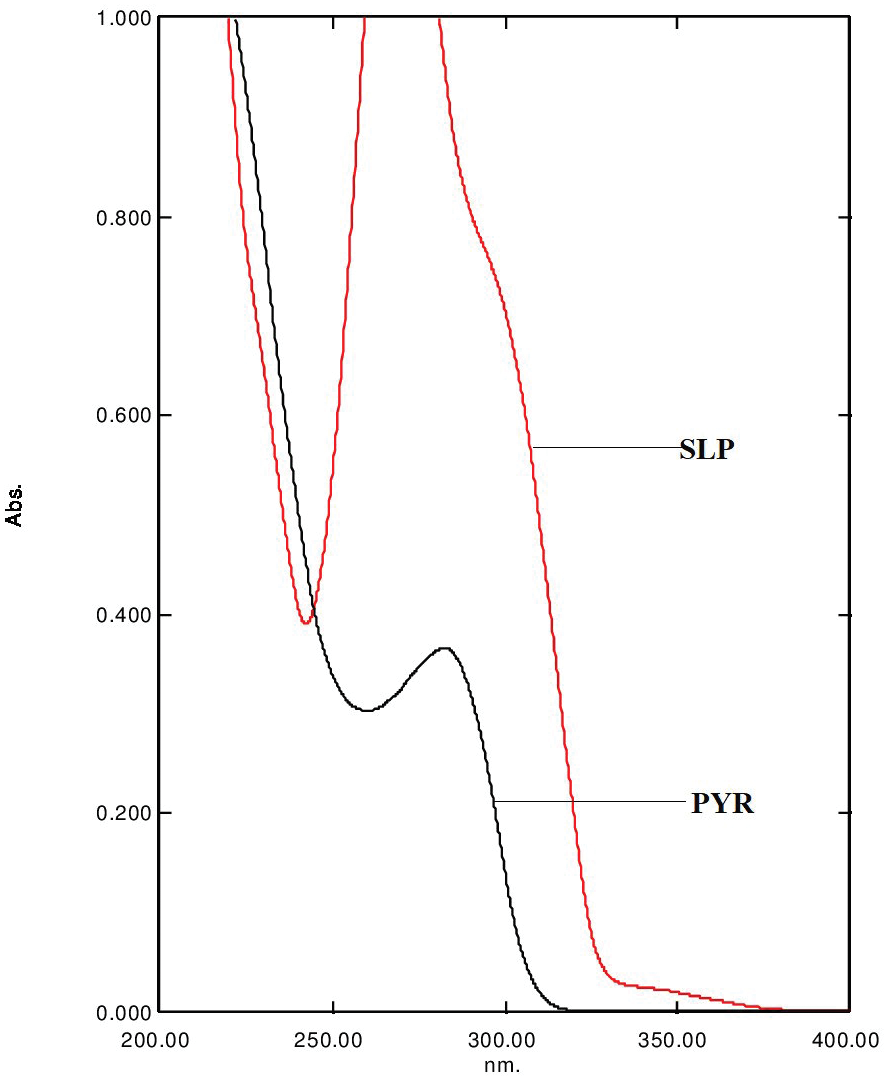
Stability indicating RP-HPLC method for simultaneous determination of pyrimethamine and sulfamethoxypyrazine in pharmaceutical formulation: Application to method validation
Shankaranahalli Gurusiddappa Keshava, Gurupadayya Bannimath, Prachi Raikar, Maruthi Reddy
DOI: 10.7324/JAPS.2020.102008Pages: 049-055
LC-MS method development for the quantitation of potential genotoxic impurity 2-Methyl-6-nitro aniline in Telmisartan API
Duvvuri Suryakala, Sivakumar Susarla, Bandlamudi Mallikarjuna Rao
DOI: 10.7324/JAPS.2020.10512Pages: 092-096
Simultaneous detection and quantification of bronchodilators in pure form and from in-vitro drug release of a novel combinational formulation
Sheena M. Raj, Vilas G. Jamakandi, Sunil S. Jalalpure, Pradeepkumar M. Ronad
DOI: 10.7324/JAPS.2020.10815Pages: 131-138

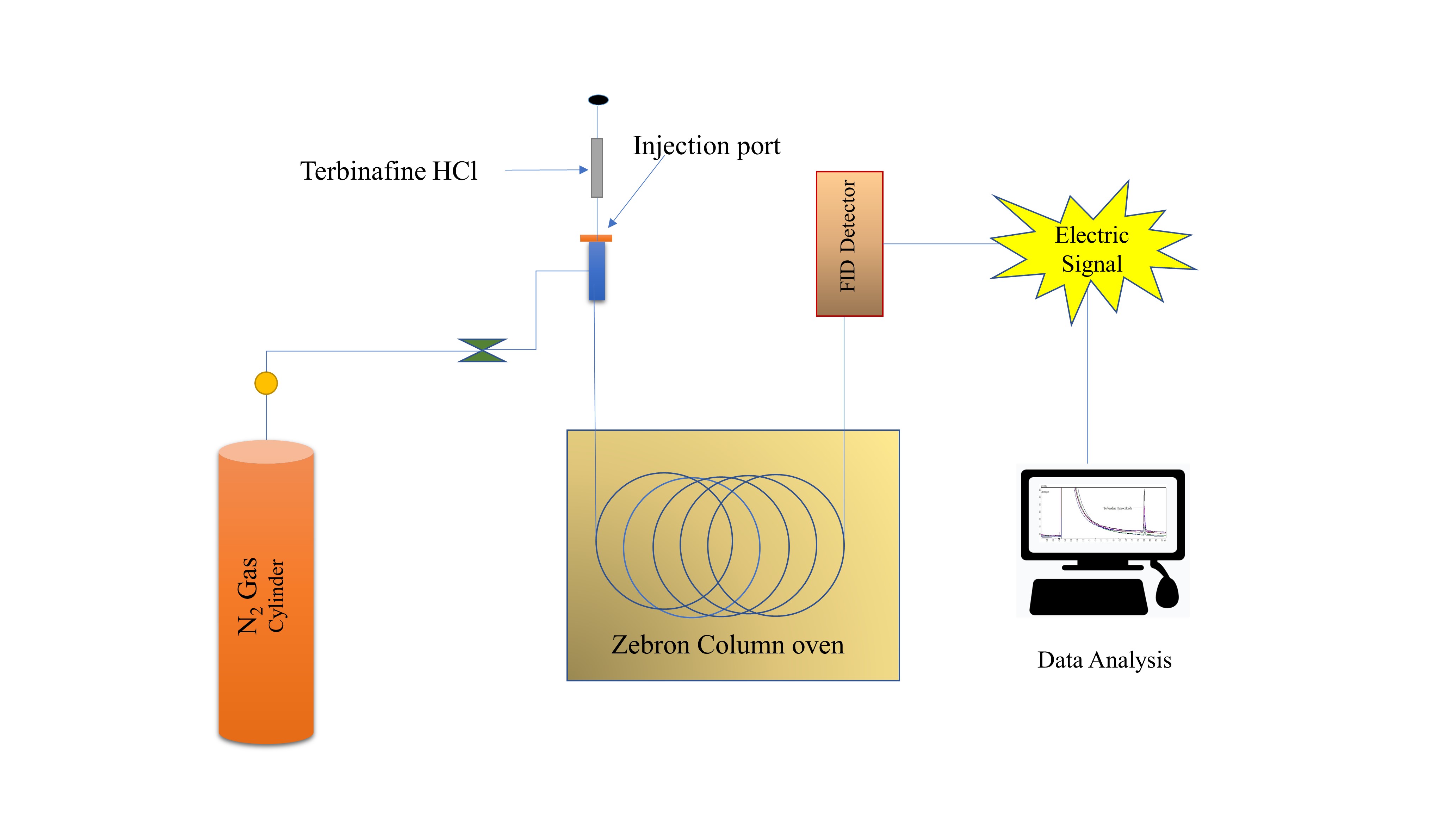
Estimation of terbinafine HCl in tablet dosage form by green gas chromatography
Kalyani Reddy, Gurupadayya Bannimath, Maruthi Reddy, Akshay Nanjundappa
DOI: 10.7324/JAPS.2021.110610Pages: 087-093
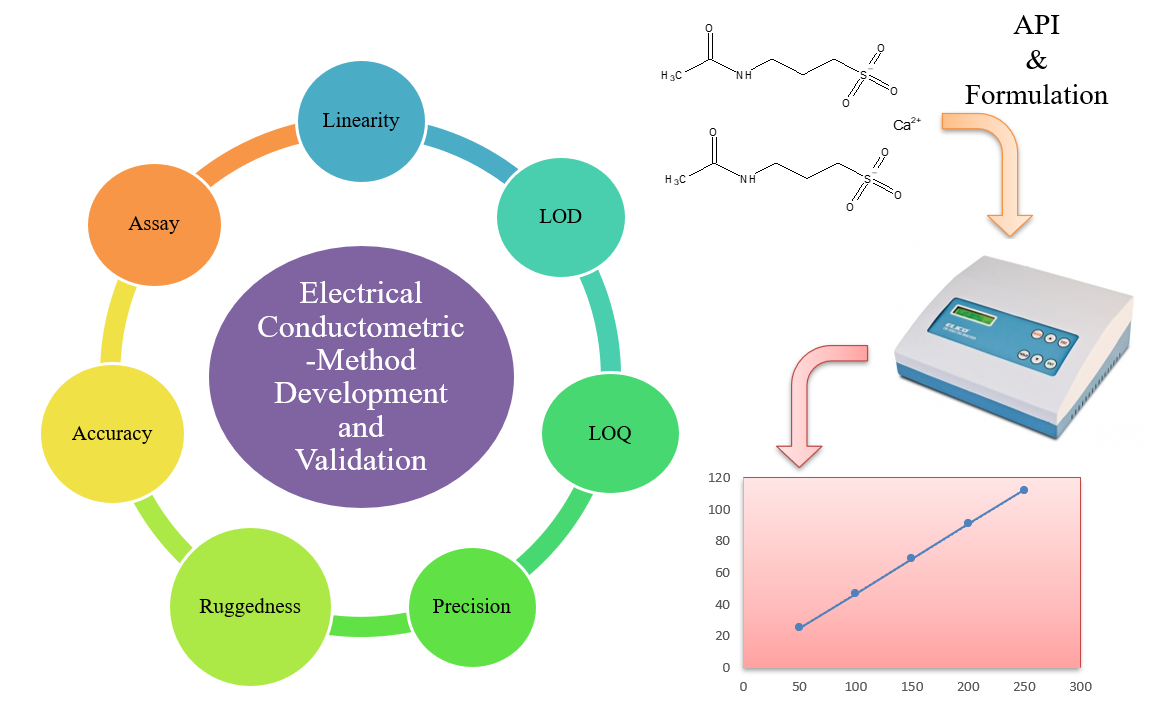
Conductometric method development and validation to estimate acamprosate calcium in API and marketed formulation
Rahul K. Yadav, Meenaxi M. Maste, Shailendra S. Surywanshi, Utkarsh Shastri
DOI: 10.7324/JAPS.2021.1101111Pages: 082–086
Development of validated stability-indicating HPTLC method for the estimation of ulipristal acetate in bulk and dosage form
Shruti Srivastava, Suneela Dhaneshwar, Neha Kawathekar
DOI: 10.7324/JAPS.2021.1101120Pages: 161-167

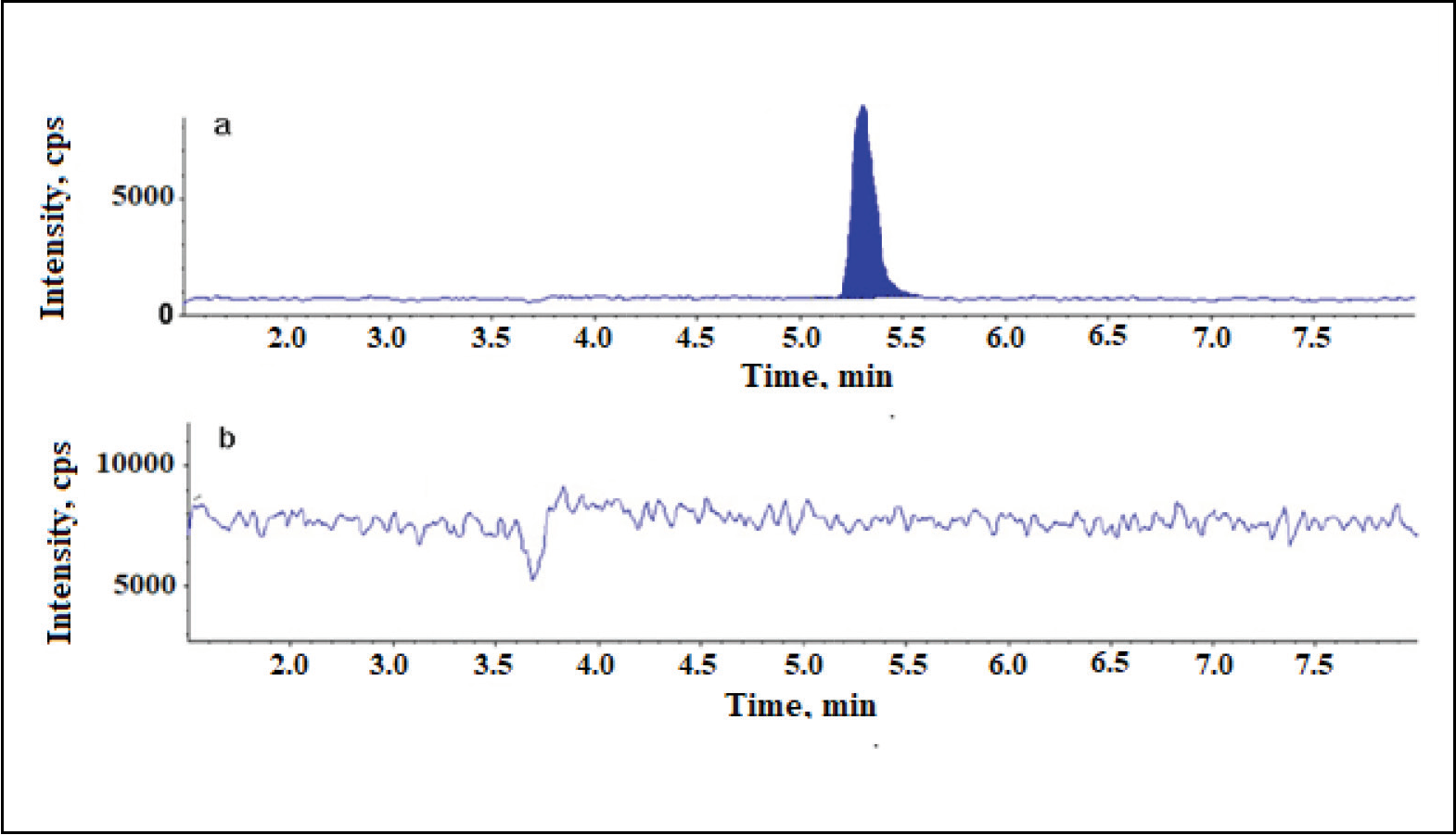
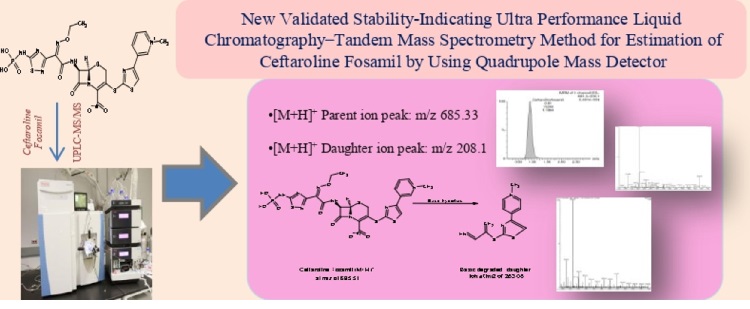
Newly validated stability-indicating ultra-performance liquid chromatography-tandem mass spectrometry method for the estimation of Ceftaroline Fosamil by using a quadrupole mass detector
Jabeen, Bangalore Venkatappa Suma
DOI: 10.7324/JAPS.2022.120621Pages: 215-223
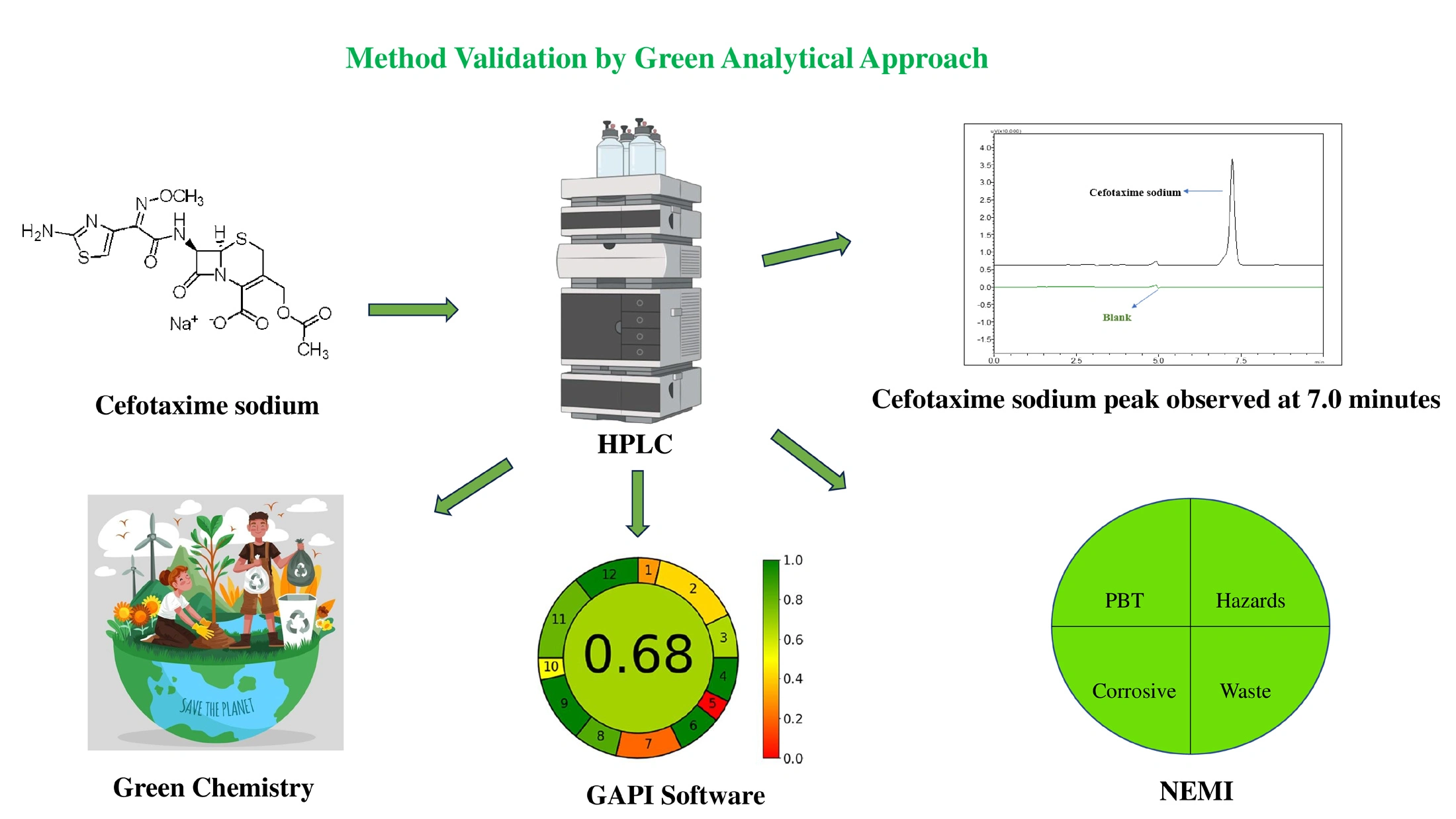
RP-HPLC method for quantification of cefotaxime sodium by using design of experiment, a green analytical approach: Analytical method development, validation, and application
Akhil Nair, H. Raghu Chandrashekhar, Usha Y. Nayak
DOI: 10.7324/JAPS.2024.192430Pages: 098-112