Analytical Method Development and Validation of Anti-HIV Drug Abacavir Sulphate
Pradeep Nagisetty, S.M.Shanta Kumar, Putta Rajesh Kumar
Pages: 85-89

Q-Absorbance Ratio Spectrophotometric Method for the Simultaneous Estimation of Prednisolone and 5-Amino Salicylic Acid in Tablet Dosage form
Gurmeet Singh, Dinesh Kumar, Deepak Sharma, Mankaran Singh, Sukhbir Kaur
DOI: 10.7324/JAPS.2012.2736Pages: 222-226

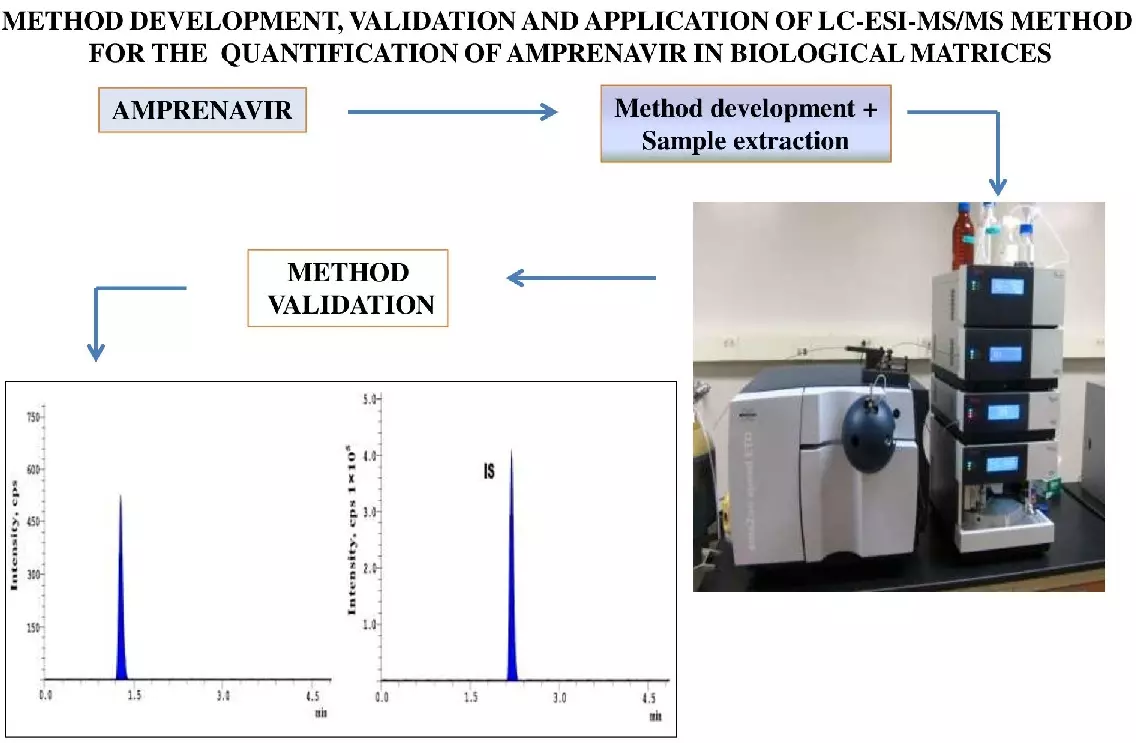
Method development, validation, and application of liquid chromatography-electrospray ionization-mass spectrometry/mass spectrometry method for the quantification of amprenavir in plasma samples
Bandaru Anil Kumar, Bomma Ramesh, Shankar Cheruku, D.V.R.N. Bhikshapathi
DOI: 10.7324/JAPS.2022.120712Pages: 115-121
Development and validation of LC-MS/MS method for alpelisib quantification in human plasma: Application to pharmacokinetics in healthy rabbits
Tandrima Majumder, Shiva Kumar Gubbiyappa
DOI: 10.7324/JAPS.2023.75269Pages: 089-096

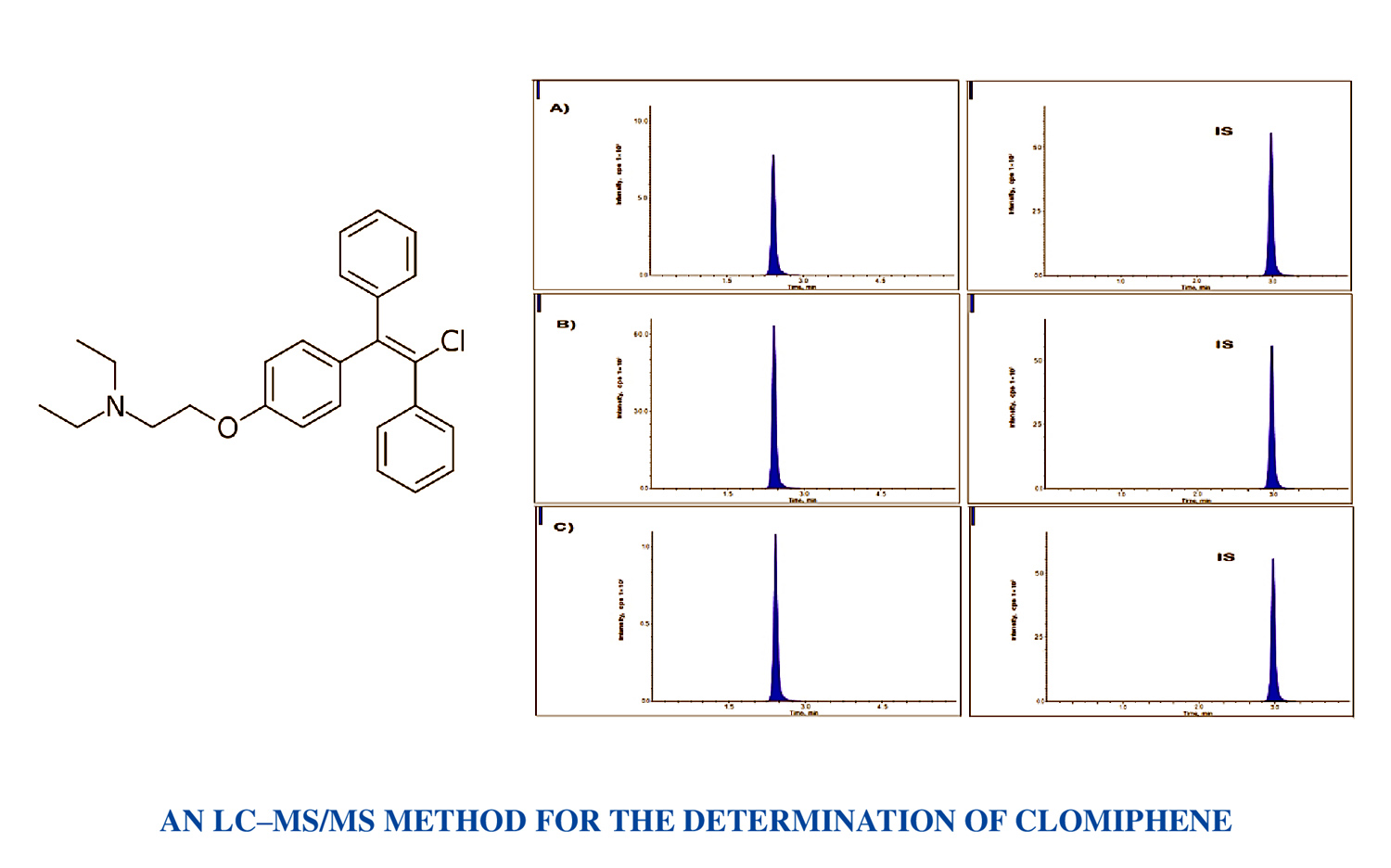
An LC–MS/MS method development and validation for the determination of clomiphene in biological matrices
Vinayaga Sundaram Krishnan, Bhikshapathi Darna
DOI: 10.7324/JAPS.2023.144778Pages: 184-189
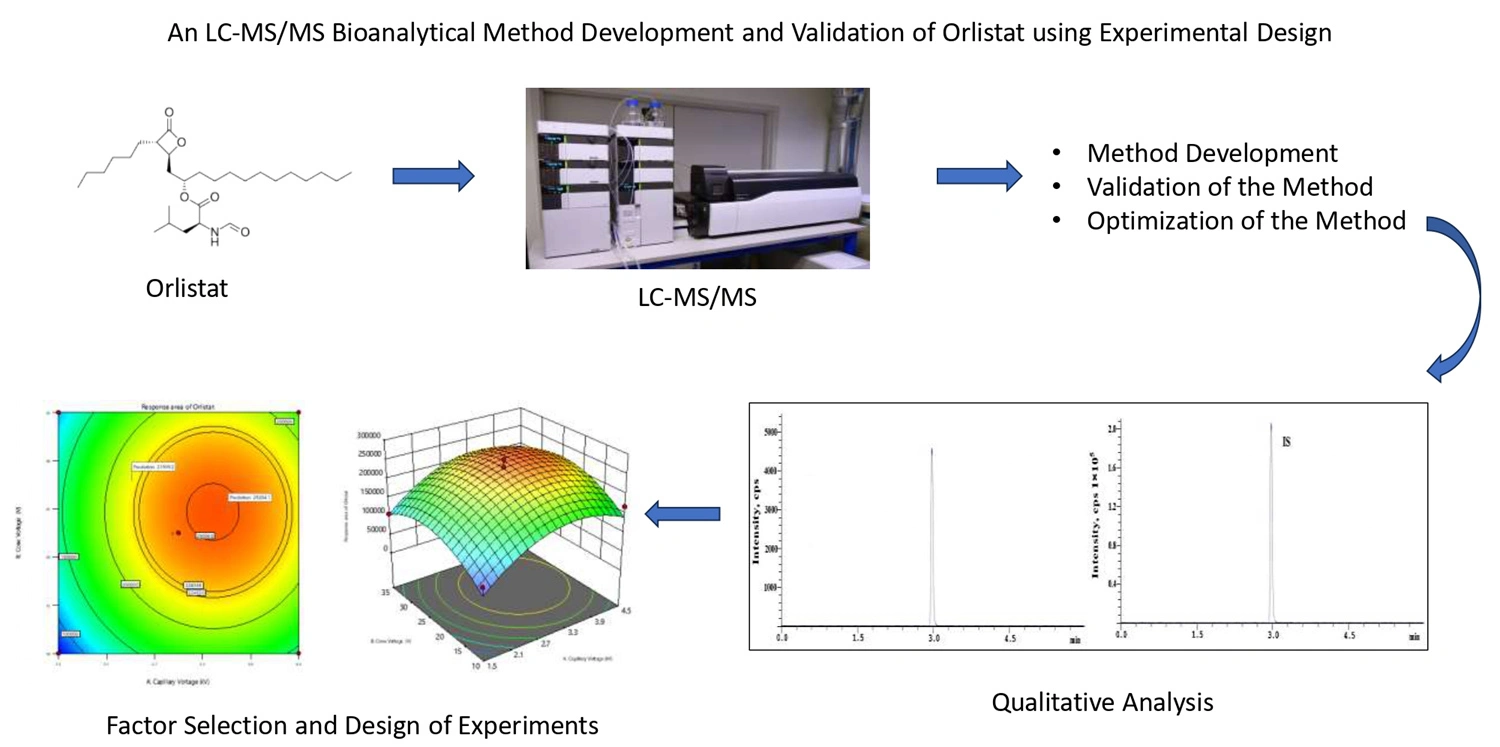
Development and validation of a new LC-MS/MS method for the determination of orlistat in biological matrices using experimental design
Rubina Kauser, Sunil Kumar Chaitanya Padavala, Venkatesan Palanivel
DOI: 10.7324/JAPS.2024.191461Pages: 196-204