Environmental biotoxicity screening of some pyrrole and 1,4-dihydropyridine heterocyclic derivatives
A. Idhayadhulla, Aseer Manilal, Behailu Merdekios and R. Surendra Kumar
DOI: 10.7324/JAPS.2015.50519Pages: 101-105

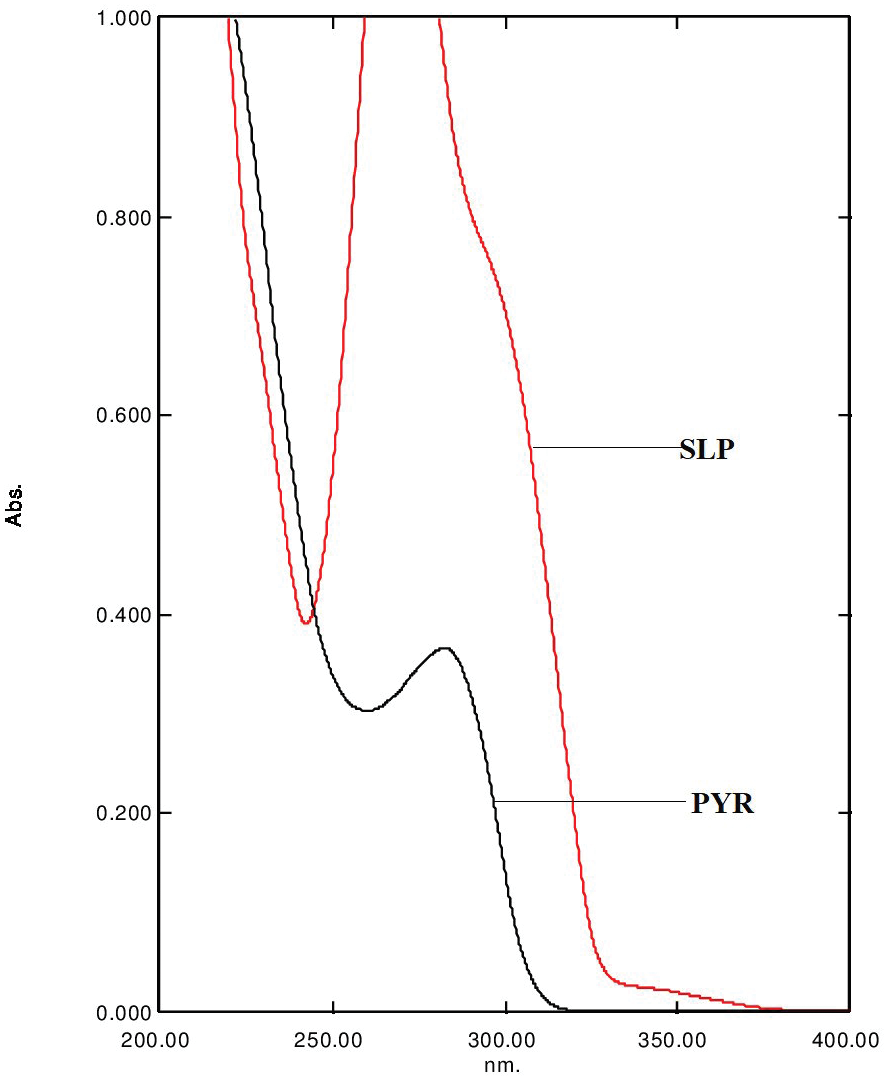
Development and Validation of UV-Spectroscopic Method for Estimation of Niacin in Bulk and Pharmaceutical Dosage Form
Indranil Chanda, Ripunjoy Bordoloi, Debarupa D. Chakraborty, Prithviraj Chakraborty, Smriti Rekha Chanda Das
DOI: 10.7324/JAPS.2017.70911Pages: 081-084

Estimation of Bosentan Monohydrate in Male Rabbit Plasma by using RP-HPLC Method
Revathi Mannam, Indira Muzib Yallamalli
DOI: 10.7324/JAPS.2017.71116Pages: 106-109

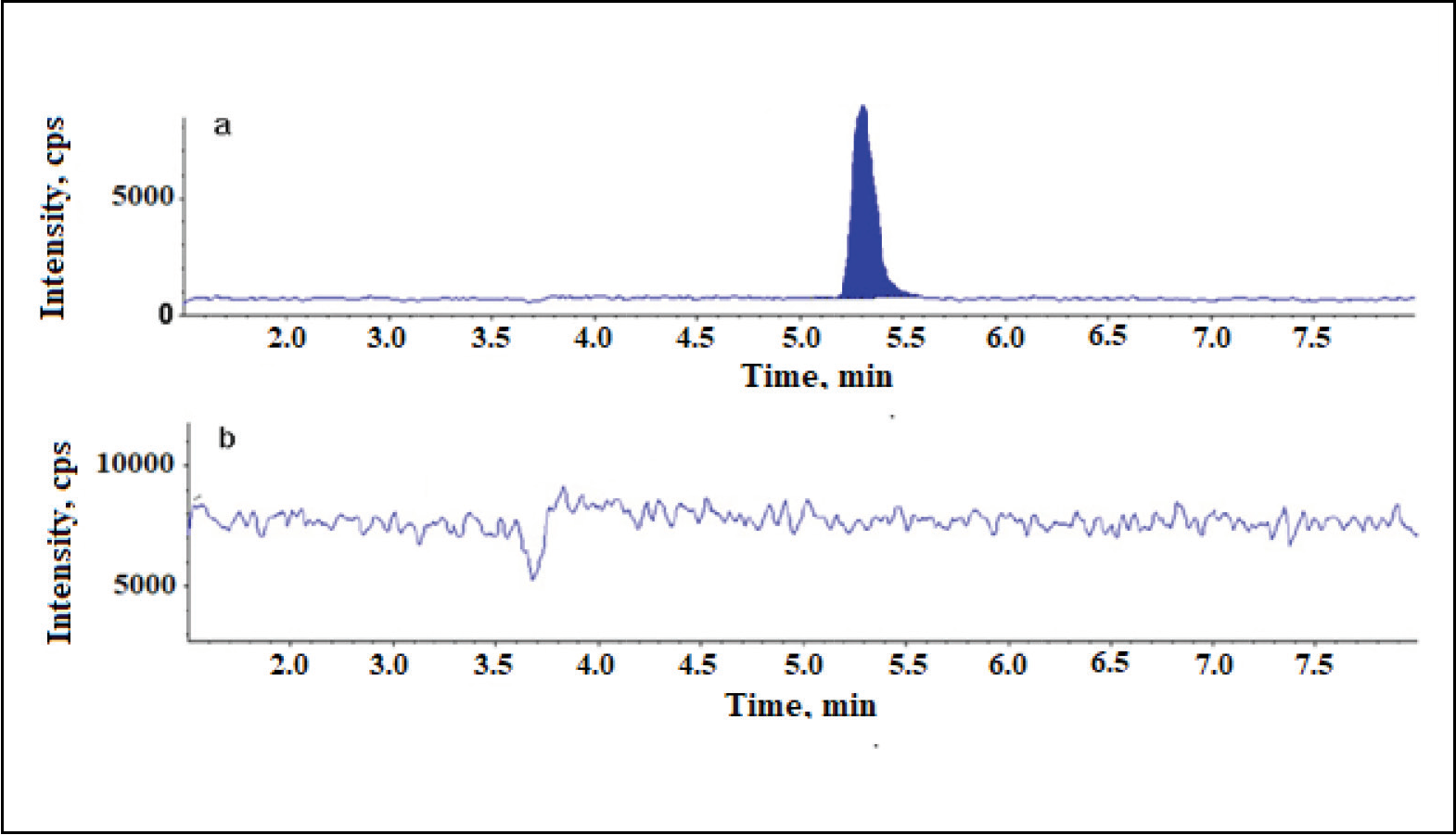
Sensitive Analytical Liquid Chromatography-Tandem Mass Spectroscopy Method for the Estimation of Dexlansoprazole in Pharmaceutical Formulations
Rinchi Bora, S.T. Narenderan, B. Babu, S.N. Meyyanathan, Abel Jacob George, M. Kalaivani
DOI: 10.7324/JAPS.2018.8706Pages: 033-036

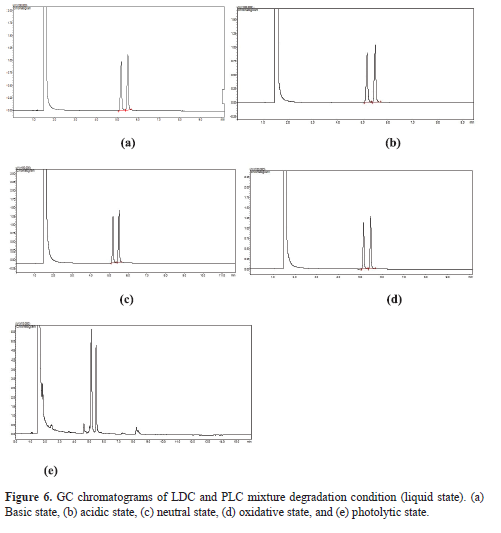
Simultaneous estimation of lidocaine and prilocaine in topical cream by green gas chromatography
Hashim Chekku Marakkarakath, Gurupadayya Bannimath, Prachi Pramesh Raikar
DOI: 10.7324/JAPS.2019.90310Pages: 066-072
A novel analytical liquid chromatography–tandem mass spectrometry method for the estimation of Ribavirin in bulk and pharmaceutical formulation
Prachi Sharma, Narenderan S. T, Meyyanathan S. N, Sangamithra R, Mohire Sourabh Sanjay, Babu B, Kalaivani M
DOI: 10.7324/JAPS.2020.101013Pages: 096-100

Stability indicating RP-HPLC method for simultaneous determination of pyrimethamine and sulfamethoxypyrazine in pharmaceutical formulation: Application to method validation
Shankaranahalli Gurusiddappa Keshava, Gurupadayya Bannimath, Prachi Raikar, Maruthi Reddy
DOI: 10.7324/JAPS.2020.102008Pages: 049-055
LC-MS method development for the quantitation of potential genotoxic impurity 2-Methyl-6-nitro aniline in Telmisartan API
Duvvuri Suryakala, Sivakumar Susarla, Bandlamudi Mallikarjuna Rao
DOI: 10.7324/JAPS.2020.10512Pages: 092-096
Simultaneous detection and quantification of bronchodilators in pure form and from in-vitro drug release of a novel combinational formulation
Sheena M. Raj, Vilas G. Jamakandi, Sunil S. Jalalpure, Pradeepkumar M. Ronad
DOI: 10.7324/JAPS.2020.10815Pages: 131-138

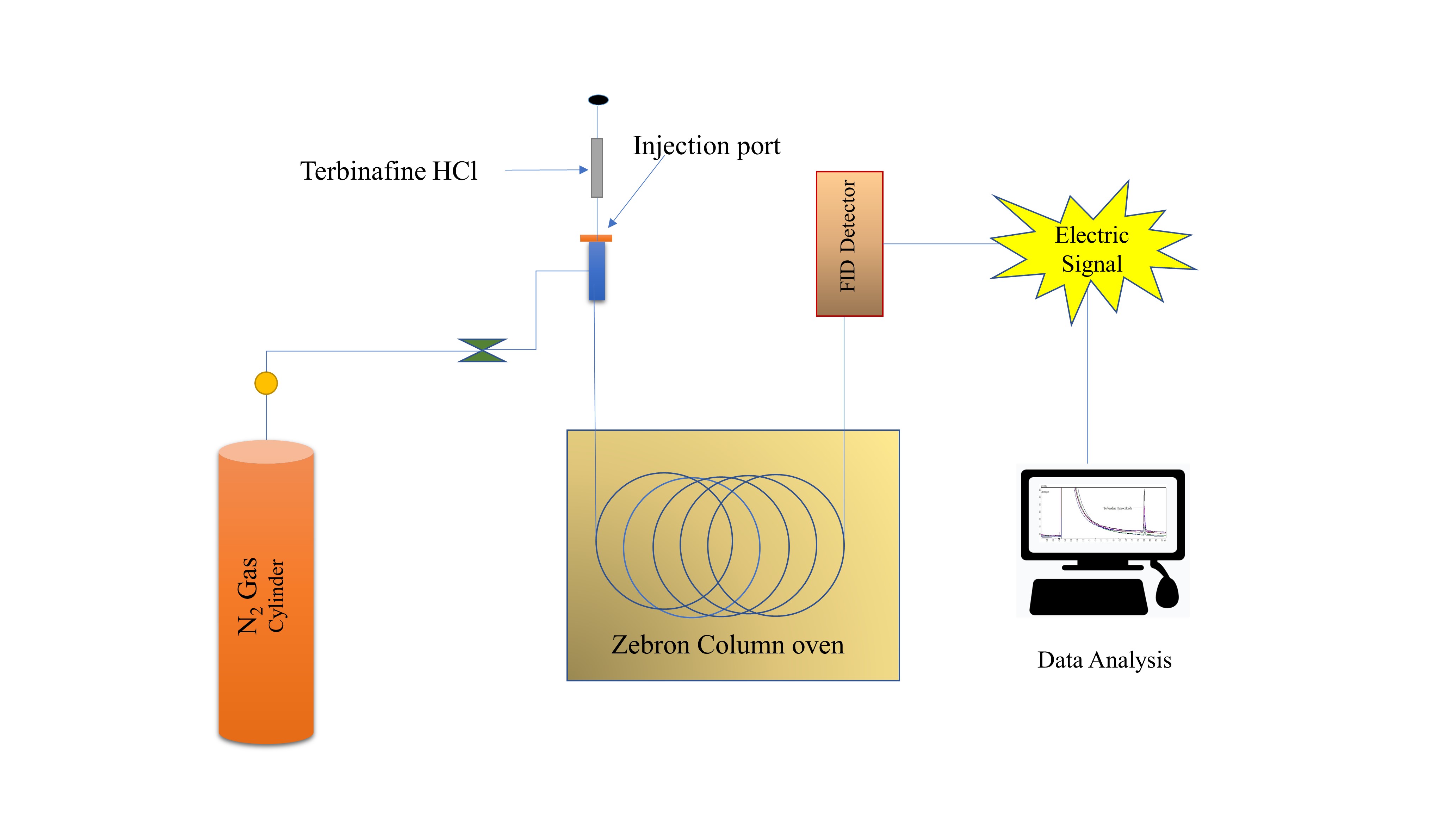
Estimation of terbinafine HCl in tablet dosage form by green gas chromatography
Kalyani Reddy, Gurupadayya Bannimath, Maruthi Reddy, Akshay Nanjundappa
DOI: 10.7324/JAPS.2021.110610Pages: 087-093
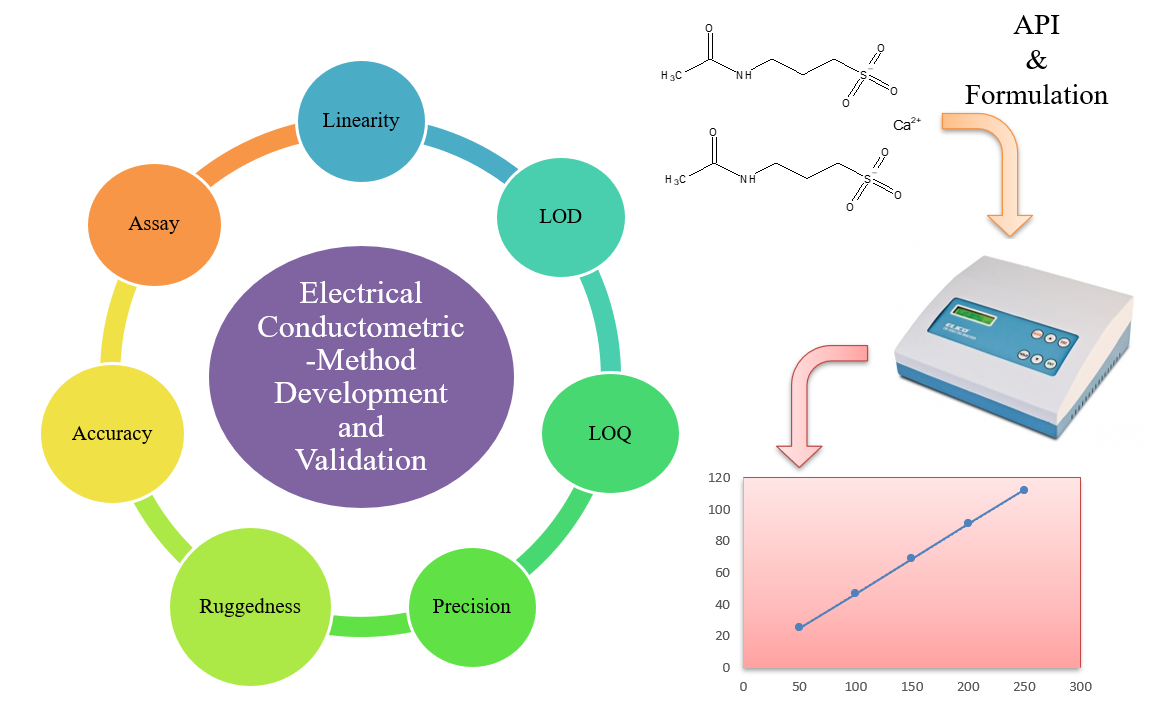
Conductometric method development and validation to estimate acamprosate calcium in API and marketed formulation
Rahul K. Yadav, Meenaxi M. Maste, Shailendra S. Surywanshi, Utkarsh Shastri
DOI: 10.7324/JAPS.2021.1101111Pages: 082–086
Development of validated stability-indicating HPTLC method for the estimation of ulipristal acetate in bulk and dosage form
Shruti Srivastava, Suneela Dhaneshwar, Neha Kawathekar
DOI: 10.7324/JAPS.2021.1101120Pages: 161-167

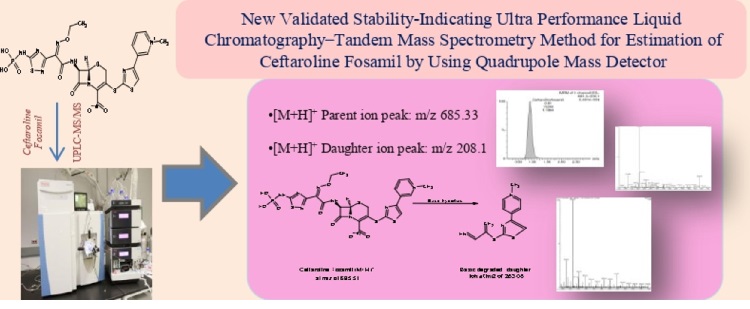
Newly validated stability-indicating ultra-performance liquid chromatography-tandem mass spectrometry method for the estimation of Ceftaroline Fosamil by using a quadrupole mass detector
Jabeen, Bangalore Venkatappa Suma
DOI: 10.7324/JAPS.2022.120621Pages: 215-223
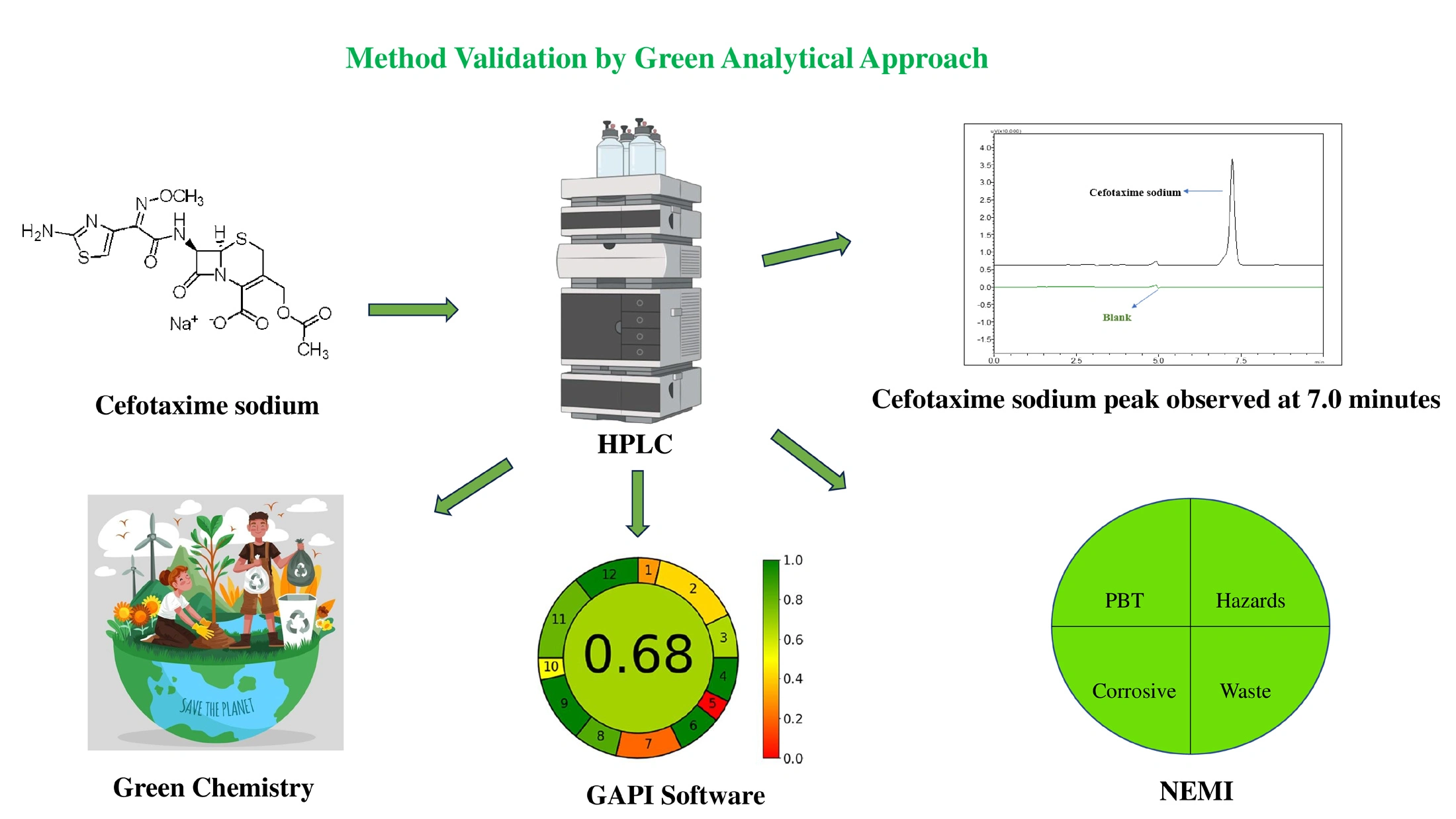
RP-HPLC method for quantification of cefotaxime sodium by using design of experiment, a green analytical approach: Analytical method development, validation, and application
Akhil Nair, H. Raghu Chandrashekhar, Usha Y. Nayak
DOI: 10.7324/JAPS.2024.192430Pages: 098-112