Stability-indicating HPLC-DAD method for the determination of Granisetron hydrochloride in its pharmaceutical preparations
Mokhtar Mabrouk, Hamed El-Fatatry, Ismail Hewala and Ehab Emam
DOI: 10.7324/JAPS.2013.3633Pages: 189-202

Development and Validation of a Stability Indicating Spectrofluorimetric Method for the Determination of Lanzoprazole via its Degradation Product
Ghaleb Oriquat, Afaf Osman, Mohammad Abdul-Azim and Sawsan Abuhamdah
DOI: 10.7324/JAPS.2014.40410Pages: 057-061

Stability Indicating Method for the Determination of Mefenamic Acid in Pharmaceutical Formulations by HPLC
Bhagyashree R. Dhumal, Kishore P. Bhusari, Madhukar R. Tajne, Mahavir H. Ghante, Nishant S. Jain
DOI: 10.7324/JAPS.2014.41211Pages: 060-064

Stability Indicating RP-HPLC Method for the Simultaneous Estimation of Pyrimethamine and Sulphadoxine in Bulk and Tablet Dosage Form
Veeragoni Anil Kumar, Vasudeva Murthy Sindgi, Shoba Rani Satla, Manish Kumar Thimmaraju
DOI: 10.7324/JAPS.2016.60312Pages: 071-076

Development and Validation of a Stability-Indicating High Performance Thin Layer Chromatography (HPTLC) Method for estimation of Canagliflozin in bulk and Pharmaceutical Dosage Form
Ishpreet Kaur, Sharad Wakode, Harsharan Pal Singh
DOI: 10.7324/JAPS.2016.60508Pages: 051-057

Enhancement of dissolution rate and intestinal stability of candesartan cilexitil
Noha Desouky Fayed, Mohamed Ali Osman, Gamal Mohammed El Maghraby
DOI: 10.7324/JAPS.2016.60516Pages: 102-111

Nano-bio hybrid system for enhanced degradation of cefdinir using Candida sp. SMN04 coated with zero-valent iron nanoparticles
Adikesavan Selvi, Nilanjana Das
DOI: 10.7324/JAPS.2016.60902Pages: 009-017

A Study of Method Development, Validation and Forced Degradation for Quantification of Buprenorphine Hydrochloride in a Microemulsion Formulation
Dhanashree Arun Mundhey, Vishal V. Rajkondawar, Anwar S. Daud, Nidhi P. Sapkal
DOI: 10.7324/JAPS.2016.601022Pages: 159-169

Development and validation of a stability-indicating RP-HPLC method for the detection and quantification of azithromycin in bulk, and self-emulsifying drug delivery system (SEDDs) formulation
Reem Abou Assi, Yusrida Darwis, Ibrahim M. Abdulbaq, Shaik Mohammed Asif
DOI: 10.7324/JAPS.2017.70903Pages: 020-029

A new rapid Stability indicating RP-PDA-UPLC method for the estimation of Assay of Pemetrexed disodium-An anti-Lung cancer drug from lyophilized parenteral formulation
Vamsi Krishna Galla, V. Archana, Rajeswari Jinka
DOI: 10.7324/JAPS.2017.71019Pages: 131-137

A Sensitive, Stability indicating UPLC method for the identification and characterization of forced degradation products for Drometrizole Trisiloxane through MSn studies
M. Ajay Babu, G. V. Krishna Mohan, J. Satish, Pradipbhai D. Kalariya, CH. Krishnam Raju, Sharad D. Mankumare
DOI: 10.7324/JAPS.2018.8609Pages: 065-074

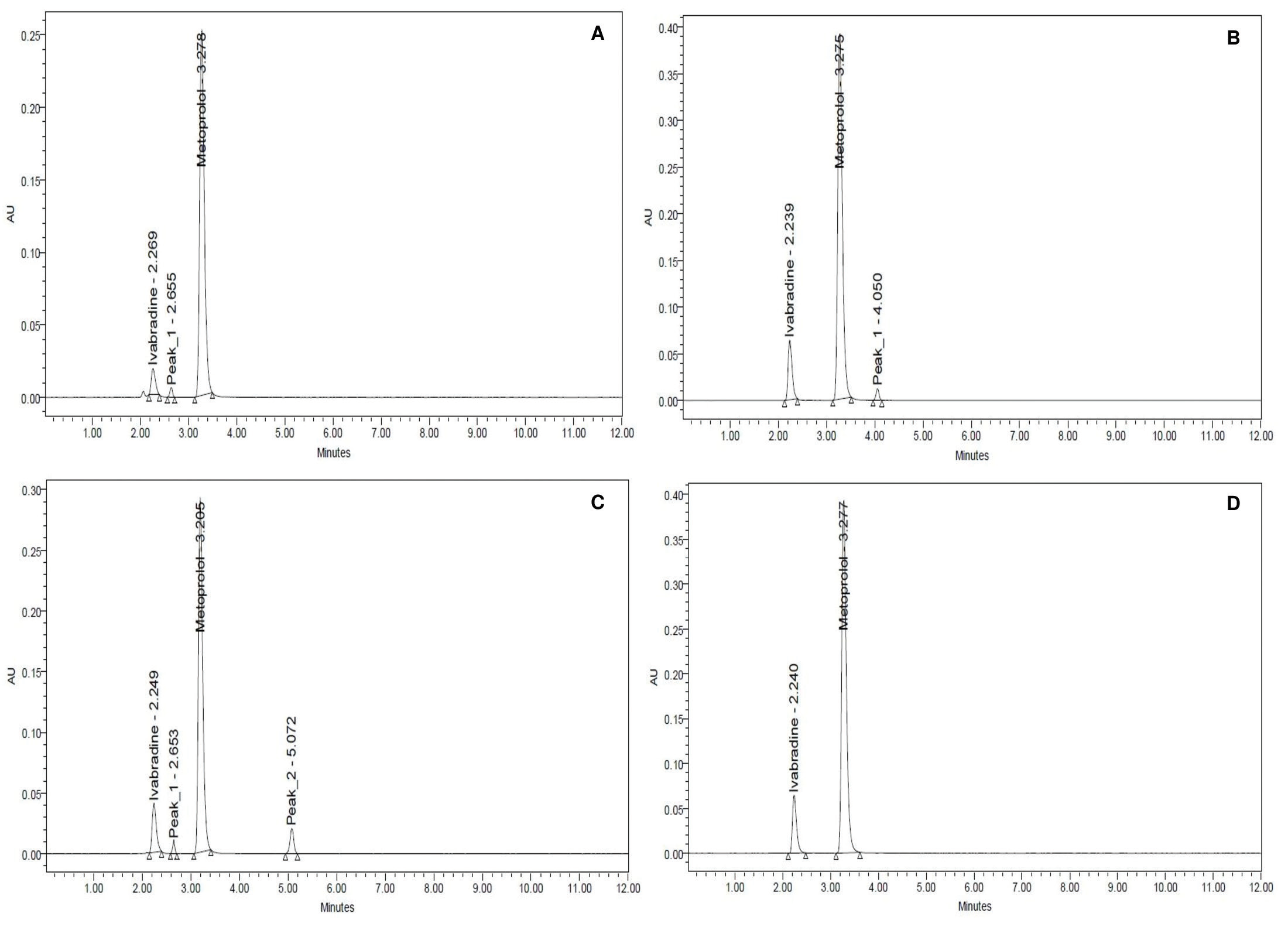
Stability indicating RP-HPLC method for the simultaneous estimation of ivabradine and metoprolol in bulk and tablet formulation
Sangameshwar B. Kanthale, Sanjay S. Thonte, Debarshi Kar Mahapatra
DOI: 10.7324/JAPS.2019.90418Pages: 137-144
Development and validation of a stability-indicating RP-HPLC method of cholecalciferol in bulk and pharmaceutical formulations: Analytical quality by design approach
Dilipkumar Suryawanshi, Durgesh Kumar Jha, Umesh Shinde, Purnima D. Amin
DOI: 10.7324/JAPS.2019.90604Pages: 021-032

Development of validated stability indicating RP-HPLC method for the estimation of glecaprevir and pibrentasvir in bulk and pharmaceutical dosage form
Sangameshwar B. Kanthale, Sanjay S. Thonte, Debarshi Kar Mahapatra
DOI: 10.7324/JAPS.2019.90607Pages: 052-060

Quantification of rasagiline mesylate by stability indicating RP-HPLC method: Development and validation
Rohith Ganapathi Bhatta, Sathesha Babu Birur Kotappa, Sadashivaiah Rudragangaiah
DOI: 10.7324/JAPS.2019.90908Pages: 059-065

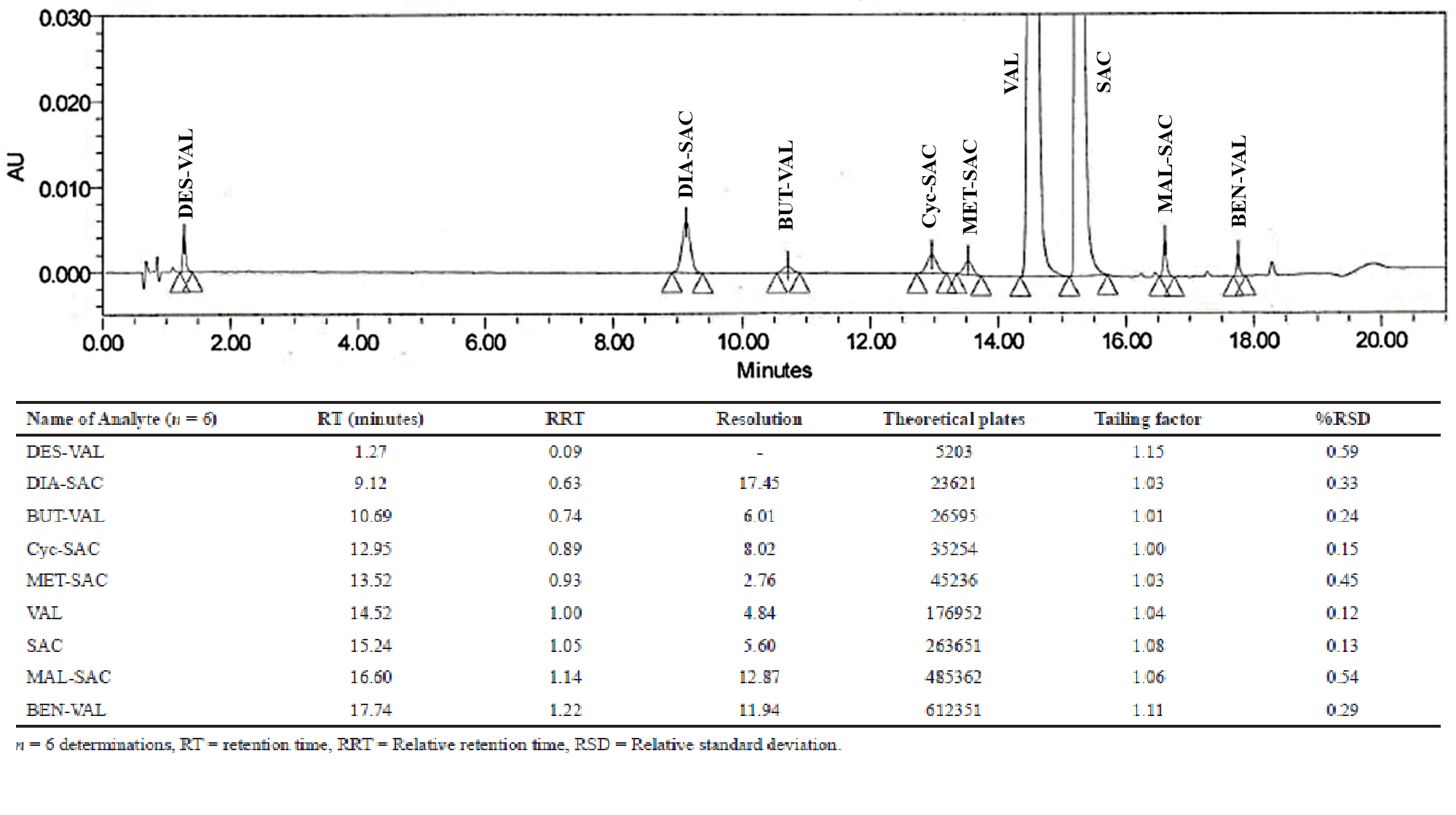
Development and validation of a stability indicating UHPLC method for Sacubitril/Valsartan complex in the presence of impurities and degradation products
Pintu Prajapati, Dhara Bhayani, Priti Mehta
DOI: 10.7324/JAPS.2020.102015Pages: 097-107
Forced degradation study of efonidipine HCl ethanolate, characterization of degradation products by LC-Q-TOF-MS and NMR
Charu P. Pandya, Sadhana J. Rajput
DOI: 10.7324/JAPS.2020.104012Pages: 075-099

Green synthesis and in silico characterization of 4-Hydroxy-3- methoxybenzaldehyde Schiff bases for insulysin inhibition – a potential lead for type 2 diabetes mellitus
Sridevi Chigurupati, Vasanth Raj Palanimuthu, Suganya Kanagaraj, Sumathi Sundaravadivelu, Venkata Ramaiah Varadharajula
DOI: 10.7324/JAPS.2021.110706Pages: 063-071

A new approach for evolution and quantification of Triamcinolone acetonide in medication shots by using RP-HPLC
Manikantha Naveen Vuddagiri, Veeraswami Boddu
DOI: 10.7324/JAPS.2021.1101216Pages: 169–174

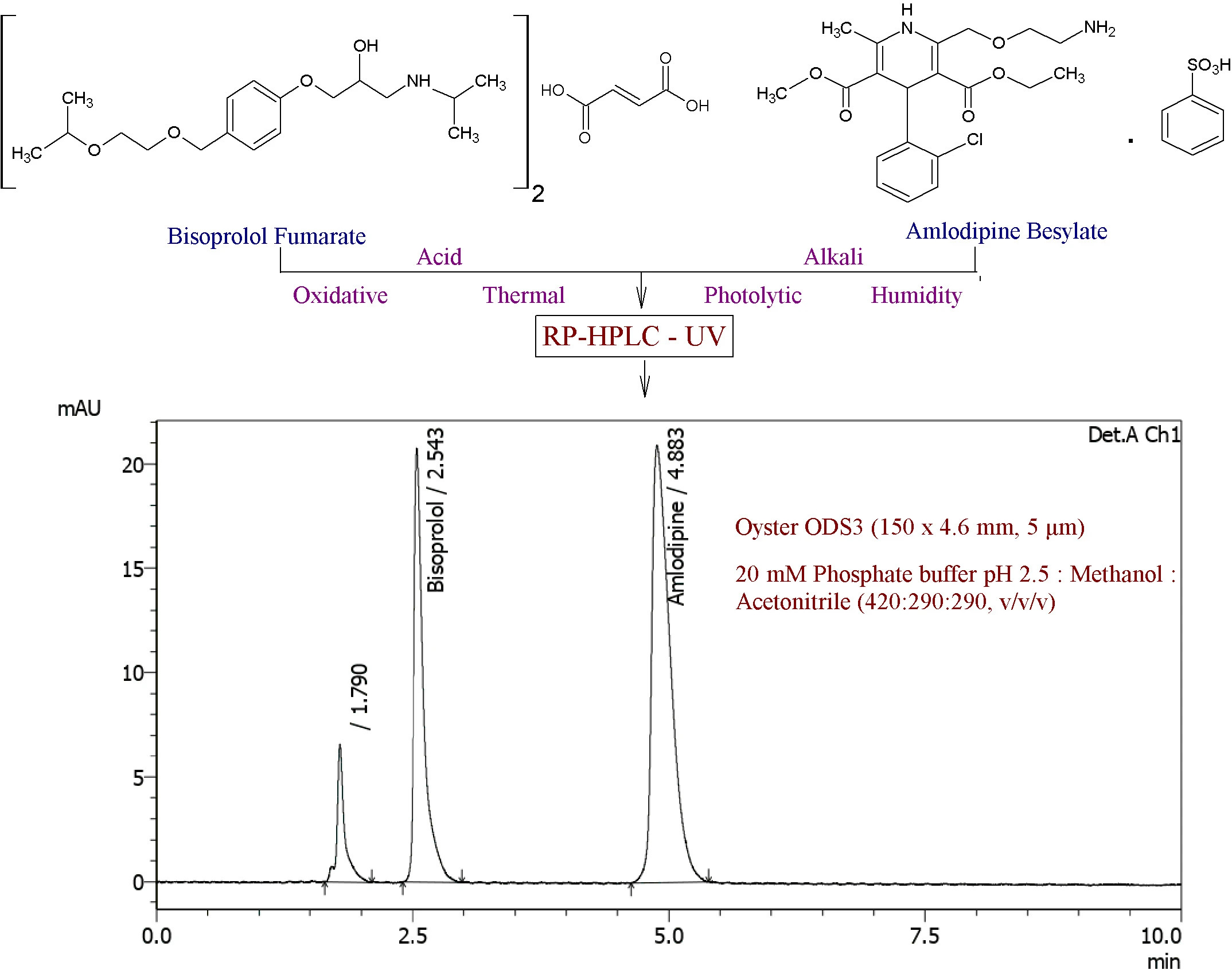
Stability-indicating RP-HPLC method development and validation for simultaneous estimation of bisoprolol fumarate and amlodipine besylate in bulk and in tablet dosage form
Rameshwar Bhausaheb Gholve, Sanjay Sudhakar Pekamwar, Tukaram Mohanrao Kalyankar
DOI: 10.7324/JAPS.2021.1101211Pages: 121–134
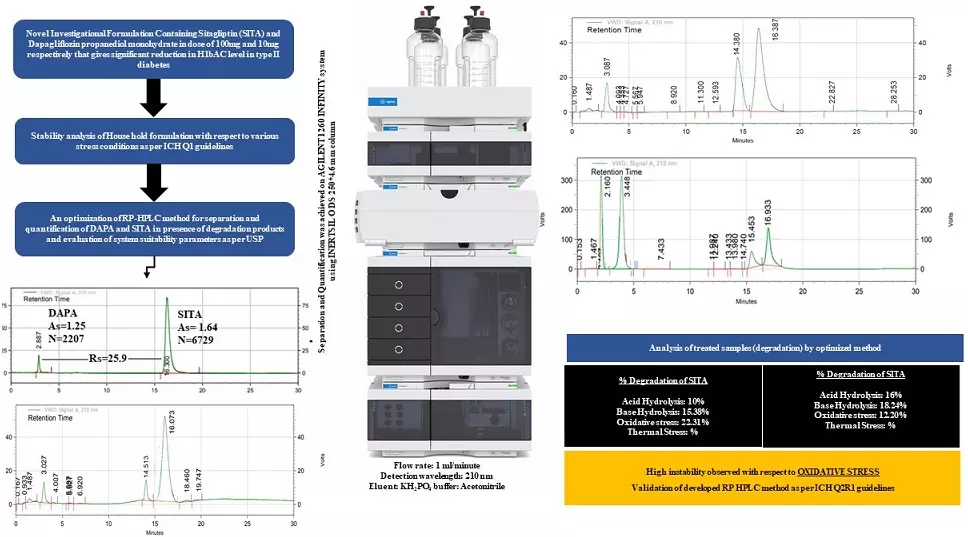
Quantitative computation and stability evaluation of phase III composition comprising sitagliptin and dapagliflozin propanediol monohydrate by RP-HPLC
Yesha Darshak Patel, Pinak Rameshbhai Patel, Jigna Bhatt, Binny Mehta, Krunal Detholia
DOI: 10.7324/JAPS.2022.120614Pages: 148-155
A novel LC-MS/MS technique for identification and characterization of degradation products of Bilastine and Montelukast sodium and its greenness assessment using AGREE tool
Sapna M. Rathod, Nisarg C. Patel, Riyaz Dantroliya, Bhupendra G. Prajapati
DOI: 10.7324/JAPS.2025.199837Pages: 142-154
_.jpg)