INTRODUCTION
Antihistamines are a family of medications used to treat allergies brought on by histamines physiological effects [1]. The current guidelines state that non-sedating antihistamines are mostly used nowadays to treat allergic rhino conjunctivitis, which includes urticaria. Bilastine (BLS) is a new second-generation H1-antihistaminic medication that is used to treat chronic urticaria and allergic rhinitis (AR) in patients over the age of 12 [2]. Its structure is represented in Figure 1A.
Cysteinyl leukotriene receptor antagonists like Montelukast (MTK) and Zafirlukast are prescribed to prevent and treat persistent asthma [3]. A cysteinyl leukotriene receptor blocker, MTK sodium is employed to manage symptoms of seasonal allergies and asthma. MTK functions by attaching to a cysteinyl leukotriene receptor in the lungs and bronchial tubes and obstructing leukotriene D4’s function on it [4]. Its structure is shown in Figure 1B.
A new combination of BLS and MTK was approved by DCGI on 09 March 2020 [5] to treat adult cases of AR. This combination is beneficial in the management of seasonal allergic rhino conjunctivitis and mild to moderate asthma. As per International Council for Harmonisation (ICH) [6] and other regulatory guidelines [7], forced degradation studies are crucial for examining how drugs and drug products change during storage and for figuring out how environmental conditions such as light exposure, oxidation, and hydrolysis (acid, base, and neutral) affect things. In contrast to general stability investigations, forced degradation must produce the greatest number of degradation products (DPs) in the shortest amount of time [8,9]. Since the degradants could have harmful qualities, it is important to understand DPs, their structures, and the processes by which they degrade. For structural analysis of DPs without isolating the molecule, hyphenated techniques namely liquid chromatography-tandem mass spectrometry (LC-MS/MS), LC-NMR, and LC-MS have all been widely used; LC-MS/MS have been used mostly because of their sensitivity [10]. In the field of drug development, the use of forced degradation in conjunction with the identification and characterization of DPs is essential to understanding drug stability [11].
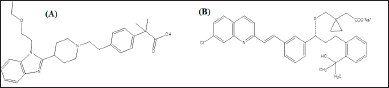 | Figure 1. Chemical structure of BLS (A) and MTK (B). [Click here to view] |
The majority of public and business sectors now place a high priority on and employ green chemistry concepts. Understanding the influence of analytical procedures on the ecosystem and economy requires evaluating their sustainability. But sustainability is a complex concept that takes into account several aspects, including waste reduction, effectiveness, safety, greenness, and cost. Sustainability cannot be fully assessed by a single method in all of these dimensions. AGREE is therefore used in this work as a methodology to facilitate a more comprehensive examination. For a personalized greenness assessment, 12 key green chemistry ideas can be customized for weighting using the AGREE tool. The measure presents the outcome as a color image and is meant to be readily comprehended [12]. Moreover, AGREE is easy to utilize because readily accessible software can be used to apply it [13]. Anywhere from dark red (0) to dark green (1) could be the color. When evaluating environmental standards-based greenness, AGREE is a priceless tool [14].
A thorough literature search reveals that some spectrophotometric [15–22], few chromatographic methods [18, 23–42], and spectrofluorometric methods [43] for the concurrent determination of MTK and BLS in API and dosage form were reported. The comprehensive characterization and molecular mechanism of DPs of BLS and MTK under stress conditions have not yet been documented in any study. The primary goals of the current investigation were to identify the primary DPs and examine the drug’s overall degrading behavior. In order to determine the fragmentation pattern of the drug and its DPs, the drug was exposed to the ICH-recommended stimuli of hydrolysis, heat, oxidation, and photolysis. The resultant solutions were then analyzed using optimized LC–MS/MS and precise mass measurements.
MATERIALS AND METHODS
Chemical and reagents
BLS (99.94% pure) and MTK (99.86% pure) were gifted from Shagun Pharmaceuticals (Indore, Madhya Pradesh, India) and Sehat Pharma Pvt Ltd (Himmatnagar, Gujarat, India) respectively. Acetonitrile, methanol and ammonium acetate (HPLC grade), sodium hydroxide, formic acid, hydrochloric acid, and hydrogen peroxide (analytical grade) were acquired from Rakesh Chemicals, Ahmedabad, Gujarat, India.
Instrumentation and mass spectrometric conditions
The instrument utilized was an API-2000 Liquid chromatography-mass spectrometer, which was outfitted with a column oven, auto injector, auto sample, ion source electron spray ionizer (ESI) with Q1, and collision energy. To separate each component, an Agilent Zorbax C18 column (150 × 4.6 mm, 5 µm) was used. The ABScix API 2000 series online ion trap MSD mass spectrometer, which was outfitted with an auto sampler and an ESI source in positive mode, was used to perform LC–MS/MS. Software called Analyst QS managed the data acquisition process. An ion trap (quadrupole) mass spectrometer was used for the MSn investigations, fitted with an ionization source (electrospray). The mass spectra were captured in the positive detection mode. With helium serving as the collision gas, collision-induced dissociation (CID) was accomplished. The conditions (ion source) were fixed as dry gas (5 l/minute), nebulizer gas (30 psi), dry temperature (400°C), electro multiplier voltage (2100 V), vaporizer temperature (400°C), capillary current (81.787 nA), corona current (4000 nA), dwell time (200 ms), and capillary exit (113.5 V). Capillary voltage of 5 kV (positive mode 4 kV), entry potential (10 V), resolution (10,000), focusing potential (400 V), and declustering potential (20 V) were the typical source settings. As a collision gas and curtain, ultra-high pure nitrogen was utilized, while zero air served as the nebulizer. The quadruple analyzer 1 was used to pick the precursor ion for the CID studies, while the quadrupole analyzer 3 examined the product ions.
LC-MS/MS analysis
Method development and optimization
The sample’s solubility, pKa, molecular weight, and type (ionic or neutral molecules) all have a role in the appropriate choice of LC-MS/MS technique. The impact of chromatographic factors such as flow rate, pH, mobile phase, and solvent ratio was investigated in order to optimize the chromatographic settings. For LC-MS/MS method development, C8 and C18 columns and different mobile phases like formic acid, methanol, water, and acetonitrile with or without buffer (ammonium acetate) in different proportions were tried. For every drug and chromatogram, the retention period and tailing factor were computed. After infusing the drug solution into the mass spectrometer, the tuning parameters (ion spray voltage, temperature, ion source gas, curtain gas, declustering potential, etc) were also adjusted to get the higher intensity of selected fragments.
Diluent preparation
The diluent is made up of water and acetonitrile in a 50:50 v/v ratio.
Standard solution preparation
Precisely weighed masses of 10 mg MTK and 20 mg BLS were added into a separate volumetric flask of 100 ml quantity and sonicated to dissolve in diluent and made to mark. This solution represents a standard stock solution. One ml of each stock solution was aliquot into a 10 ml volumetric flask. Diluent was utilized to make up the volume. Furthermore, dilution with diluent was done to prepare a combined working standard solution comprising of MTK 1 μg/ml and BLS 2 μg/ml.
Preparation of eluent
In 25:75 v/v acetonitrile and water (pH 4; adjusted with formic acid), prepare a 10 mM ammonium acetate buffer. Stir thoroughly and use sonication to degas.
Preparation of sample solution
Weigh and powder 10 tablets. Weigh the quantity of powder equivalent to 10 mg MTK (20 mg BLS) in a 100 ml volumetric flask. This solution represents a sample stock solution. After adding the required amount of diluent, place the flask in a sonicator for 15 minutes. Dilute it upto the mark and filtration was carried out. Further dilutions were made to get the concentration within the linearity range. 20 µl volume was injected into the system and outcomes were reported.
Forced degradation studies
In accordance with ICH recommendations, stress degradation experiments of BLS and MTK were conducted. Every sample that was under stress was removed at appropriate intervals and diluted with mobile phase within the linearity range. Prior to LC-MS/MS analysis, all of the samples were passed through a 0.22 µm membrane filter.
Acid degradation
In two distinct 100 ml volumetric flasks, each ml of stock solution (standard) and sample stock solution were taken separately. Add 1 N of HCl in both flasks and were refluxed. For 4 hours, keep the mixture in a water bath at 60°C. The content was neutralized by adding an equal amount of 1 N NaOH, followed by the addition of a diluent to achieve MTK 1 μg/ml and BLS 2 μg/ml.
Base degradation
In two distinct 100 ml volumetric flasks, each ml of stock solution (standard) and sample stock solution were taken separately. Add 1 N of NaOH in both flasks and were refluxed. For 4 hours, keep the mixture in a water bath at 60°C. The content was neutralized by adding an equal amount of 1 N HCl, followed by the addition of a diluent to achieve MTK 1 μg/ml and BLS 2 μg/ml.
Oxidative degradation
In two distinct 100 ml volumetric flasks, each ml of stock solution (standard) and sample stock solution were taken separately. Add 1 ml of the 30% H2O2 solution in both flasks and were refluxed to conduct oxidative breakdown investigations. For 5 hours, keep the contents at 60°C in a water bath. Next, diluent was used to achieve MTK 1 μg/ml and BLS 2 μg/ml.
Thermal degradation and photolytic degradation
Place Petri dishes containing approximately 100 mg of the drug standard in a hot air oven; set to 105°C (Thermal) and in a photostability chamber (Photolytic) for 5 days individually for respective degradation. Five days later, weigh and transfer roughly 10 mg of MTK and 20 mg of BLS into separate 100 ml volumetric flasks, adding diluent to make up the volume. Dilution was made further to get 1 μg/ml MTK and 2 μg/ml BLS. The same procedure was followed for the formulation.
After making final solutions for all the degradation studies, it is injected with a syringe into the mass spectrometer to identify and characterize the DPs of the Drug, and its fragmentation pathway is identified by MRM scan (Q1-Q3).
Method validation
Specificity
The blank solution, working standard solution, and working sample solution of MTK (1 μg/ml) and BLS (2 μg/ml) are injected into the LC-MS/MS system. The chromatogram of blank, standard, and sample was checked for interference.
Calibration curve
Different aliquots like 0.5, 0.75, 1.0, 1.25, and 1.5 ml from the standard stock solution of BLS and MTK were transferred to a series of 100 ml volumetric flasks to obtain the concentration in the range of 0.5–1.5 μg/ml for MTK and 1–3 μg/ml BLS correspondingly. Diluent was utilized for the dilution.
Precision
Repeatability
A standard solution comprising MTK (1 µg/ml) and BLS (2 µg/ml) was injected six times and areas of peaks were measured and % RSD was computed.
Intermediate precision
A standard solution containing (0.5, 1, 1.5 µg/ml) MTK and (1, 2, and 3 µg/ml) BLS were analyzed three times on the same day and on three consecutive days for the determination of intraday and inter day variations correspondingly. The outcomes were noted.
Accuracy
The recovery study was accomplished at three levels (80%, 100%, and 120%) by adopting the standard addition method. The standard solutions of BLS (1.6, 2, and 2.4 µg/ml) and MTK (0.8, 1, and 1.2 µg/ml) were spiked to the predetermined tablet solution (1 µg/ml MTK and 2 µg/ml BLS). The determinations were made thrice and outcomes were reported.
LOD and LOQ
It was determined by substituting the respective values in the equations described in ICH guideline [44].
Robustness
Variations in method parameters like flow rate (± 0.2 ml/minute), ratio of mobile phase (± 2%), and pH (± 0.2) were done to assess the robustness of the proposed approach.
RESULTS AND DISCUSSION
Optimization of chromatographic conditions
Preliminary chromatographic experiments were tried on Agilent ZORBEX C8 (150 × 4.6 mm, 5 µm) and Agilent ZORBEX C18 column (150 × 4.6 mm, 5 µm) and different solvents (water, methanol, formic acid, acetonitrile, acetate buffer) were tried as eluent for the resolution of BLS, MTK and its DPs. While using water: methanol (20:80 v/v), broad peak of BLS is observed. In the case of water: acetonitrile (20:80 v/v), no peaks were observed for both drugs. Upon the addition of formic acid, in both mobile phases, drug peaks were observed but retention time increases as well as tailing were observed for BLS. Hence, ammonium acetate buffer (pH 4 adjusted with formic acid) was employed in place of water to achieve a satisfactory resolution between the drug and its DPs. Aqueous ammonium acetate buffer (pH 4): acetonitrile (25:75, v/v) in isocratic mode and Agilent ZORBEX C18 (150 × 4.6 mm, 5 µm) column was finalized as sharp peak and good resolution was obtained for cited drugs and their DPs. There was a 10-minute run time, 35°C column temperature, and a 1 ml/minute flow rate. These ideal chromatographic conditions were applied to the separation of MTK, BLS, and their respective DPs. The procedure was verified in accordance with the specifications given in ICH standards Q2 (R1). To provide a better signal and high sensitivity for LC-MS experiments, the Triple Quadrupole with ESI source conditions was optimized. In order to identify and characterize the DPs, a number of parameters, including drying gas temperature, capillary voltage, skimmer voltage, spray voltage, nebulizing gas flow, and drying gas flow were optimized to maximize ionization in the source and sensitivity even at extremely low quantities.
Degradation behavior of MTK and BLS
The DPs can be identified using the optimized LC-MS approach. Under varied stress circumstances, the LC–ESI–MS total ion chromatograms (TICs) were acquired. Tandem mass spectrometric analysis was used to identify and characterize a total of six DPs. The DPs generated under hydrolysis and oxidative degradation were given different notations, viz. DP1, DP2, and DP3 for MTK and DP1, DP2, and DP3 for BLS, individually. The drug (MTK and BLS) is hydrolyzed in an acidic and basic manner to produce two degradants, DP1 and DP2. Under the oxidative condition (30% H2O2; 60°C, 5 hours), one DP(DP3) was formed for both the drugs. It was discovered that both drugs remained stable when exposed to thermal stress and UV light in both their solid and solution forms. Figures 2 and 3 represent the typical chromatograms of BLS and MTK (standard) and their DPs formed under hydrolysis and oxidative stimuli correspondingly. Figures 4 and 5 represent the typical chromatograms of BLS and MTK (sample) and their DPs formed under hydrolysis and oxidative stimuli correspondingly.
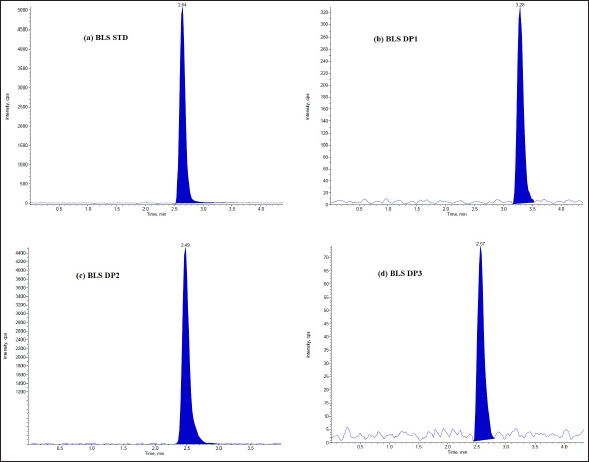 | Figure 2. LC/ESI-MS TIC of (a) BLS standard drug (b) Acid degradant (DP1) (c) Base degradant (DP2) (d) Oxidative degradant (DP3). [Click here to view] |
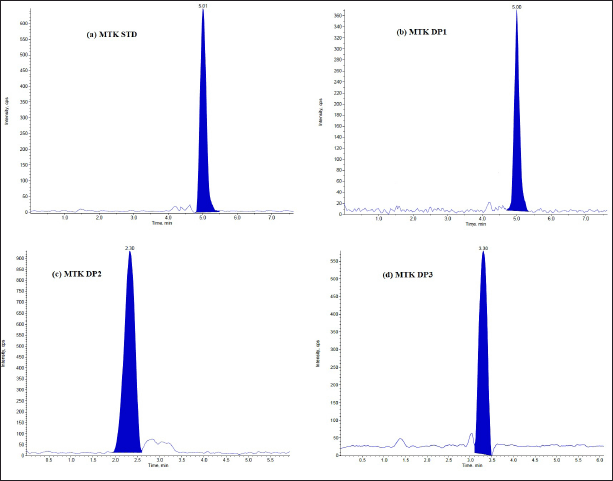 | Figure 3. LC/ESI-MS TIC of (a) MTK standard drug (b) Acid degradant (DP1) (c) Base degradant (DP2) (d) Oxidative degradant (DP3). [Click here to view |
Identification and characterization of DPs by LCMS/MS
With the aid of their fragments from LC-MS/MS investigations and a comparison with the drug’s fragmentation pattern from MS/MS and MSn analysis, all of the DPs were identified. The stressed solution exposed to different conditions was exposed to LC-MS/MS analysis for characterization of the DPs.
MSn study of MTK and BLS
The MSn spectra of MTK and BLS were performed at reduced collision energy (0.2 mA), drugs underwent protonation and the molecular ion peak at m/z 586.3 Da was noted. For MTK, the ESI/MS of [M+H]+ ion at m/z 586.3 Da demonstrates abundant fragment ion at m/z 568.4 Da (Removal of - OH from m/z 586.3), Mass Difference (MD) of 17, m/z 528.1 Da (Removal of – C3H5 from m/z 568.4), MD of 41, m/z 423.6 Da (Removal of – C8H9 from m/z 528.1), MD of 105, m/z 265.3 Da (Removal of – C7H10O2S from m/z 423.6), MD of 158. For BLS, the ESI/MS of [M+H]+ ion at m/z 464.3 Da illustrates abundant fragment ion at m/z 378.2 Da (Removal of - C4H7O2 from m/z 464.3), MD of 87, m/z 272.3 Da (Removal of – C8H9 from m/z 378.2), MD of 105, m/z 190.9 Da (Removal of – C5H9N from m/z 272.3), MD of 83. From the findings of the MS/MS analysis utilizing optimal mass parameters and the LC/ESI/MS in positive mode, the mass fragmentation pathway of the BLS and MTK (Fig. 6) was identified.
Characterization of DPs of MTK
Characterization of acid DP; DP1
The ESI-MS/MS of MTK exhibited an abundant protonated molecular ion at m/z 586.300 Da. DP1 appeared at m/z 602.100 Da ([M+H]+; C35H36ClNO4S) and was eluted at 5 minutes (Fig. 7). DP1 formed as a result of hydrolysis of the sodium acetate group in the presence of acid. The LC-ESI-MS/MS spectrum of DP1 represents abundant fragment ion at m/z 544.300 Da (removal of C3H8O from m/z 602.1), MD of 58, fragment 2 at m/z 439.500 Da (loss of - C8H10 from m/z 544.300), MD of 105, and m/z 281.6 (loss of – C7H9SO2 from m/z 439.500).
Characterization of base DP; DP2
The ESI-MS/MS of MTK exhibited an abundant protonated molecular ion at m/z 586.300 Da. DP2 appeared at m/z 568.100 Da ([M+H] +; C35H36ClNO4S) and was eluted at 2.30 minutes (Fig. 7). DP2 formed as a result of hydrolysis of chloroquinoline moiety in the presence of a base. The LC-ESI-MS/MS spectrum of DP2 shows abundant fragment ion at m/z 510.300 Da (removal of C3H8O from m/z 568.1), MD of 58, fragment 2 at m/z 406.400 Da (loss of - C8H10 from m/z 510.300), MD of 104, and m/z 237.2 (loss of - C11H8NO from m/z 406.400), MD of 170.
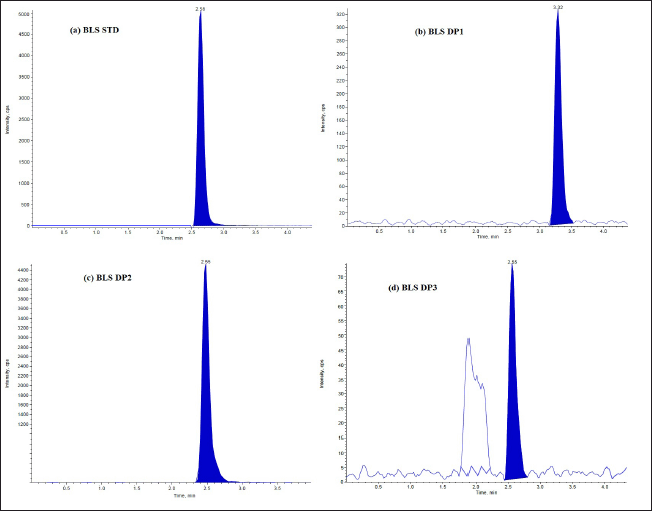 | Figure 4. LC/ESI-MS TIC of (a) BLS standard drug (b) Acid degradant (DP1) (c) Base degradant (DP2) (d) Oxidative degradant (DP3) of Tablet dosage form. [Click here to view] |
Characterization of oxidative DP; DP3
The ESI-MS/MS of MTK shows an abundant protonated molecular ion at m/z 586.300 Da. DP3 appeared at m/z 602.300 Da ([M+H]+; C35H35ClNO4S) was eluted at 3.30 minutes (Fig. 7). DP3 formed due to oxidation of sulphur (sulfoxide formed) in the presence of H2O2. The LC-ESI-MS/MS spectrum of DP3 demonstrates abundant fragment ion at m/z 585.300 Da (removal of -OH from m/z 602.3), MD of 17, and fragment 2 at m/z 543.600 Da (removal of - C3H7 from m/z 585.300), MD of 42.
The proposed structures of MTK and its DPs along with the degradation pathway are represented in Figure 8.
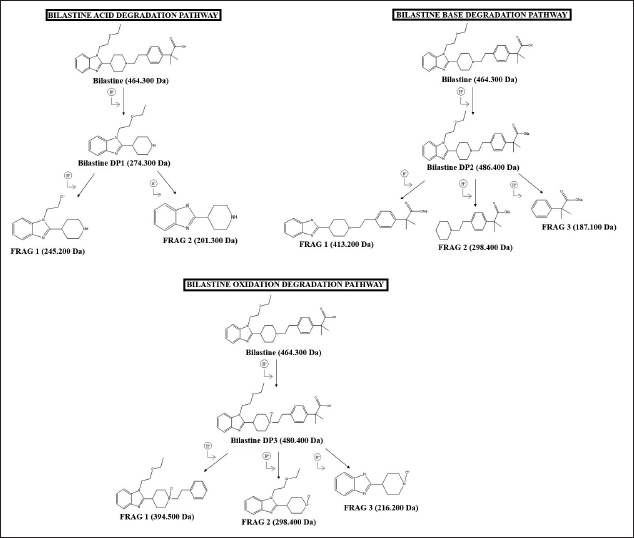
Characterization of DPs of BLS
Characterization of acid DP; DP1
The ESI-MS/MS of BLS represents an abundant protonated molecular ion at m/z 464.300 Da. DP1 appeared at m/z 274.300 Da ([M+H]+; C6H19N3O) and was eluted at 2.64 minutes (Fig. 7). DP1 formed due to hydrolysis of piperidine and cleavage of –ethyl-phenyl-2-methyl propanoic acid. The LC-ESI-MS/MS spectrum of DP1 represents abundant fragment ions at m/z 245.200 Da (removal of C2H5 from m/z 274.3), MD of 29, and fragment 2 at 201.300 Da. (loss of - C4H9O from m/z 274.3), MD of 73.
Characterization of base DP; DP2
The ESI-MS/MS of BLS shows an abundant protonated molecular ion at m/z 464.300 Da. DP2 appeared at m/z 486.400 Da ([M+H] +; C28H36N3ONa) was eluted at 2.49 minutes (Fig. 7). DP2 formed due to hydrolysis of carboxylic acid to form sodium salt. The LC-ESI-MS/MS spectrum of DP2 represents abundant fragment ion at m/z 413.200 Da (removal of - C4H9O from m/z 486.400), MD of 73, fragment 2 at 298.400 Da (loss of C7H6N2 from m/z 413.2 Da), MD of 115 and 187.100 Da (loss of C7H14N from m/z 298.4), MD of 112.
Characterization of oxidative DP; DP3
The ESI-MS/MS of BLS shows an abundant protonated molecular ion at m/z 464.300 Da. DP3 appeared at m/z 480.400 Da ([M+H]+; C28H37N3O4) was eluted at 2.57 minutes (Fig. 7). DP3 formed as a result of the oxidation of nitrogen of the piperidine ring. The LC-ESI-MS/MS spectrum of DP3 shows abundant fragment ion at m/z 394.500 Da (loss of C4H8O2 from m/z 480.4), MD of 86 and fragment 2 at 289.400 Da (loss of - C8H10 from m/z 394.500), MD of 105 and 216.200 Da (loss of - C4H9O from m/z 289.400), MD of 73.
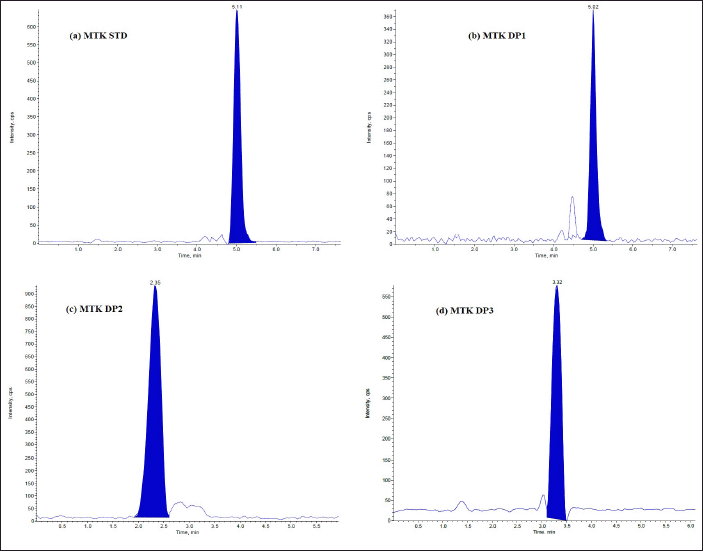 | Figure 5. LC/ESI-MS TIC of (a) MTK standard drug (b) Acid degradant (DP1) (c) Base degradant (DP2) (d) Oxidative degradant (DP3) of Tablet dosage form. [Click here to view] |
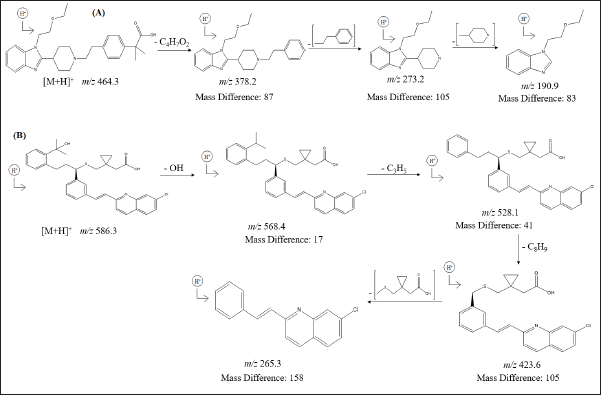 | Figure 6. Proposed MS/MS fragmentation pathway of (A) BLS and (B) MTK. [Click here to view] |
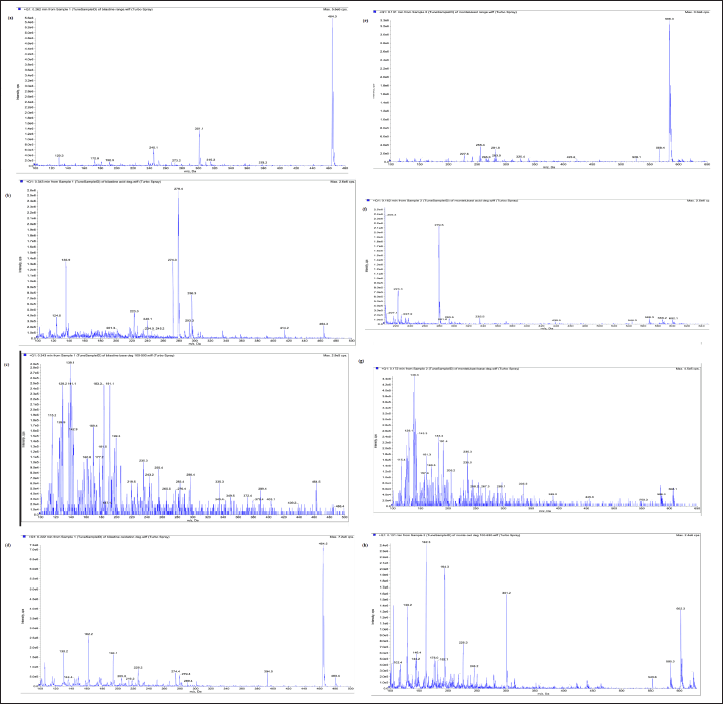 | Figure 7. LC/ESI-MS/MS (product ion tandem mass) spectra of [M+H] + ions of (a) BLS standard drug, (b) DP1 of BLS, (c) DP2 of BLS, (d) DP3 of BLS, (e) MTK standard drug, (f) DP1 of MTK, (g) DP2 of MTK, (h) DP3 of MTK. [Click here to view] |
The proposed structures of BLS and its DPs along with the degradation pathway are represented in Figure 9.
Method validation
The optimized LC–MS/MS method was validated with respect to various parameters summarized in the ICH guidelines [44]. The speci?city of the method was established by comparing the chromatograms of blank, placebo, sample, and standard, and no interference was observed (Fig. 10). The mass detector showed an excellent purity for BLS and MTK and every DP, which unambiguously proves the speci?city of the method. The linearity was established in the range of 0.5–1.5 µg/ml MTK and 1–3 µg/ml BLS. The data were subjected to statistical analysis using a linear regression model; the linear regression equation and correlation coef?cient (R2) were y =7027.3x + 78.271, 0.9993 for MTK and y = 19434x + 9379.8, 0.9991 for BLS correspondingly. The determinations indicate a good correlation exists between the concentration of the drug and response (peak area). The LOD values were found to be 0.098 and 0.236 µg/ml for MTK and BLS correspondingly. The LOQ values were found to be 0.297 and 0.716 µg/ml for MTK and BLS correspondingly. Table 1 shows that the % RSD for intra and inter-day precision was <2%, indicating that the method was precise. Recovery results were within an acceptable limit (98%–102%) which indicates that none of the excipients components have any interference peak under the peak of cited drugs. The outcomes of robustness of the proposed approach showed % RSD <2%, indicates that the method is robust with respect to variations in selected parameters within range. The outcomes of all the validation parameters are summarized in Table 1.
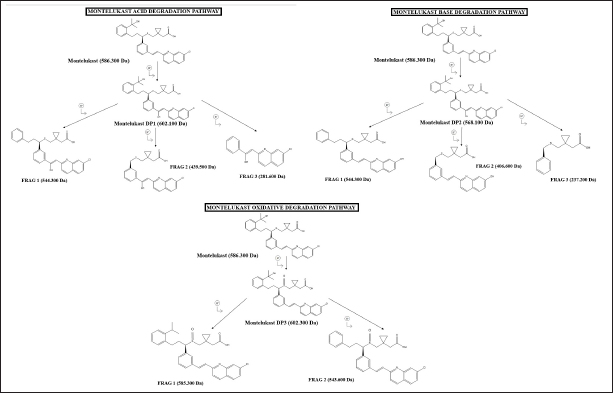 | Figure 8. Proposed degradation pathway of MTK. [Click here to view] |
 | Figure 9. Proposed degradation pathway of BLS. [Click here to view] |
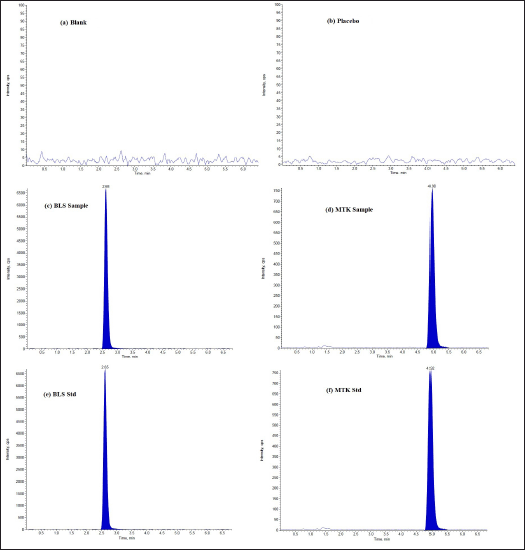 | Figure 10. LC/ESI-MS TIC of (a) Blank (b) Placebo (DP1) (c) BLS sample (d) MTK sample (e) BLS standard (f) MTK standard. [Click here to view] |
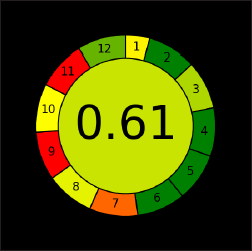 | Figure 11. AGREE assessment pictogram. [Click here to view] |
Assessment of method greenness
The most recent tool for evaluating greenness, “AGREE”, was released in 2020. The AGREE tool was utilized to evaluate the created method’s greenness. It is an advanced and updated method for figuring out how eco-friendly analytical procedures are. It is a qualitative analysis of the method’s interactions with the surroundings. The outcome of AGREE will be a circular pictogram with 12 pieces, each of which will stand for 1 of the 12 green analytical chemistry principles. A number of 0–1 is assigned to each principle or component; a score of 1 indicates the most environmentally friendly. The outcome was a score of 0.61 based on the present AGREE tool approach (Fig. 11). Because of their problems and potential health risks, the most popular organic solvents, such as methanol and acetonitrile, ought to be used less frequently. Acetonitrile, for example, is poisonous, volatile, and combustible [ICH Q3C (R8)]. Therefore, in the AGREE pictogram, section 11 (Toxicity of the Reagents) is shown in red. The suggested approach’s Sections 7 (amount of garbage generated) and 9 (energy usage) are red, which impacts the greenness of the method. According to the assessment, the suggested strategy is overall green.
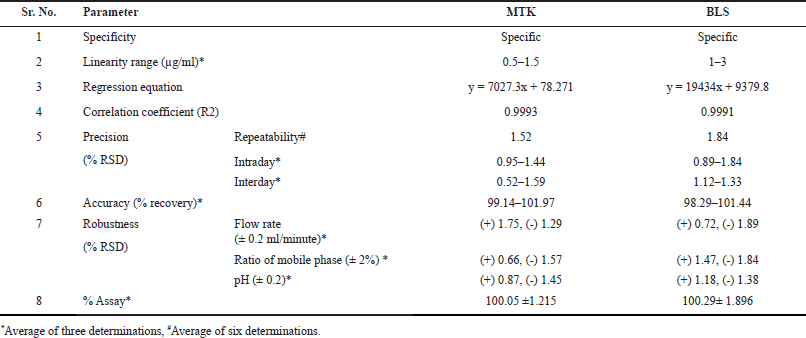 | Table 1. Outcomes of validation parameters. [Click here to view] |
CONCLUSION
In accordance with ICH recommendations, the degradation behavior of MTK and BLS under photolysis, thermal, oxidation, and hydrolysis (acid and base) stress was investigated. Under different stress environments, the current method can resolve all DPs from MTK and BLS as well as from each other. While both drugs were stable under thermal and photolytic stress conditions, they both showed significant changes under acid, base hydrolysis, and oxidative stress. Based on LC-MS/MS data, a total of six DPs were identified, and fragmentation mechanisms were suggested.
ACKNOWLEDGMENTS
The authors thank APMC College of Pharmaceutical Education and Research for facility access.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICT OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
This study paper includes all generated and analyzed data.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Min YG. The pathophysiology, diagnosis and treatment of allergic rhinitis. Allergy Asthma Immunol Res. 2010;2(2):65–76. CrossRef
2. Sharma S, Hatware K, Bhadane P, Patil K. Chemistry, pharmacokinetics, pharmacodynamics and analytical methods of Bilastine, a Histamine H1 receptor antagonist: an update. Mini Rev Med Chem. 2021;21(20):3183–90. CrossRef
3. Johnson J, Abraham T, Sandhu M, Jhaveri D, Hostoffer R, Sher T. Differential diagnosis of asthma. In: Mahmoudi M, editor. Allergy and asthma. Cham, Switzerland: Springer; 2019. pp. 383–400. CrossRef
4. Marques CF, Marques MM, Justino GC. Leukotrienes vs. Montelukast-activity, metabolism, and toxicity hints for repurposing. Pharmaceuticals (Basel). 2022;15(9):1039. CrossRef
5. FDC New Drugs Marketing [Internet]. Gov.in. [cited 2024 Apr 15]. Available from: https://cdsco.gov.in/opencms/opencms/en/Approval_new/FDC-New-Drugs-Marketing/
6. ICH.org. [cited 2024 Apr 15]. Available from: https://database.ich.org/sites/default/files/Q1A%28R2%29%20Guideline.pdf.
7. TRS 1010—Annex 10: WHO guidelines on stability testing of active pharmaceutical ingredients and finished pharmaceutical products [Internet]. WHO.int. [cited 2024 Apr 15]. Available from: https://www.who.int/publications/m/item/trs1010-annex10
8. Blessy M, Patel RD, Prajapati PN, Agrawal Y. Development of forced degradation and stability indicating studies of drugs—a review. J Pharm Anal. 2014;4(3):159–65. CrossRef
9. Bakshi M, Singh S. Development of validated stability-indicating assay methods—critical review. J Pharm Biomed Anal. 2002;28(6):1011–40. CrossRef
10. Patel KN, Patel JK, Patel MP, Rajput GC, Patel HA. Introduction to hyphenated techniques and their applications in pharmacy. Pharm Methods. 2010;1(1):2–13. CrossRef
11. Pan C, Liu F, Ji Q, Wang W, Drinkwater D, Vivilecchia R. The use of LC/MS, GC/MS, and LC/NMR hyphenated techniques to identify a drug degradation product in pharmaceutical development. J Pharm Biomed Anal. 2006;40(3):581–90. CrossRef
12. Youssef YM, Mahrouse MA, Mostafa EA. Assessment of environmental impact of a novel stability-indicating RP-HPLC method and reported methods for the determination of selexipag in bulk and dosage form: a comparative study using different greenness assessment tools. Microchem J. 2023;185:108256. CrossRef
13. Pena-Pereira F, Wojnowski W, Tobiszewski M. AGREE—analytical GREEnness metric approach and software. Anal Chem. 2020;92:10076–82. CrossRef
14. Almalki AH, Alsalahat I, Alharthi MA, Panda DS, Almahri A, Naguib IA. Evaluation of greenness of LC-MS chromatographic methods for simultaneous analysis of mixtures of serotonin, dopamine, acetylcholine, GABA and glutamate: AGREE tool application. Separations. 2022;9(6):147. CrossRef
15. Detroja K, Vekariya H. Advanced derivative spectroscopic method for estimation of Montelukast and Bilastine in their tablet dosage form. Int J Pharm Sci Drug Res. 2021;13(3):268–74. CrossRef
16. Yadav M, Kant R, Yadav S, Chetna, Verma S. Analytical method development and validation by simultaneous estimation of Montelukast sodium and Bilastine by UV spectrophotometry. Eur Chem Bull. 2023;12(Special Issue 10):2082–88. CrossRef
17. Thakur S. Spectroscopy- based development and validation of analytical technique for simultaneous estimation of Bilastine Montelukast. Res Sq. 2022;1:1–18. CrossRef
18. Mistry R, Mashru R. Analytical method development and validation for simultaneous estimation of Bilastine and Montelukast sodium in their combined dosage form by derivative UV-spectroscopy and RP – HPLC method. Int J Pharma Res Health Sci. 2021;9:3313–18. CrossRef
19. Kothari S, Pandya N, Dharu N. Development and validation of analytical methods for simultaneous estimation of Bilastine and Montelukast sodium in combined tablet dosage form. Int J All Res Edu Sci Methods. 2021;9:2830–42.
20. Raj RM, Sankar ASK, Vetrichelvan T. Analytical method development and validation for simultaneous estimation of Bilastine and Montelukast sodium by UV spectrophotometry. World J Pharm Pharma Sci. 2021;10(1):680–87. CrossRef
21. Prajapati P, Tamboli J, Surati P, Mishra A. Risk assessment-based enhanced analytical quality-by-design approach to eco-friendly and economical multicomponent spectrophotometric methods for simultaneous estimation of montelukast sodium and Bilastine. J AOAC Int. 2021;104(5):1453–63. CrossRef
22. Patel P, Panchal ZA, Patel S, Shaikh A. Simultaneous estimation of Montelukast sodium and Bilastine in tablet dosage form by simultaneous equation spectrophotometric method. Int J Educ Appl Sci Res. 2022;9(2):34–43. doi: http://doi.org/10.5281/zenodo. 7307826
23. Chandra U, Kumar M, Sharma S, Gupta P, Gurg A. Development and validation of reverse phase high performance liquid chromatography method for in-vitro dissolution testing of Bilastine and Montelukast sodium rablets. Int J Pharm Sci Drug Res. 2021;13(3):281–87. CrossRef
24. Shinde A, Gajeli GB, Ubale S, Matole V. Simultaneous HPLC method development and validation of Bilastine and Montelukast in bulk and formulation. Res J Pharm Technol. 2021;14(11):6061–65. CrossRef
25. Kancharla V, Bethapudi SA, Bolineni NR. Quantification of Bilastine and Montelukast combination in formulations utilizing liquid chromatography: stability studies. Int J App Pharm. 2021;13(6):210–15. CrossRef
26. Padhiyar V, Patani P, Tiwari N. Development and validation of stability indicating RP-HPLC method for simultaneous estimation of Montelukast sodium and Bilastine from its pharmaceutical dosage form. J Emerg Technol Innov Res. 2021;8(6):B865–77.
27. Andhale SM, Anna PG, Nikalje G. Simultaneous estimation of Bilastine and Montelukast in bulk by RP-HPLC and assessment of its applicability in marketed tablet dosage form. J Pharm Res Int. 2022;34:8–25. CrossRef
28. Vyas P, Thakur VS, Basedia D, Dubey B. Development and validation of RP-HPLC based analytical method for simultaneous estimation of Montelukast and Bilastine in tablet dosage form. Cur Res Pharm Sci. 2022;12(1):68–73. CrossRef
29. Sunkara R, Ajitha M. Stability indicating RP-HPLC method development and validation for simultaneous estimation of Montelukast and Bilastine in bulk and pharmaceutical dosage form. World J Pharm Sci. 2022;10(1):90–7. CrossRef
30. Nizamuddin S, Raju SA. Development and validation RP-HPLC method for simultaneous estimation of Bilastine and Montelukast in bulk and pharmaceutical dosage. Asian Pac J Health Sci. 2022;9(3):242–47.
31. Sudhakar B, Akshaya K, Sri SR. Analytical method development and validation for the simultaneous estimation of Bilastine and Montelukast by RP-HPLC. Asian J Res Pharm Sci. 2023;13(2):79–84.
32. Bhanu MS, Dadi V, Rao YS, Rao VP. RP-HPLC method for quantification of Bilastine and Monteleukast sodium in pharmaceutical dosage form. Res J Pharm Technol. 2023;16(3):1079–84.
33. Patil CV, Khan S, Sharma R, Patel J, Patel R. Stability indicating RP-HPLC method development and validation for simultaneous estimation of Bilastine and Montelukast in bulk and tablet dosage form. Int J Pharm Sci Med. 2023;8(3):132–49. CrossRef
34. Shah DA, Patel PA, Chhalotiya U. Thin-layer chromatographic?densitometric method of analysis for the estimation of Montelukast and Bilastine in combination. J Planar Chromatogr. 2021;34:289–95. CrossRef
35. Rathod KG, Denial V, Saluja MS, Patil DA. Development and validation of stability indicating RP-HPLC method for determination of Bilastine and Montelukast sodium from its combined tablet dosage form. NeuroQuantology. 2022;20(11):9299–314. CrossRef
36. Roshdy A, Salam RA, Hadad G, Belal F, Elmansi H. Green quality by design HPLC approach for the simultaneous determination of Bilastine and Montelukast. BMC Chem. 2023;17(43):1–23. CrossRef
37. Balar YN, Dedania ZR, Dedania RR. A validated stability indicating HPTLC method for Bilastine and Montelukast in pharmacuetical dosage form. Res J Pharm Technol. 2023;16(10):4498–504. CrossRef
38. Vijayalakshmi KA, Andrews BS, Rao BN. Quantification of Bilastine and Montelukast combination in formulations utilizing liquid chromatography: stability studies. Int J App Pharm. 2021;13:210–15.
39. Chandra U, Kumar M, Garg A, Sharma S, Gupta P. An innovative impurity profiling method development for identification and quantitation of Bilastine and Montelukast sodium with related impurities by RP-HPLC. Int J Pharm Res. 2021;13(3):754. CrossRef
40. Patel KK, Patel AM, Patel CN. A new simple RP-HPLC method development, validation and forced degradation studies of Bilastine. Asian J Pharm Anal. 2021;11(3):183–87. CrossRef
41. Prajapati P, Tamboli J, Mishra A. Risk and DoE-based analytical failure mode effect analysis (AFMEA) to simultaneous estimation of Montelukast sodium and Bilastine by HPTLC method using enhanced AQbD approach. J Chromatogr Sci. 2022;60(6):595–605. CrossRef
42. Akansha, Singhla S, Dana S, Palia P, Goyal S. Development & validation of stability indicating RP—HPLC method for determination of related substance of Bilastine & Montelukast in suspension dosage form. World J Pharm Res. 2022;11(12):1021–49.
43. Derayea SM, Badr El-Din KM, Ahmed AS, Khorshed KA, Oraby M. Development of a green synchronous spectrofluorimetric technique for simultaneous determination of Montelukast sodium and Bilastine in pharmaceutical formulations. BMC Chem. 2024;18(18):1–13. CrossRef
44. ICH Harmonised Tripartite Guideline. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use [Internet]. Ich.org. [cited 2024 Sep 30]. Available from: https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf