INTRODUCTION
Diabetes mellitus (DM) is a major international health emergency that affects 415 million persons aged 20–70 years, and its prevalence is increasing [1]. The most common forms of DM include type 2 DM (T2DM), gestational DM, and type 1 DM (T1DM) [2]. T1DM is characterized by absolute insulin deficiency and results from autoimmune damage to pancreatic β-cells [3]. T2DM, which leads to at least 90% of DM occurrences, is characterized by long-term hyperglycemia, altered insulin secretion, and insulin resistance (IR) [4]. In addition to chronic hyperglycemia, lipid accumulation is also closely associated with IR [5].
The key organ in glucose, lipid, and protein metabolism and the primary target of insulin is the liver, which plays a substantial task in the improvement of IR in people with T2DM [6]. DM may produce hepatic injury and liver cirrhosis [7], this action may be due to inflammation that developed in fatty tissues and the liver through increases in mitochondrial oxidative stress and inflammatory chemical mediators such as adiponectin, leptin, interleukin (IL)-6, and TNF-α [8,9]. The formation of these chemical mediators in adipose tissues is substantially stimulated by IR. A deeper comprehension of the connection between these adipocytokines and DM may provide new insight regarding the physiopathology of diabetes [10].
Carbohydrate metabolism disorders in DM lead to high levels of free radicals that cause oxidative damage to biomolecules within the body, gradually resulting in premature aging and chronic illnesses such as hepatic disorder, atherosclerosis, and cancer. However, the use of antioxidant-rich plants and plant constituents may improve the antioxidant protection system, thereby defending against oxidative stress and damage produced by free radicals in DM [11].
Oleuropein, which is responsible for the distinctive bitter flavor of olive fruits, is the highest common phenolic element in the flesh of olives (Olea europaea). It is composed of 2%–3% (w/w) phenolic compounds [12]. Oleuropein was shown to have a maximum content of dry matter in olive fruit and 6%–9% dry material in olive leaves. However, some sources claim that olive leaves have an oleuropein concentration of up to 19% (w/w) [13]. Some preparations of olive oil, the consumption of which is associated with declined risks of DM, obesity, and cardiovascular disease [14,15] have been shown to contain up to 9.0 mg/l of oleuropein [16]. Following metabolic degradation, oleuropein can produce other bioactive constituents, namely hydroxytyrosol and tyrosol. Oleuropein and its degradation product, hydroxytyrosol, have been shown to possess anti-proliferative, anti-apoptotic, anti-inflammatory, and anti-obesity properties [17]. The present study will examine the roles of oleuropein, on hyperlipidemia, oxidative stress, inflammatory status, and liver dysfunction in diabetic male Wistar rats induced by streptozotocin (STZ).
MATERIALS AND METHODS
Chemicals
STZ and oleuropein were delivered from Sigma-Aldrich Co. in Germany. Entirety other chemicals were commercially available, and they were of ultragrade.
Experimental animals
Male albino rats (mean body weight, 180 ± 20 g) of the Wistar strain were gained from the College of Medicine, Alexandria University, Alexandria, Egypt. The environment in which the animals were kept was managed to have a 12-hour cycle of light and darkness as well as a constant temperature and humidity. The experiment’s concept accepted Institutional Animal Care and Use Committee (IACUC) approval in April 2017, and the steps were followed by the committee’s guidelines and standards for the management of animals.
Induction of DM
To induce DM, male Wistar rats were fasting overnight and given a single fresh preparation of 40 mg/kg BW STZ (pH 4.5) intraperitoneally. Using an Accu-Chek Sensor Comfort glucometer, the glucose level of blood from the lateral tail vein was measured 1 week after the injection to check for hyperglycemia. The rats were judged diabetic and participated in the experimental study if their blood glucose concentrations at 2 hours postprandial were more than 200 mg/dl [18].
Experimental design
Following the accommodation period and induction of DM, the rats were allocated to the following groups (Fig. 1).
Normal negative control group: The animals were healthy normal rats that were supplied with the same volume of distilled water (vehicle in which oleuropein was dissolved) by oral gavage daily for 15 days. The animals in this group were considered as controls to those in the STZ animals group.
STZ animals positive control group: This group included STZ animals rats that were supplied with the corresponding amount of distilled water (vehicle in which oleuropein was dissolved) by oral gavage daily for 15 days. In this group, rats were considered as STZ animals’ control over those in the STZ animals group that were treated with oleuropein.
STZ animals + oleuropein group: The STZ animal rats were supplied with oleuropein (5 mg/kg BW/day) for 15 consecutive days using oral gavage.
Blood sampling and tissue sampling
The rats were denied food and water, and blood sampling was assembled from the jugular vein into dry centrifuge tubes while being lightly sedated with inhalation anesthesia. For the blood to coagulate, room temperature (24°C–26°C) was used. The serum was then isolated from the clot by centrifuging for 10 minutes at 3,000 ×g. When not in use, the sera were placed in sterile, clean Eppendorf tubes and kept at −20°C.
Following the blood sampling, the rats were dissected, by decapitation, and livers were rapidly excised and perfused in sterile isotonic saline (0.9% NaCl). A half gram of liver was homogenized in 5 ml of phosphate-buffered saline at pH 7.4. Then, homogenates were centrifuged at 10,000 ×g for 15 minutes and cooled to 4°C. Liver homogenate supernatants were prepared to determine enzymatic and nonenzymatic antioxidants as well as lipid peroxidation (LPO).
 | Figure 1. Scheme of animal grouping and schedule of doses. [Click here to view] |
Biochemical analysis
Based on the technique developed by Bergmeyer et al. [19], the activity of ALT and AST were assayed. In accordance with the International Federation of Clinical Chemistry, alkaline phosphatase (ALP) activity was determined using kits provided by BioSystems S.A. Costa Brava 30, Barcelona, Spain. Liver glucose-6-phosphate dehydrogenase (G6PDH) was measured by rat G6PDH ELISA kit (E-EL-R0428) according to the instruction of the manufacturer (Elabscience Biotechnology Inc., USA). Serum leptin level was measured by using Abcam’s rat leptin ELISA kit (ab100773) obtained from Abcam, Cambridge, United Kingdom, according to the manufacturer’s instructions.
Serum adiponectin was measured by using a rat adiponectin ELISA kit (#JIM-K4903-100) attained from MBL International Corporation (Woburn, MA) following the manufacturer’s instruction. Total serum bilirubin was measured as per [20]. The levels of TC, TG, LDL-C, and HDL-C were assayed by the enzymatic procedure in a Labmax Plenno® biochemical analyzer using Labtest® kits (Labtest Inc. Lagoa Santa, MG, Brazil). To calculate vLDL-C values, the formula vLDL-C = TG/5 was used [21].
Detection of LPO and antioxidant defense biomarkers
The hepatic thiobarbituric acid reactive substance (TBARS) as an indicator of LPO and reduced glutathione (GSH) level was assayed using a spectrophotometer (Chem-7 Semi-Auto Chemistry Analyzer, Erba Diagnostics Mannheim GmbH, Germany). The catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), and Glutathione-S-transferase (GST), activities were estimated in the hepatic tissues by using kits.
Determination of the TNF-α and COX-2 by quantitative RT-PCR assay (qRT-PCR) in the liver
TNF-α and COX-2 genes mRNA expression levels were evaluated using qRT-PCR. As described in other studies, hepatic tissues’ total RNAs were extracted by means of Biozol reagent using the GStractTM RNA separation kit II guanidinium thiocyanate technique. The fold change was computed using the formulation 2−ΔΔct concerning β-actin as revealed by Livak and Schmittgen [22]. The primer sequences used for β-actin, TNF-α, and COX-2 [23] are described in the previous publications.
Statistical analysis
Data analysis was performed by SPSS version 22.0 (Chicago, IL). Multiple group comparisons were made using the post hoc Tukey’s test, and the changes were judged significant at p < 0.05. Symbol “a” is used to refer to a comparison with the normal control and Symbol “b” is used to indicate a comparison with the STZ animal control.
Results
Effects of oleuropein on liver function biomarker
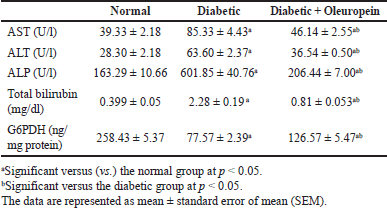
Hepatic function test, AST, ALT, ALP, and total bilirubin quantities in STZ animals’ rats were significantly (p < 0.05) superior, whereas liver G6PDH activity was significantly (p < 0.05) lesser than those in the control group. Oral giving of oleuropein to STZ animals rats significantly (p < 0.05) ameliorated hepatic enzyme activities (ALT, AST, and ALP) and total bilirubin level, while oleuropein, induced an increase (p < 0.05) in liver G6PDH activity compared to the STZ animals control group (Table 1).
Effects of oleuropein on lipid profile, adiponectin, and leptin
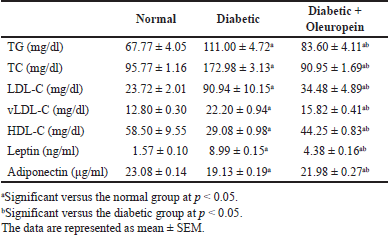
The STZ animals group showed a significant elevation (p < 0.05) in cholesterol, LDL-C, VLDL-C, TG, and leptin, while a significant decrease (p < 0.05) in HDL-C and adiponectin when compared with the normal control group (Table 2).
Oleuropein administration in STZ animals rats caused a significant improvement in cholesterol, LDL-C, TG, VLDL-C, and leptin (p < 0.05) and enhanced HDL-C and adiponectin levels when compared with the STZ rats.
Effects of oleuropein on oxidative stress and antioxidant defense biomarkers
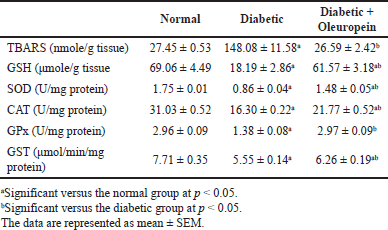
Table 3 reveals that hepatic CAT, SOD, GPx, GST activities, and GSH levels considerably reduced (p < 0.05), whereas TBARS significantly heightened (p < 0.05) in the STZ animals in comparison with the normal group. The STZ animals’ group that received oral oleuropein had considerably lower TBARS (p < 0.05), higher glutathione levels, and higher antioxidant enzyme activities when matched to the only STZ animals group.
 | Table 1. Effects of oleuropein on serum AST, ALT, ALP, total bilirubin, and liver G6PDH levels in male diabetic rats. [Click here to view] |
 | Table 2. Effects of oleuropein on serum lipid profile, leptin and diponectin in diabetic male rats. [Click here to view] |
 | Table 3. Effects of oleuropein on oxidative stress markers and antioxidant enzyme activities in the livers of male diabetic rats. [Click here to view] |
 | Table 4. The effects of oleuropein on TNF-α and COX-2 mRNA expression in the liver of male diabetic rats. [Click here to view] |
Effects of oleuropein on liver TNF-α and COX-2 mRNA expression
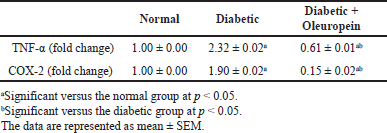
Serum TNF-α and COX-2 were considerably higher (p < 0.05) in the diabetes group in comparison with the normal group. Oleuropein-treated STZ rats showed a substantial improvement (p < 0.05) in TNF-α and COX-2 levels when evaluated with the STZ animals’ group (Table 4).
DISCUSSION
Serums ALT, AST, and the total bilirubin levels were the precise bioindicators applied to monitor hepatic disorders [24]. The elevation in these parameters in the STZ rats may be due to hepatic cell membrane damage or necrosis, which releases these enzymes and bilirubin into the circulatory system [25]. According to Swamy et al. [26], these increased values indicate cellular leaking and a loss of the functional integrity of the cell membranes. In addition, people with DM are more likely than those without DM to have abnormal liver function tests [27]. The development of NASH to cirrhosis and chronic liver disease have also been linked to DM. While the elevation of serum ALP in conjunction with an increase in serum bilirubin levels indicates hepatobiliary illness, the rise in the activity of serums ALT and AST in diabetic rats suggests damage to hepatocytes caused by STZ-induced DM [28].
Oleuropein’s ability to stabilize membranes may explain why it can reduce the rise in serum liver enzyme levels by preventing the release of membrane-bonding enzymes as well as leakages of intracellular enzymes. Furthermore, the protective effect of oleuropein against hepatic disorders may be due to its ability to maintain liver cell integrity. Moreover, olive oil’s phenolic hydroxytyrosol and tyrosol compounds (the breakdown products of oleuropein) ameliorate hepatotoxicity in rats by repressing oxidative stress and programmed cell death [29].
The current results demonstrate that oleuropein therapy significantly reduced the elevated serum bilirubin levels. This finding agrees with Karakoç and Sekkin [30], who found that the administration of oleuropein to cyclophosphamide and epirubicin-injected rats significantly improved the elevated total bilirubin level in conjunction with the decrease in the elevated serum transaminases’ activities. However, these results are in discordance with Domitrovi? et al. [31] who demonstrated that oleuropein, in vivo, induced the liver heme oxygenase, which stimulates the breakdown of heme to iron, bilirubin, and carbon monoxide.
According to the current investigation, insulin insufficiency in the STZ rats that was left untreated may be the cause of the decreased liver G6PDH activity, which is important as a rate-limiting enzyme of the pentose phosphate pathway for nicotinamide adenine dinucleotide phosphate (NADPH) synthesis. This contributes significantly to maintaining the antioxidant defense mechanism [32]. It was hypothesized that the decrease in G6PDH activity in DM is the main reason for the redox imbalance [33].
Moreover, Choukem et al. [34] stated that G6PDH deficiency may be related to oxidative stress in T2DM owing to the insufficient or limited production of reduced NADPH that recovers GSH (a physiologic antioxidant) to eradicate glucose-generated free radicals. It is worth noting that oleuropein treatment upregulated the expression of hepatic G6PDH by enhancing the synthesis of insulin. Oleuropein administration had a positive effect on glucose metabolism and the consecutive metabolic correlations between elevated glycolysis and lowered gluconeogenesis, elucidating the biochemical mechanisms by which regulation of glucose homeostasis is achieved [35,36].
Serum lipids levels in diabetic rats increased [37], leading to a rise in the mobilizations of free fatty acids (FFAs) from the peripheral depot fat which is primarily responsible for the abnormally high concentration of plasma lipids and lipoproteins in DM [38]. A deficiency of insulin diminishes lipoprotein lipase activity, resulting in disturbances in the metabolism of lipoprotein in DM. The increase in LDL levels in STZ animals might be because of the overproduction of LDL by the hepatic tissues as a result of the stimulation of hepatic triglyceride synthesis by the free fatty acid influx [39].
Decreases in plasma HDL-C in a rat model of DM and patients with DM were due to defects in reverse cholesterol transport. Oleuropein exhibited a hypolipidemic effect that may be due to decreased cholesterol formation and fatty acid synthesis [40]. Oleuropein also has an anti-diabetic impact by improving glucose absorption and utilization, insulin secretory response, and antioxidant activities [41]. Mice supplied with a high-sugar food, with O. europaea fruit resulted in lower levels of triglycerides and VLDL-C; this effect is presumably caused by the reduced IR and an anti-diabetic action [42]. In addition, studies have shown that oleuropein decreases triglyceride formation inside cells and both the quantity and size of lipid drops treated with FFAs [43].
Leptin is a peptide hormone, produced from adipose tissues and encoded by the obese gene [44]. Increased IR and vascular damage caused by leptin may have a role in the development of DM and cardiovascular disorders [45]. Leptin has been shown to have antisteatotic properties, but in some situations, such as hyperleptinemia, this hormone may also promote the aggravation of liver steatosis.
Leptin also promotes nonalcoholic fatty liver disease (NAFLD) which contributes to the development of nonalcoholic steatohepatitis and liver fibrosis [46]. Leptin appears to have a dual impact on NAFLD in experimental animals, having anti-steatotic, pro-inflammatory, pro-fibrogenic, and perhaps carcinogenic properties [47]. In the current study, the serum leptin level was significantly raised in STZ-induced diabetic rats, which may be due to decreased pancreatic insulin secretion, that has been destroyed by STZ, leading to impaired negative feedback control [48].
Local and circulating levels of adiponectin decrease during obesity, IR, T2DM, and atherosclerosis. This action is because it suppresses glucose-6-phosphatase transcription, which reduces gluconeogenesis. The high-molecular-weight adiponectin complex causes a more pronounced reduction in glucose levels in mice [49]. Adiponectin is an efficient preventive agent against several types of liver damage, according to animal-based research, and some data point to adiponectin’s direct opposition to TNF-α’s necrotic and destructive effects on liver tissues [50] where it binds to its receptor in hepatocytes, which increases aldehyde oxidase-1 activity and lowers intracellular ROS levels via increasing PPARα activation [51].
However, in DM oxidative stress is detrimental to adiponectin action; specifically, oxidative stress in adipose tissue suppresses adiponectin secretion [52], resulting in a decline in adiponectin levels and losing its ability to reduce the risky effects of TNF-α within the liver tissue, as demonstrated in the current study. Particularly, an inflammatory state raises the cytokines as TNF-α and leptin, predisposing tissues to hepatic illnesses and causing a concurrent downregulation of protective adipocytokines like adiponectin [53]. In the current study, serum adiponectin significantly diminished in STZ-induced diabetic rats [48]. The increased intracellular ROS production following mitochondrial dysfunction, STZ-induced hyperglycemia, and exacerbated fatty acid oxidation may disturb adipocyte functions and suppress adiponectin secretion [54,55].
Leptin concentrations were significantly reduced in the oleuropein while the opposite results were observed in adiponectin. These results are in concurrence with Fki et al. [56] who reported a significant decrease in plasma leptin due to treatment of high-fat supplemented rats with oleuropein. In addition, adiponectin in patients who received oral ascorbic acid supplementation improves insulin sensitivity which could be associated with increased FFAs’ oxidation as well as decreased glucose synthesis in the liver [57].
Oxidative stress is a principal factor in the etiology of DM, so antioxidants may help treat this condition (Fig. 2). Free radical levels rise as a result of the antioxidant defense mechanism being insufficient. Increased LPO, oxidative damage to membranes, and disturbances in essential cell activities may result from elevated free radical levels [58]. The current investigation demonstrated that higher LPO (TBARS) and reduced antioxidant enzymes were related to diabetes-induced liver damage (GSH, SOD, GPx, and CAT). In the current study, the antioxidant enzymes’ decreased activity resulted in higher ROS generation in diabetic rats not receiving treatment, which exacerbated oxidative stress.
The progress of diabetes problems may be related to the elevated TBARS concentration in STZ rats. Hazardous free radicals are scavenged by various enzymatic antioxidants involving SOD and CAT [59]. Hyperglycemia enhances the production of reactive oxygen species by increasing glucose auto-oxidation. As a result, the antioxidant defense mechanism is less active, which might damage the liver cells. Since various tissues are more susceptible to oxidative damage, which may cause several complications in chronic DM, the enhancement of antioxidant condition is a vital component to consider when evaluating the advantages of antidiabetic medicines [60,61].
Several bioactive ingredients in olive oil are associated with antioxidative and anti-inflammatory preventive functions, particularly those from biophenols, such as oleuropein and its degradation product, hydroxytyrosol. Oleuropein incubated with the cells displayed a significant decrease in cytokine-induced ROS generation and alleviated the attenuated antioxidant protection system [62]. The antioxidant ability of oleuropein is to remove ROS. Oleuropein-treated rats showed a significant reduction in LPO and oleuropein has beneficial antioxidant properties against gastric damage and reduces hepatic oxidative stress in rats [63].
Hyperglycemia can stimulate stress signaling as well as pro-inflammatory pathways. Nuclear factor-kappaB (NF-κB) signaling to form NF-κB p65 and NF-κB p50 is the main signal transduction pathway, which is connected with the gene control and activation of pro-inflammatory cytokines, including COX-2, inducible nitric oxide synthase (iNOS), TNF-α and IL-1β, [64]. Increased synthesis of chemokines and cytokines from activated Kupffer cells recruit the neutrophils and other inflammatory cells to inflamed liver and activate endothelial cells, resulting in more production of ROS and the progress of liver necrosis and damage [65].
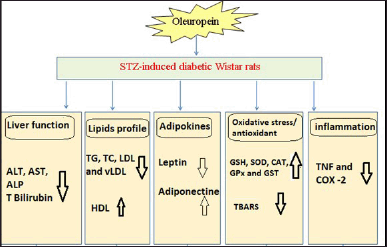
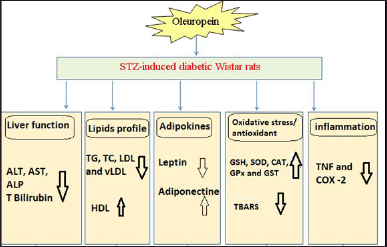 | Figure 2. Schematic figure depicting the effects and modes of action of oleuropein in STZ-induced rats. [Click here to view] |
Oleuropein oral administration to STZ rats reduced COX-2 and TNF-α expression in the diabetic rats’ livers (Fig. 2). According to Wardyn et al. [66], oleuropein decreased NF-κB p65, phospho-p65, COX-2, and TNF-α, production in the kidneys as a result of cisplatin therapy. The degradative metabolic product of oleuropein, hydroxytyrosol, also showed anti-inflammatory characteristics, decreasing iNOS, COX-2, TNF-α, and nitric oxide release in the lipopolysaccharide-activated human monocytic cell line [67]. Oleuropein, a polyphenolic substance, allegedly reduced NF-κB phosphorylation in models of spinal cord injury and ileum ischemia/reperfusion in mice [68]. Oleuropein specifically reduced the expression of IL-1β and IL-6 in the colon, and in diabetic rats [69].
Overall, oleuropein has potent ameliorative effects on liver function, lipid profile, leptin and adiponectin, inflammation, and oxidative stress (Fig. 2). In our opinion, the improvement in lipid profile and adipocytokines (leptin and adiponectin) levels as well as the inhibition of oxidative stress and improvement of antioxidant defense system may show a vital effect in the prevention of liver disorders in DM.
In conclusion, the oral administration of oleuropein could prevent liver dysfunctions in diabetic rats through its anti-hyperlipidemic, antioxidant, and anti-inflammatory effects (Fig. 2).
ACKNOWLEDGMENTS
The authors thank Imam Abdulrahman Bin Faisal University, Dammam, Saudi Arabia, for its support. The authors are also thankful to the Molecular Biology Lab at the High Institute of Public Health, Alexandria University, Egypt.
AUTHOR CONTRIBUTIONS
Conceptualization, NAM, OMA, and HMA; project administration, NAM, OMA, SSA, HMA, KAA, and HMA; supervision, NAM and SSA; funding acquisition, MMH, SSA, HMA, and KAA; methodology, NAM, MMH, and HMA; data curation, NAM, MMH, and HMA; statistical analysis, NAM, MMH, OMA, and HMA; software, MMH; validation and visualization, NAM, MMH, OMA, SSA, HMA, KAA, and HMA; formal analysis, NAM, MMH, and HMA; writing the original draft, NAM, MMH, OMA, and HMA; revising and editing, NAM, MMH, OMA, SSA, HMA, KAA, and HMA. All authors have read and agreed to the published version of the manuscript.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Institutional Animal Care and Use Committee of College of Medicine, Alexandria University, Egypt with approval number Alex/FS/2017/4 and date: 7/2/2024.
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Hunt D, Hemmingsen B, Matzke A, Varghese C, Hammerich A, Luciani S, et al. The WHO global diabetes compact: a new initiative to support people living with diabetes. Lancet Diabetes Endocrinol. 2021;9(6):325–7. CrossRef
2. Pihoker C, Gilliam LK, Hampe CS, Lernmark A. Autoantibodies in diabetes. Diabetes. 2005;54(suppl2):S52–61.
3. Solis-Herrera C, Triplitt C, Reasner, C, DeFronzo RA. Classification of diabetes mellitus. In: Feingold KR, Anawalt B, Boyce A, et al., editors. Endotext. South Dartmouth, MA: MDText.com, Inc.; 2018.
4. Borse SP, Chhipa AS, Sharma V, Singh DP, Nivsarkar M. Management of type 2 diabetes: current strategies, unfocussed aspects, challenges, and alternatives. Med Princ Pract. 2021;30(2):109–21. CrossRef
5. Kakehi Y, Tamura K, Takeno K, Sakurai Y, Kawaguchi M, Watanabe T, et al. Increased intramyocellular lipid/impaired insulin sensitivity is associated with altered lipid metabolic genes in muscle of high responders to a high-fat diet. Am J Physiol- Endocrinol and Metab. 2016;310:32–40.
6. Keith KG, Fonseca V, Tan MH, Dalpiaz A. Narrative review: hepatobiliary disease in type 2 diabetes mellitus. Ann Intern Med. 2017;141:946–56.
7. Chung W, Promrat K, Wands J. Clinical implications, diagnosis, and management of diabetes in patients with chronic liver diseases. World J Hepatol. 2020;12(9):533–57. CrossRef
8. Clark JL, Taylor CG, Zahradka P. Exploring the cardio-metabolic relevance of T-cadherin: a pleiotropic adiponectin receptor. Endocr Metab and Immune Disord Drug Targets. 2017;17:200–6. CrossRef
9. Abdel Aziz SM, Ahmed OM, Abd El-Twab SM, Al-Muzafar HM, Amin KA, Abdel-Gabbar M. Antihyperglycemic effects and mode of actions of Musa paradisiaca leaf and fruit peel hydroethanolic extracts in nicotinamide/streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2020;2020:9276343.
10. Rehman K, Akash MS. Nutrition and diabetes mellitus: how are they interlinked? Crit Rev Eukaryot Gene Expr. 2016;26(4):317–32. CrossRef
11. Ahmed OM, Abd El-Twab SM, Al-Muzafar HM, Adel Amin K, Abdel Aziz SM, Abdel-Gabbar M. Musa paradisiaca L. leaf and fruit peel hydroethanolic extracts improved the lipid profile, glycemic index and oxidative stress in nicotinamide/streptozotocin-induced diabetic rats. Vet Med Sci. 2021;7(2):500–11. CrossRef
12. Ozdemir Y, Guven E, Ozturk A. Understanding the characteristics of oleuropein for table olive processing. J Food Process Technol. 2014;5:328.
13. Rashed SA, Saad TI, El-Darier SM. Potential aptitude of four olive cultivars as anticancer and antioxidant agents: oleuropein content. Rend Fis Acc Lincei. 2022;33:195–203.
14. Bartimoccia S, Cammisotto V, Nocella C, Del Ben M, D’Amico A, Castellani V, et al. Extra virgin olive oil reduces gut permeability and metabolic endotoxemia in diabetic patients. Nutrients. 2022;14(10):2153.
15. Mizouri R, Abir E, Yamoun R, Mahjoub F, Nsir S, Amorri A, Othman RB, et al. Mediterranean diet rich in olive oil and vascular diabetes complications. Endocr Abstr. 2022;81:EP443.
16. Britti D, Impellizzeri D, Procopio A, Cuzzocrea S. Oleuropein an olive oil compound in acute and chronic inflammation models: facts and perspectives. In: Muzzalupo I, editor. Olive germplasm. The olive cultivation, table olive and olive oil industry in Italy. Londo, UK: IntechOpen; 2012. Available from: https://www.intechopen.com/chapters/41347; doi: https://doi.org/10.5772/51889
17. Martínez-Navarro ME, Cebrián-Tarancón C, Oliva J, Salinas MR, Alonso GL. Oleuropein degradation kinetics in olive leaf and its aqueous extracts. Antioxidants. 2021;10:1963.
18. Mohamed NA, Ahmed OM, Hozayen WG, Ahmed MA. Ameliorative effects of bee pollen and date palm pollen on the glycemic state and male sexual dysfunctions in streptozotocin-induced diabetic Wistar rats. Biomed Pharmacother. 2018;97:9–18.
19. Bergmeyer HU, Horder M, Rey J. Approved recommendation on IFCC methods for the measurement of catalytic enzymes. Part 2: IFCC method for aspartate aminotransferase. J Clin Chem Clin Bioch. 1986;24:497–510.
20. Jendrassik L, Grof P. Vereinfachte, photometrische methoden zur bestimmung des blutbilirubins. Biochem Z. 1938;297:81–9.
21. Scheen AJ. Pharmacokinetic and toxicological considerations for the treatment of diabetes in patients with liver disease. Expert Opin Drug Metab Toxicol. 2014;10:839–57.
22. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T) method. Methods 2001;25:402–8.
23. Zaky A, Mohammad B, Moftah M, Kandeel KM, Bassiouny AR. Apurinic/apyrimidinic endonuclease 1 is a key modulator of aluminum-induced neuroinflammation. BMC Neurosci. 2013;14:26.
24. Afrisham R, Aberomand M, Ghaffari MA, Siahpoosh A, Jamalan M. Inhibitory effect of Heracleum persicum and Ziziphus jujuba on the activity of alpha-amylase. J Bot 2015;10:1155.
25. Mohamed AH, Abd El-Hameed MN, Khamis HH, Sharkawy GK, Samir AH. Anti-diabetic and anti-inflammatory effects of two Fabaceae extracts against streptozotocin induced diabetic impairment in male rats. World J of Adv Res Rev. 2020;6(3):012–29.
26. Swamy SK, Nagalakshmi NC, Santhosh K, Yogesh HS. Hypoglycemic activity of ethanol extract of Jasminum grandiflorum flowers in vivo and cytotoxicity of its chloroform isolate in vitro. J Diabetes Metab Disor. 2018;3:1–9.
27. Kumar M. A review on phytochemical constituents and pharmacological activities of Ricinus communis L. Plant. Intern J Pharmacogn Phytochem Res. 2017;9(4):466–72.
28. Ahmed OM. Histopathological and biochemical evaluation of liver and kidney lesions in streptozotocin-diabetic rats treated with glimepiride and various plant extracts. J Union Arab Biol. 2001;16A:585–625.
29. Kalaiselvan I, Samuthirapandi M, Govindaraju A, Sheeja D, Malar P, Kasi D. Olive oil and its phenolic compounds (hydroxytyrosol and tyrosol) ameliorated TCDD-induced heptotoxicity in rats via inhibition of oxidative stress and apoptosis. Pharm Biol. 2016;54:338–46.
30. Karakoç MD, Sekkin S. Effects of oleuropein on epirubicin and cyclophosphamide combination treatment in rats. Turk J Pharm Sci. 2021;18(4):420–9.
31. Domitrovi? R, Jakovac H, Marchesi VV, Šain I, Romi? Ž, Raheli? D. Preventive and therapeutic effects of oleuropein against carbon tetrachloride-induced liver damage in mice. Pharmacol Res. 2012;65:451–64.
32. Ge T, Yang J, Zhou S, Wang Y, Li Y, Tong X. The role of the pentose phosphate pathway in diabetes and cancer. Front Endocrinol (Lausanne). 2020;9(11):365.
33. Zhang Z, Yang Z, Zhu B, Hu J, Liew CW, Zhang Y, et al. Increasing glucose 6-phosphate dehydrogenase activity restores redox balance in vascular endothelial cells exposed to high glucose. PLoS One. 2012;7(11):e49128. CrossRef
34. Choukem SP, Sobngwi E, Garnier JP, Letellier S, Mauvais-Jarvis F, Calvo F, et al. Hyperglycaemia per se does not affect erythrocyte glucose-6-phosphate dehydrogenase activity in ketosis-prone diabetes. Diabetes Metab J. 2015;41:326–30.
35. Constantin RP, Constantin RP, Bracht A, Yamamoto NS, Ishii-Iwamoto EL, Constantin J. Molecular mechanisms of citrus flavanones on hepatic gluconeogenesis. Fitoterapia. 2014;92:148–62.
36. Miyamoto T, Amrein H. Gluconeogenesis: an ancient biochemical pathway with a new twist. Fly (Austin). 2017;11:218–23.
37. Al-Mahmood SM, Razak TA, Abdullah ST, Fatnoon NA, Mohamed AH, Al-Ani IM, et al. A comprehensive study of chronic diabetes complications in streptozotocin-induced diabetic rat. Makara J Health Res. 2016;20:48–56.
38. Daisy P, Kani GF. Hypolipidemic and hepatoprotective effects of Cassia auriculata Linn bark extracts on streptozotocin induced diabetics in male Wister albino rats. Asian J Pharm Clin Res. 2013; 6:43–8.
39. Gandhimathi S, Viji Stella Bai G. Antidiabetic activity of Randia dumetorum against streptozotocin (STZ) induced diabetics in rats. Int J Pharm Res. 2014;3:126–9.
40. Hur W, Kim SW, Lee YK, Choi JE, Hong SW, Song MJ. Oleuropein reduces free fatty acid-induced lipogenesis via lowered extracellular signal-regulated kinase activation in hepatocytes. Nutr Res. 2012;32:778–86.
41. Zheng S, Huang K, Tong T. Efficacy and mechanisms of oleuropein in mitigating diabetes and diabetes complications. J Agric Food Chem. 2021;69(22):6145–55. CrossRef
42. Ahangarpour A, Ramezani-Ali akbari F. Protective effects of Olea europaea fruit extracts on metabolic disorders associated with sucrose-induced metabolic syndrome in rats. Avicenna J Med Biochem. 2018;6:8–14.
43. Oi-Kano Y, Kawada T, Watanabe T, Koyama F, Watanabe K, Senbongi R. Extra virgin olive oil increases uncoupling protein 1 content in brown adipose tissue and enhances noradrenaline and adrenaline secretions in rats. J Nutr Bioch. 2017;18:685–92.
44. Obradovic M, Sudar-Milovanovic E, Soskic S, Essack M, Arya S, Stewart AJ, et al. Leptin and obesity: role and clinical implication. Front Endocrinol. 2021;12:585887.
45. Poetsch MS, Strano A, Guan K. Role of leptin in cardiovascular diseases. Front Endocrinol (Lausanne). 2020;11:354.
46. Jiménez-Cortegana C, García-Galey A, Tami M, Del Pino P, Carmona I, López S, et al. Role of leptin in non-alcoholic fatty liver disease. Biomedicines. 2021;9(7):762.
47. Polyzos SA, Kountouras J, Mantzoros CS. Leptin in nonalcoholic fatty liver disease: a narrative review. Metabolism. 2015;64(1):60–78. CrossRef
48. Akta? ?H, Pençe HH, Özçelik F, Sayir N, Sapmaz T, Kutlu O, et al. Vaspin, adiponectin and leptin levels in type 1 diabetic rats induced by streptozotocin. Acta Endocrinol (Buchar). 2020;16(2):136–41.
49. Wang ZV, Scherer PE. Adiponectin, the past two decades. J Mol Cell Biol. 2016;8:93–100.
50. Shabalala SC, Dludla PV, Mabasa L, Kappo AP, Basson AK, Pheiffer C, et al. The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomed Pharmacother. 2020;131:110785. CrossRef
51. Neumeier M, Weigert J, Schäffler A, Weiss TS, Schmidl C, Büttner R, et al. Aldehyde oxidase 1 is highly abundant in hepatic steatosis and is downregulated by adiponectin and fenofibric acid in hepatocytes in vitro. Biochem Biophys Res Commun. 2006;350(3):731–5. CrossRef
52. Yokoyama H, Saito S, Daitoku K, Fukuda I, Higuma T, Hanada H. et al. Effects of pravastatin and rosuvastatin on the generation of adiponectin in the visceral adipose tissue in patients with coronary artery disease. Fundam Clin Pharmacol. 2011;25:378–87.
53. Sethi JK, Hotamisligil GS. Metabolic Messengers: tumor necrosis factor. Nat Metab. 2021;3(10):1302–12. CrossRef
54. Wang CH, Wang CC, Huang HC, Wei YH. Mitochondrial dysfunction leads to impairment of insulin sensitivity and adiponectin secretion in adipocytes. FEBS J. 2013;280:1039–50.
55. Samaha MM, Helal MG, El-Sherbiny M, Said E, Salem HA. Indapamide increases IRS1 expression and modifies adiponectin/NLRP3/PPARγ crosstalk in type 2 diabetic rats. Antioxidants. 2022;11:691.
56. Fki I, Sayadi S, Mahmoudi A, Daoued I, Marrekchi R, Ghorbel H. Comparative study on beneficial effects of hydroxytyrosol- and oleuropein-rich olive leaf extracts on high-fat diet-induced lipid metabolism disturbance and liver injury in rats. BioMed Res Int. 2020;2020:1315202.
57. Nguyen TMD. Adiponectin: role in physiology and pathophysiology. Int J Prev Med. 2020;11:136. CrossRef
58. Dos JM, Santos DS, de Oliveira ML, Moreli Benite-Ribeiro SA. The role of mitochondrial DNA damage at skeletal muscle oxidative stress on the development of type 2 diabetes.” Mol Cell Bioch. 2018; 449,251-255.
59. Zhu Y, Wang K, Jia X, Fu C, Yu H, Wang Y. Antioxidant peptides, the guardian of life from oxidative stress. Med Res Rev. 2024 Jan;44(1):275–364. CrossRef
60. Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999 Jan;48(1):1–9. CrossRef
61. Zaky AS, Kandeil M, Abdel-Gabbar M, Fahmy EM, Almehmadi MM, Ali TM, et al. The antidiabetic effects and modes of action of the Balanites aegyptiaca fruit and seed aqueous extracts in NA/STZ-Induced diabetic rats. Pharmaceutics. 2022;14(2):263. CrossRef
62. Cumao?lu A, Ari N, Kartal M, Karasu Ç. Polyphenolic extracts from Olea europea L. protect against cytokine-induced β-cell damage through maintenance of redox homeostasis. Rejuvenation Res. 2011;14(3):325–34. CrossRef
63. Nediani C, Ruzzolini J, Romani A, Calorini L. Oleuropein, a bioactive compound from Olea europaea L., as a potential preventive and therapeutic agent in non-communicable diseases. Antioxidants (Basel). 2019;8(12):578. CrossRef
64. Aly RH, Ahmed AE, Hozayen WG, Rabea AM, Ali TM, El Askary A, et al. Patterns of toll-like receptor expressions and inflammatory cytokine levels and their implications in the progress of insulin resistance and diabetic nephropathy in type 2 diabetic patients. Front Physiol. 2020;11:609223. CrossRef
65. Ahmed OM, Abdel Fattah AA, Abdul-Hamid M, Abdel-Aziz AM, Sakr HI, Damanhory AA, et al. Antidiabetic and liver histological and ultrastructural effects of Cynara scolymus leaf and flower head hydroethanolic extracts in nicotinamide/streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med. 2023;2023:4223026. CrossRef
66. Wardyn JD, Ponsford AH, Sanderson CM. Dissecting molecular cross-talk between Nrf2 and NF-κB response pathways. Biochem Soc Trans. 2015;43(4):621–6. CrossRef
67. Zheng Y, Wang CY, Wang SY, Zheng W. Effect of high-oxygen atmospheres on blueberry phenolics, anthocyanins, and antioxidant capacity. J Agric Food Chem. 2003;51(24):7162–9. CrossRef
68. Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Bramanti P, et al. The effects of a polyphenol present in olive oil, oleuropein aglycone, in an experimental model of spinal cord injury in mice. Biochem Pharmacol. 2012;83(10):1413–26. CrossRef
69. Ryu SJ, Choi HS, Yoon KY, Lee OH, Kim KJ, Lee BY. Oleuropein suppresses LPS-induced inflammatory responses in RAW 264.7 cell and zebrafish. J Agric Food Chem. 2015;63(7):2098–105. CrossRef