INTRODUCTION
Zinc deficiency can be caused by several factors including insufficient zinc intake, increased zinc demand, malabsorption, and impairments of zinc function [1,2]. Due to the various functions of zinc in life processes, zinc inadequacy leads to zinc deficiency which is the upstream of the dysfunction of body systems and functions. Zinc deficiency can be caused by several factors including insufficient zinc intake, increased zinc demand, malabsorption, and impairments of zinc function. Being micronutrients as more than 1,000 cofactors of enzymatic reactions, its role in various functions in the structure and regulation of the human body makes zinc (Zn2+) one of the essential minerals needed by the body [3]. Estimated that there are more than 2 billion humans who have a zinc deficit which leads to health problems such as growth retardation, neurodegenerative diseases, and immune system damage [4]. The availability of zinc in the body decreases with age due to insufficient intake and excessive excretion. This condition will cause health problems because the body cannot store zinc minerals. Therefore, the daily requirement is sourced from food or zinc supplements [5].
Due to the need for zinc minerals, there are three types of zinc supplements, first-generation zinc supplements; inorganic zinc such as zinc sulfate, zinc oxide, and zinc carbonate. The first-generation zinc supplement that is commonly used is zinc sulfate. However, zinc sulfate can induce gastrointestinal reactions such as nausea and vomiting at high doses [6]. The second generation of zinc supplements is organic zinc such as zinc gluconate, zinc oxalate, zinc citrate, zinc lactate, and so on. The two generations mentioned above provide zinc bound to chemical compounds with absorption levels depending on their solubility. Second-generation zinc supplements reduce gastrointestinal reactions [7]. Meanwhile, third-generation zinc supplements are an approach to reducing side effects on zinc absorption and bioavailability in the body. Therefore, third-generation zinc supplements are zinc-chelated with peptide-like molecules. Research proves that zinc chelating peptides (ZCPs) are supplements with high solubility and stability in the gastrointestinal track [5]. Furthermore, research related to this third-generation zinc supplement is currently being widely studied; hence, it is not widely used as a standard therapy for the treatment of zinc deficiency.
Peptides are protein hydrolysates that can chelate minerals, potentially as carriers and providers of minerals. ZCPs form soluble complexes with divalent cations and resist secondary hydrolysis [5]. The source of peptides to chelate zinc includes aquatic, plant, and dairy products [8–13]. ZCP has been reported that peptides derived from food resources increase bioaccessibility and can facilitate zinc transport and absorption [14–16]. The acquisition of peptides is through hydrolysis, purification, sequencing, and synthesis of metal-peptide complexes [17]. The study explained that ZCP could improve zinc transport compared with ZnSO4 [14]. Several peptides have been successfully sequenced, especially from marine natural resources. Sticopus japonicus (sea cucumber) produces several ZCPs, one of which is Trp-Leu-Thr-Pro-Thr-Tyr-Pro-Glu (WLTPTYPE), which is capable of binding zinc of 56.93% with a peptide molecular weight of 1,005.5 Da [10]. Holothuria scabra is another marine sample and a sea cucumber that has been successfully cultivated in Indonesia [18,19]. With high protein content, there is potential for peptide hydrolysates that can be beneficially used as ZCPs [20,21].
Although there have been several studies on zinc-binding peptides from several protein sources from animals and plants, research related to peptides from H. scabra has not been reported before. Previous research on extracting collagen peptides from H. scabra has been conducted to obtain the optimal collagen yield and its characterization. This study used collagen protein from H. scabra to produce zinc-binding peptides [21]. Thus, the present study aimed to discover a new alternative source of ZCP from H. scabra. This study investigates the ZCPs characterize and absorption ability in an ex vivo evaluation; everted gut sac model method.
MATERIAL AND METHODS
Collagen extraction from H. scabra and its hydrolysis
Holothuria scabra collagen (HsC) material was obtained from our previous study under solvent selection, extraction phase, temperature, and extraction time with the optimum conditions for HsC extraction [21]. Hydrolysis of HsC was performed according to the method described with some modifications [22]. The freeze-dried HsC was mixed with Milli-Q water with a ratio of 1:50 (w/v). The solution was homogenized at room temperature and heated at 80°C for 10 minutes. Hydrolysis was performed using bromelain enzyme to the substrate in which each is in equal concentrations and the pH of the mixture was adjusted to 7. Enzymatic hydrolysis was performed for 24 hours. The HsC hydrolysates were heated at 80°C for 10 minutes in a water bath and cooled to room temperature.
Hydrolysate fractionation based on molecular weight
The HsC hydrolysates were fractionated by ultrafiltration following the method stated by Park and Jo [23] with slight modification. The fractionation was conducted using Amicon Ultra-0.5 Centrifugal Filter Unit with 30,000 Da (30 kDa) and 3,000 Da (3 kDa) molecular weight cut-off (MWCO). The concentrates and filtrates from each ultrafiltration were collected as the >30, 3–30, and <3 kDa H. scabra peptides fractions (HsP), namely HsP1, HsP2, and HsP3. The HsPs were characterized by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) in 4%–20% Mini-PROTEAN® TGXTM precast protein gels. The HsPs obtained from the fractionation process were then stored at 4°C for further assay. Peptide variations: HsP1, HsP2, and HsP3 were reacted with mineral zinc to determine the capability of chelating peptides by the next step method of Xie et al. [24].
Synthesis of ZCPs
This synthesis was conducted based on the report by Xie et al. [24] with some modifications. The peptides were reacted with 1 ml of ZnCl2 0.4 M. A pH meter measured the solution mixture to obtain pH 6.0 (0.5 M NaOH or 0.5 M HCl was added for pH adjustment), then incubated for 45 minutes at 30°C to the Zn-peptide complexes (white precipitates). The mixture was centrifuged at 8,000 × g and 20°C for 10 minutes. The precipitates were washed twice with 1 ml of ethanol 90%. The ZCP precipitate was lyophilized with a freeze dryer. The capacity of the zinc peptide complex was determined by ICP-OES. Henceforth, ZCPs are abbreviated to ZCP.
Peptides isolation and purification using reversed-phase high-performance liquid chromatography (RP-HPLC)
HPLC instrument used was HPLC Hitachi (Chromaster 5420 UV-Vis detector, Chromaster 5310 column oven, Chromaster 5210 autosampler, and Chromaster 5110 pump). Operation conditions of RP-HPLC were adapted from Xie et al. [24] with slight modification. The column was Partisil 10 ODS-3 4.6 × 250 mm, and the mobile phase was a mixture of acetonitrile, water, and trifluoracetic acid (7:3:0.05, respectively) with a flow rate of 0.8 ml/minute, and peaks were observed at a wavelength of 221 nm. HsP hydrolysates were prepared and filtered through a 0.45 μm syringe filter. The fractions of HsP1 (HsP1-1 and HsP1-2) were collected based on peaks from the chromatogram. Peptide fractions: HsP1-1 and HsP1-2 were reacted with zinc to determine the binding ability.
Peptide identification using liquid chromatography-tandem mass spectrometry (LC-MS/MS)
The purified peptides fraction of HsP1 (HsP1-1), which has a higher Zn (II)-chelating capacity compared to the other fraction (HsP1-2), was analyzed using LCMS-8060 Triple Quadrupole LC-MS/MS. The conditions of LC-MS/MS were based on the method of Liao et al. [25] with slight modification. The sample was injected into Phenomenex HPLC C18 column, 100 mm l × 2.1 mm ID, 2.6 μm, 100 Å, with a 0.3 ml/minute flow rate of 1.8 kV electrospray voltage, and range of mass of 350 until 1250 m/z. Determination of the peptide sequences using the database at MassBank (https://massbank.jp/) based on the MS ionization chromatogram of each peptide.
Topography and morphology of ZCP with scanning electron microscope-energy dispersion spectroscopy (SEM-EDS)
HsP1, a peptide fraction with the highest zinc chelating (ZCP) was analyzed for topography and morphology using a SEM (Thermo-Scientific Quattro S completed with EDS detector). A total of 1 mg of dry ZCP samples without coating process were visualized observing the ZCP surface at an acceleration potential of 5 kV with 50,000-fold magnification. As a comparison of the morphology and topography of ZCP, peptides without reacting with zinc were selected. Observations were measured through an EDS.
Group function analysis with Fourier-transform infrared (FTIR) spectroscopy
Analysis of functional groups in dry ZCP via IR was carried out at room temperature using an FTIR spectrometer (PerkinElmer’s Spectrum Two IR). Analysis of changes in ZCP functional groups was performed by comparing them to peptides. The spectra were recorded over a wavenumber region between 400 and 400 cm−1 at a resolution of 4 cm−1 [21].
ZCP absorption investigations by everted rat gut sacs
The present ex vivo study was done in the Animal Research Facility, Faculty of Medicine, University of Indonesia, with ethical approval in Faculty of Medicine, University of Indonesia, protocol number: 22-03-0342. Twelve male Spraque Dawley rats, aged 10 weeks old, weighing 200–250 g were used to obtain intestinal segments for absorption study. Rats were fasted overnight and injected with ketamine-xylazine (10:1) for sacrifice, and all intestinal segments were removed through the midline incision of the abdomen. The duodenum and jejunum segments were separated. The segments were washed with 0.9% NaCl to remove the intestinal [26,27]. Clean segments of duodenum, ileum, and jejunum were stored in MEM solution media (Gibco) and kept at 4°C for ready use.
Before the experiments, the duodenum, ileum, and jejunum intestinal segments were everted with the inner side to the outside and washed again with MEM media. One side of the intestine was tied with a thin thread, and the other was filled with MEM media with a syringe. After being filled, the other end of the intestine was tied. The intestine section that has been tied on both sides was placed into 9 ml MEM media of 0.74 mg/ml ZCP and incubated for 120 minutes. Samples were taken from the media at intervals of 0, 10, 30, 60, 90, and 120 minutes. For comparison, ZnSO4 was subjected to the same treatment as ZCP. Samples taken at the time intervals were calculated for zinc concentration with ICP-OES.
Zinc concentration analysis
Zinc concentration was determined using an ICP-OES analyzer, Agilent Technologies 700, series 1984 according to the method described by Tiffany et al. [28] with slight modifications. The measurement parameters were radio frequency power of 20 rpm, nebulizer gas flow rate of 0.6 l/minute, plasma gas flow rate-Argon of 10 l/minute, and sample flow rate of 1.50 ml/minute.
RESULTS AND DISCUSSION
The primary zinc requirement is sourced from food with a risk of inadequate zinc intake in the world population of up to 17%, especially in developing countries [5]. Vegetarians, people who live in inadequate/slum areas, elderly people, and pregnant and lactating women are all vulnerable to zinc deficiency [29]. There is evidence that zinc deficiency causes a variety of health problems both directly and indirectly, some of the diseases cause such as neuropsychiatric illnesses, immunological disorders, and physiological problems [30]. Zinc sufficiency in men is useful for reproduction, both sperm maturation and hormonal regulation [31]. In women, zinc sufficiency plays a major role during pregnancy, especially during fetal development and maternal health [32,33]. Zinc also helps the digestive system, which protects and prevents diarrhea, food allergies, and GI cancers [34]. Child growth failure, hair loss, and bone fragility are also affected by zinc deficiency [6,35–38].
As previously reported, zinc deficiency is a crucial health issue and difficult to resolve with inorganic and organic zinc supplements that are commonly and standardly used nowadays. The absorption rate is low and not optimal in the digestive system is problematic for both supplements form. Low absorption rate and usage in high doses will be harmful to human health. These supplements are unstable and irritating to the intestines if consumed for prolonged durations because they can interfere with the absorption of other dietary nutrients [5,39]. Inorganic and organic zinc supplements have the possibility for zinc ions binding with food antinutrients in food such as phytate, tannin, and oxalate that form complexes with zinc ions, thereby inhibiting zinc absorption [40]. Therefore, this study aims to characterize and identify a new zinc supplement resource with optimal absorption ability in the gastrointestinal system through ZCP. This study successfully purified and characterized ZCP from the collagen of H. scabra and investigated the absorption level through ex vivo evaluation.
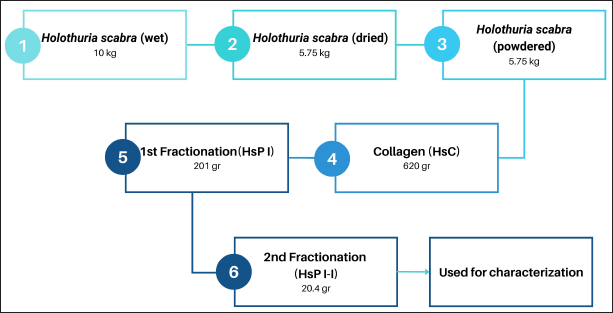 | Figure 1. The process of producing collagen and collagen peptides and their yields. [Click here to view] |
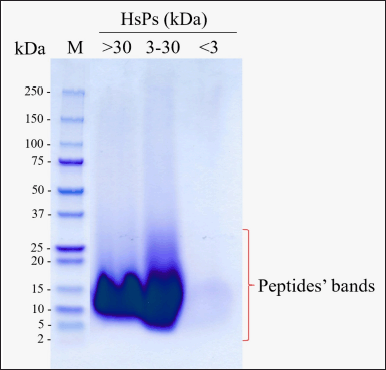 | Figure 2. Tricine-SDS-PAGE analysis of HsPs from HsC hydrolysates fractionation using 3 and 30 kDa MWCO ultrafiltration membranes. [Click here to view] |
Fractionation results with rich collagen peptides from H. scabra
Collagen and collagen peptide fractions with a weight of 620 and 20.4 g, respectively, were successfully obtained from the weight of sea cucumber flour (curing at 500°C to dry and mashed) of 4.5 kg. The collagen obtained was 10.7% and the HsP1-1 peptide fraction obtained was 0.35% of sea cucumber flour. The HsP1-1 fraction has identified six peptides namely DDAFQAFC, TDNL, LGC, PGT, SC, and PY (Table 3) which are further evaluated in silico, ex vivo, and in vivo (Fig. 1).
Bromelain successfully hydrolyzed collagen peptides by producing peptides up to 30 kDa in size. HsC hydrolysates in this study were fractionated into three selected different MW HsPs fractions, i.e., fraction I (>30 kDa), fraction II (3–30 kDa), and fraction III (<3 kDa), referred as HsP1, HsP2, and HsP3, respectively (Fig. 2).
The SDS-PAGE evaluation (Fig. 2) revealed that HsC were successfully hydrolyzed to low molecular weight peptides by bromelain enzyme, and HsC hydrolysates have been separated according to the selected separated (3 and 30 kDa) molecular weight ultrafiltration membrane [41]. Nevertheless, each concentrate residue of the 3 and 30 kDa molecular weight ultrafiltration membranes contained a significant proportion of the lower molecular weight HsPs [42] (with modifications). A multistep acid-base extraction procedure was used to successfully extract collagen from H. scabra [21,43]. Collagen is a triple helix protein of approximately 300 kDa with about 1,140 amino acids per helix chain, with the primary amino acids being glycine (33%), proline, and hydroxyproline (22%). Collagen possesses an overall length of 280 nm and a diameter of 1.4 nm [43]. So far, no collagen has been collected as a mineral chelator, especially for zinc. Mineral supplements frequently consist of minerals chelated by collagen peptides derived from various sources of food, mainly marine products [9,44,45]. In our study, we successfully obtained peptides with the best binding activity from the collection of fractions through molecular weight ultrafiltration.
Increased zinc chelation activity of fractionated collagen peptides from H. scabra
In comparison with without fractionation, the ability of the peptide as a zinc chelator increased to more than four-fold (HsC vs. HsP1). In HsP1, a peptide with high molecular weight (>30 kDa) has the highest zinc chelator ability compared to other fractions (HsP2 and HsP3). As shown in Table 1, the highest zinc-chelating activity of HsP was obtained for the HsP1 peptide fractions, which reach 92.11 mg/100 mg HsP1, compared to HsC and other lower theoretical molecular weight peptide fractions (Hsp2 and HsP3).
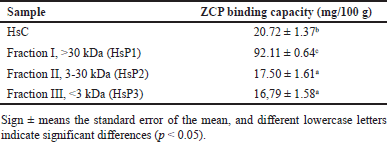 | Table 1. The results of the Zn-chelating capacity assay of different molecular weight peptides obtained by ultrafiltration. [Click here to view] |
This result was further confirmed by the Zn-chelating activity analysis of the RP-HPLC-purified peptides fractions of HsP1 (HsP1-1 and HsP1-2), which both of their peaks have similar retention times (MW). For purification, HsP1-1 was collected at a retention time of 2,480–3,250, while HsP1-2 was collected at a retention time 3,260–4,300 as shown in Figure 3. Each collected fraction was reacted with zinc compounds to determine its ability as a zinc chelator.
When viewed from the SDS-PAGE results in Figure 2, it is known that the peptide is abundant in size > 30 kDa with a zinc-binding ability of 92.11 mg/100-g sample (Table 1). When compared with native collagen, the ability to bind zinc can be four times more, so the ability of the peptide to bind zinc is better than native collagen. Peptides with lower molecular weight also have the ability to bind zinc but their ability is lower than peptides with high molecular weight. Research that reveals the size of peptides with high molecular weight can bind calcium minerals by peptides from soybean with a size of 10–30 kDa compared to peptides with lower molecular weight [46]. Another study revealed that peptides from chicken muscle with a size of >10 kDa were able to bind iron, and only 10% of iron was bound to small peptides [47]. Based on physicochemical analyses, the lower size molecular weight might be due to the coagulation of peptides during preparation [48]. Peptides could aggregate during hydrolysis, promoted by hydrophobic interaction between peptides or proteins [49,50]. or form polymers [51]. This study recommends options for future peptide production purposes, for industrial purposes it is allowed to use peptide hydrolysate fractions based on molecular weight size with the highest zinc binding ability. To find out which peptide is responsible for mineral binding in a fraction, further investigation is needed with further purification by chromatography.
The results showed that HsP1-1 and HsP1-2 have Zn-chelating capacity (Table 2). However, HsP1-1 has a two-fold capacity to bind zinc, and LC/MS-MS further sequenced HsP1-1. Thus, further characterization was done using HsP1-1.
According to previous studies, various enzyme combinations can hydrolyze proteins to obtain metal-chelating hydrolysates. This study the native collagen using a bromelain enzyme to generate ZCPs. Theoretically, bromelain cleaves the peptide chain at Arg-Ala and Ala-Glu bonds, showing a preference for Glu, Asp, Lys, or Arg in the pocket 1 (P1) site [52]. Bromelain is a cysteine or thiol protease with a preferential cleavage site at the carbonyl end of Lys, Ala, Tyr, and Gly [53]. Bromelain was selected as the hydrolytic enzyme used in this research since bromelain was an effective protease for hydrolyzing proteins into low-MW peptides [41] and was one of the enzymes that derived protein hydrolysates with a relatively high zinc-binding capacity [12] and highest iron-chelating activity [54,55]. Bromelain was also used for protein hydrolysis to generate the angiotensin I-converting enzyme (ACE)-inhibitory peptides, which involved zinc-cofactor (in the ACE binding domain) interaction with peptides [56]. The protein source and hydrolysis conditions, such as enzyme concentrations and hydrolysis times [41], and enzyme selection, especially in sequential hydrolysis of protein [57], determine the peptides released by hydrolysis which could significantly affect the chelation property or other biological activities of hydrolysates.
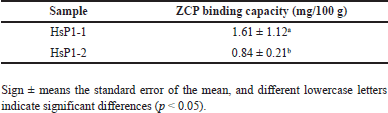 | Table 2. The results of the Zn-chelating capacity assay of the RP-HPLC-purified HsP1 peptide fractions. [Click here to view] |
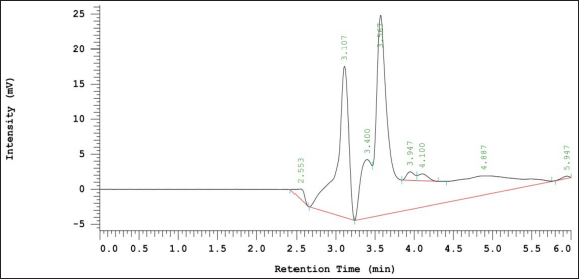 | Figure 3. Purification results of HsC by RP-HPLC. The blue box shows the retention time used to collect the HsP1-1 peptide fraction, while the red color is for the HsP1-2 peptide fraction. [Click here to view] |
ZCP sequences from HsP1-1 and its characterization
The HsP1-1 peptide fraction was scanned in LC/MS, resulting in six peaks (HsP1-1A to HsP1-1F), each of which was sequenced (Fig. 4). Meanwhile, the peptides obtained in six peaks are continued by identifying the fragments of each peptide with precursor ion scan mass spectrum MS/MS. Peptide fragmentation through scanning the MS/MS spectrum is used to determine the amino acid sequence forming a peptide. MS/MS spectra of six peaks can be seen in Supplementary Data 1. Characteristics of peptides in zinc binding are summarised in Table 3. The six peptides discovered in this study have been registered patent with Indonesian patent registration number: P00202211622.
Table 3 shows that the m/z known from scanning peptide fragmentation is 1,064.65 in peptide DDAFQAFC for the peptide with the highest m/z, while peptide SC is 255. The most dominant zinc-binding functional groups in peptide HsP1-1A to HsP1-1F are the –NH2 and –COO– functional groups. As for the characteristics of the polarity proportion of each peptide: HsP1-1A and HSP1-1E are peptides with neutral character. In addition, peptides with hydrophobic properties are HsP1-1C and HsP1-1D. The nature of HsP1-1A is a peptide with an acidic hydrophobic character, while HsP1-1F has a neutral hydrophobic peptide character.
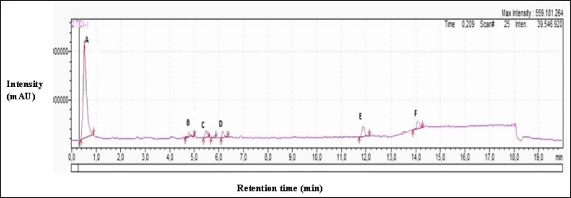 | Figure 4. Peptide scanning chromatogram with LC/MS-MS on HsP1-1, there are six peaks (HsP1-1A to HSP1-1F) that are continued to determine the amino acid constituents. [Click here to view] |
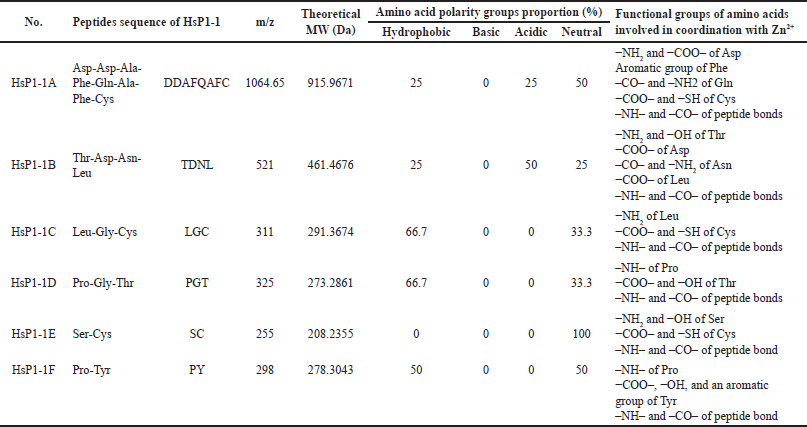 | Table 3. The chemical characteristics of the peptide obtained by LC/MS MS are related to its zinc binding ability. [Click here to view] |
These amino acid groups have functional groups which play a role in metal ions binding to peptides. The functional groups of amino acid groups involved in coordination with Zn2+ were also shown in Table 3. The amino (–NH2) and carboxyl (–COO–) groups of Asp, aromatic group of Phe, carbonyl (–CO–) and amino (–NH2) groups of Gln, carboxyl (−COO–) and sulfhydryl (−SH) groups of Cys, and imino (–NH–) and carbonyl (–CO–) groups of peptide bonds in Asp-Asp-Ala-Phe-Gln-Ala-Phe-Cys; amino and hydroxyl (−OH–) groups of Thr, carboxyl group of Asp, carbonyl and amino groups of Asn, carboxyl group of Leu, and imino and carbonyl groups of peptide bonds in Thr-Asp-Asn-Leu; amino group of Leu, carboxyl and sulfhydryl groups of Cys, and imino and carbonyl groups of peptide bonds in Leu-Gly-Cys; imino group of Pro, carboxyl and hydroxyl groups of Thr, and imino and carbonyl groups of peptide bonds in Pro-Gly-Thr; amino and hydroxyl groups of Ser, carboxyl and sulfhydryl groups of Cys, and imino and carbonyl groups of peptide bond in Ser-Cys; and imino group of Pro, carboxyl, hydroxyl, and aromatic groups of Tyr, and imino and carbonyl groups of peptide bond in Pro-Tyr might contribute to the Zn-chelating activity in this study. To find out more information about the character of the HsP1-1 peptide bound with zinc ions, it is necessary to conduct a study with FTIR which is compared with the peptide without being bound by zinc ions.
Table 3 also shows the proportion of the polarity-based amino acid groups of the six peptides of the HsPs1-1. These six low theoretical molecular weight peptides of the HsP1-1 had no positively charged/basic amino acid group. However, they had relatively high proportions of hydrophobic (Ala, Leu, Gly, and Pro), negatively charged or acidic (Asp and Asn), and uncharged or neutral (Phe, Gln, Cys, Thr, Ser, and Tyr) amino acid groups, in which these amino acid groups might contribute to the Zn-chelating activity of peptides in this study as discussed according to the previous research. The peptides acidic amino acid group (Asp and Glu) might generate high metal affinity [58]. The acidic (Asp and Glu), positively charged or basic (Lys, Arg, and His), neutral (Gln), and hydrophobic (Pro) amino acid groups determined the calcium-binding affinity [59]. Acidic (Asp and Glu) and hydrophobic (Pro) amino acid groups determined the iron-binding capacity of peptides [60]. The acidic (Asp, Glu, and Asn), basic (His), and neutral (Gln, Ser, and Cys) amino acid groups have been reported to bind divalent metals [61]. Peptides with more acidic amino acid residues could bind more Zn2+ than those with fewer acidic amino acid residues [62]. Acidic (Glu and Asp), neutral (Tyr), and basic (His and Arg) amino acid groups of the ACE-binding site formed coordinated binding with Zn2+-cofactor in the ACE-binding domain. This Zn2+-cofactor was also involved in the interaction with ACE-inhibitor peptides which had dominantly hydrophobic (Ala, Leu, Gly, Pro, Val, Met, and Ile) than neutral (Gln, Phe, Tyr, and Thr), basic (Lys and His), and acidic (Glu) amino acid groups [56]. The acidic amino acid group (Glu and Asp) remarkably contributed to the Zn2+ chelation [63]. Based on the analysis of zinc-binding amino acids, the peptides found in this study are relatively hydrophobic, with some negatively charged (acidic) and uncharged (neutral), which affects the stability. With conditions that are in accordance with the pH in the gastrointestinal, zinc can be well delivered to the gastrointestinal tract. The amino acid composition majorly determines the functionality and bioactivity of peptides [23,48,56,63] and the orientation of amino acids within their sequences [56]. Besides, it also depends on the protein substrate, the enzyme used for the proteolysis, the hydrolysis conditions, and the degree of hydrolysis. For health applications zinc-binding peptide is important to use as a chelator of zinc, enabling zinc bioavailability to be maintained in the body by sustaining their stability by amino acid composition.
Previous research reported that metal ions (Cu2+, Zn2+, and Ni2+) prefer to bind with oxygen- and nitrogen-rich groups [8]. The principal site of iron-binding corresponded primarily to the carboxylate groups and a lesser extent, to the peptide bonds [64]. The carboxylates group of Asn, peptide imino, and carbonyl groups in Asn-Cys-Ser and Leu-Ala-Asn (or Ile-Ala-Asn) might coordinate with zinc [65]. The functional groups of carboxyl, hydroxyl, and sulfhydryl groups in Ser-Met and Asn-Cys-Ser showed the strongest bonding abilities with Zn2+ compared to imino nitrogen and oxygen of the peptide bond (or the amide bond of Asn), and the carbonyl group of the peptide bond and oxygen (O) element in the water regularly participate in coordination by weaker interactions with Zn2+ [10]. Carbonyl groups of Asp and Glu formed the coordinated covalent bindings, while Pro placed constraints in the angles of the peptide backbone, favoring the iron-binding to peptides [60]. The amino group of Asn, the imino and carbonyl groups of the peptide amide groups, the hydroxyl group of Ser, and the carboxyl group of Met were speculated to have important roles in the complexation between Asn-Ser-Met and zinc, in which the side chain amino group of Asn might promote the zinc-chelating ability [24]. The high metal affinity of Asp and Glu is due to the carboxylate groups in the side chain [8].
The results obtained are peptides with the ability to bind with Zn is the result of FTIR analysis as seen in (Fig. 5, Table 4). After being bound to zinc, there is a wavenumber shift in several functional groups, namely in N–H stretching from 3,288.26 to 3,410 cm−1, vibration of C=O 1,700.46 to 1,638.68 cm−1, C–N stretching from 1,553.90 to 1,564.31 cm−1, N–H bending 1,406.99 to 1,415.56 cm−1, COO– from 1,202.39 to 1,342.70 cm−1, and C–O from 854.26 to 795.64 cm−1. Based on the study, reported that there are vibrations in amide and amide I on (C=O) in the form of tensile vibration [8]. In this study, the peptide spectra for the amide-I group appeared at 1,700.46 cm−1 and amide-II appeared at 1,406.99 cm−1. After the peptide binds to zinc, there is a wavenumber shift at 1,638.68 cm−1 and 1,415.56 cm−1 which indicates the presence of antisymmetric tensile vibration (COO–) and symmetric tensile vibration on carboxyl ions [15]. After reacting with zinc ions, absorption of the –NH functional group on –NH–C=O appears at 1,342.70 cm−1, indicating that the amino acids nitrogen atom (NH2) binds to Zn2+ ions. The band’s appearance at 3,410.52 cm−1 on the ZCP is an interaction with the –NH group which caused a wavenumber shift in the peptide spectra which appeared at 3,288.26 cm−1. These results show that zinc ions (Zn2+) are bound to carboxyl groups and also interact with nitrogen atoms in amino acids and nitrogen atoms in amides. This is in line with research on other zinc-binding peptides, where nitrogen atoms have the ability to bind zinc [8,15].
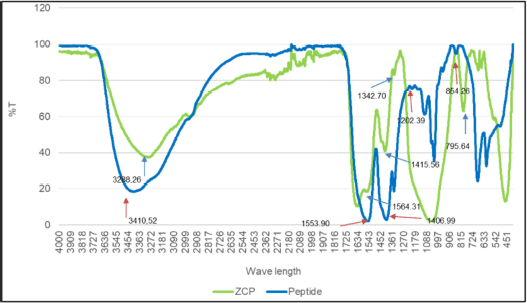 | Figure 5. FTIR spectra of ZCP and peptide in the wavenumber 4,000 to 400 cm−1. For blue line is peptide spectra and the green line is ZCP spectra. [Click here to view] |
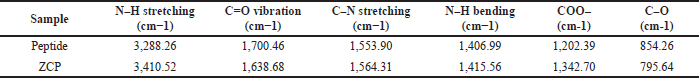 | Table 4. The shifting of wavenumber in ZCP and peptide is important in the functional group. [Click here to view] |
After investigating the functional groups that play a role in zinc binding by FTIR, morphological and topographical observations on ZCP and peptides were made using SEM-EDS. The data shows that, visually, the surface of the peptide has a smoother surface (Fig. 6A). In contrast, the ZCP has a granule-like structure, which is spread over the entire surface of the ZCP (Fig. 6B). It was discovered that the peptide that is not bound with zinc, consists of 51.5% oxygen (O) element, while 40.8% is the zinc element in ZCP. Therefore, it can be said that the synthesis of ZCP has been successfully carried out. The visualization of morphologically and topographically, ZCP has a different surface structure from the peptide [66]. The reaction process of zinc with peptides to form aggregates [67]. Peptide dimerization and stability result from the formation of aggregates by zinc ions. Aggregation progress is made possible by intermolecular forces, surface tension, anisotropy, and sulfhydryl, carboxyl, and amino group functionalities on the peptide [66,67]. Based on SEM-EDS, the elemental composition of ZCP contains the element zinc (40.8%), which indicates that zinc ions have been bound to the peptide to form a ZCP complex (Fig. 6B). As for the peptide without zinc bound, it is known that there is no zinc element (Fig. 6A). This research is in line with previous studies, which show differences in the morphology of zinc-binding peptides and peptides, where zinc ions are able to form more compact particle formations.
Higher absorption of zinc from ZCP versus commonly used zinc supplements in all segments of the small intestine
The whole small intestine segment was used in this study, namely the duodenum, ileum, and jejunum. These segments were used to determine the zinc absorption of ZCP compared to zinc organic and zinc inorganic (ZnSO4 and ZnCl2). In the duodenum segment, the zinc level of ZCP in the media was absorbed starting before minute 20 and there was an increase in absorption from minute 20 to minute 60. Afterward, the zinc absorption rate began to stationary after minute 60 until the end of the ex vivo evaluation process (120 minutes) with the final cumulative zinc level in the media of approximately 60%, with approximately 40% zinc being able to be absorbed in the duodenum. The zinc content of ZnSO4 and ZnCl2 in the media is almost 80% with as much as 20% zinc absorbed in the duodenum (Fig. 7A).
In the jejunum and ileum segments (Fig. 7A and B), the zinc cumulative level in the media from ZCP is approximately 70% and 80%, respectively. Therefore, 30% and 20% of the zinc level was absorbed in the jejunum and ileum, respectively. In contrast to the ZnSO4 and ZnCl2 comparisons, in the jejunum and ileum segments, the zinc absorption rate was lower at around 10%, with the remaining zinc content in the media being 90%. Based on the graph in Figure 5, zinc absorption in ZCP, ZnSO4, and ZnCl2 occurs in all segments with the duodenum as the most absorbed segment. However, zinc can also be absorbed in the jejunum and ileum segments with lower levels. In ex vivo evaluation, the level of zinc absorption in ZCP is more than that of ZnSO4 and ZnCl2.
 | Figure 6. SEM-EDS images of peptide (A) and ZCP (B) and their elemental compositions. [Click here to view] |
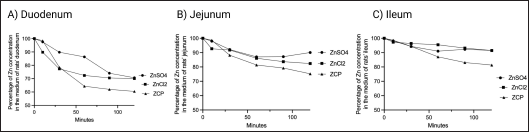 | Figure 7. Cumulative absorption of ZCP across the intestinal segments (A) duodenum, (B) jejunum, and (C) ileum. ZnCl2 and ZnSO4 are molecules as compared to ZCP to observe absorption percent. [Click here to view] |
Absorption of drugs through oral administration is mainly in the small intestine segment. Many models for investigating drug absorption have been developed as an early stage of drug development and formulation, one of which is the everted gut sac model, which has been widely used with reliable results [27]. The advantages of this method are that it is simple, quick, reproducible, and inexpensive [68]. Recently, the everted gut sac model method has been widely applied to drug delivery systems such as liposomes, nanoparticles, and vesicles [27]. Investigating the ability of ZCP to enhance zinc absorption and transport requires an intestinal simulation bioassay as an initiation phase to evaluate the capacity of ZCP in the binding and transport of zinc. Zinc absorption using the everted gut sac method has been done in several kinds of research to determine a molecule’s transport and absorption ability [26,27,68]. Previous zinc absorption studies reported that zinc is absorbed through the small intestine, with the highest absorption in the duodenum and ileum or only in the ileum or the jejunum [69].
Meanwhile, in human intestines, the duodenum and jejunum are the major segments for zinc absorption [70,71]. Zinc absorption occurs mostly in the duodenum compared to the jejunum and ileum due to differences in villosity and micro villosity in the duodenal membrane [27]. According to previous research, ZCP can increase absorption compared to organic zinc salts and inorganic zinc salts. Casein phosphopeptides increased Zn retention, transport, and absorption [14]; mung bean peptide (SSEDQPFNLR) reported that the peptide has capabilities to increase zinc transport and absorption [15]; peptide HNAPNPGLPYAA from wheat germ reported higher rate for zinc transport and absorption compared with ZnSO4 [8]. The simulation in this study was carried out for 120 minutes; in ZCP, the results showed that absorption in the duodenum occurred significantly at minute 20 and continued to accumulate zinc absorption until minute 60, around 40%, and the absorption stationary until the end of the simulation. Zinc absorption in ZnCl2 and ZnSO4 occurs lower than ZCP. It can be confirmed by data stating that until the end of the simulation, the absorbed zinc is around 20%. This study is in line with previous research which showed that ZCP has the capability to enhance zinc absorption. The enhanced absorption of zinc due to ZCP is certainly due to factors such as the peptide structure produced, the concentration of the peptide, the biomarker used, and the inhibitor amount of zinc absorption during simulation.
Two processes of zinc homeostasis occur simultaneously in the digestive tract. First is the absorption of zinc obtained from food (exogenous). The second is gastrointestinal secretion and excretion-reabsorption of zinc (endogenous). Zinc transporters, permeable channels, and metallothionein (MT) regulate the homeostasis process in the body [72]. Absorption is the zinc passing into the enterocyte through the basolateral membrane, and then circulating transport occurs [73]. MT is an intracellular mineral-binding protein that plays a transport mechanism for minerals, including zinc [74]. MT synthesis in the hepatic and intestine is stimulated by dietary zinc supplementation (exogenous). The limitation of zinc supplementation is that it reduces MT synthesis [75]. Inadequate zinc absorption also occurs due to the availability of inhibitors, such as phytate, that form complexes with zinc. The human body lacks the phytase enzyme. Therefore, complexing phytate with zinc reduces zinc absorption in the body [69]. The importance of zinc supplementation to mitigate zinc limitation is required to decrease the impact of zinc deficiency. The efficacy of both generations of zinc supplements depends on their solubility. Second-generation zinc supplements significantly reduce the stimulation of gastrointestinal reactions and increase oral consumption. The third generation is zinc chelating to molecules like peptides, creating a complex with soluble characteristics and high stability in the intestine [1,5]. This study has successfully elaborated the results of collagen acquisition and fractionation of zinc-binding peptides as well as knowing the resulting peptide sequences that were evaluated ex vivo with the entire small intestine segment. The limitation of this study is that it does not take into account the gastrointestinal conditions from the mouth to the intestine. However, if affected by stomach acid, it can be overcome by making the preparation film coated so that it can overcome acidic conditions in the stomach.
CONCLUSION
This research shows that the potential of peptides from H. scabra as ZCPs has been successfully purified and characterized. Protein hydrolysis uses bromelain enzyme which can hydrolyze proteins by forming peptides. Furthermore, the peptides obtained were tested for zinc binding with HsP1 peptide with a zinc-binding capacity of 92.11 mg/100 g, followed by peptide fractionation. RP-HPLC was carried out with an isocratic mobile phase to obtain pure peptides by producing two fractionations, namely HsP1-1, which has a better zinc binding ability of 1.61 mg/100 g. The peptide sequencing process to determine the sequence of zinc-binding amino acids used LC/MS-MS, resulting in six zinc-binding peptides: Asp-Asp-Ala-Phe-Gln-Ala-Phe-Cys; Thr-Asp-Asn-Leu; Leu-Gly-Cys; Pro-Gly-Thr; Ser-Cys; and Pro-Tyr. In ZCP, the accumulation of absorption was highest at 60 minutes, with 40% of zinc absorbed into intestinal cells. Compared with ZnCl2 and ZnSO4, the absorption in the small intestine rate of ZCP was twofold higher. This research has successfully purified peptides from H. scabra that have the potential for zinc binding and the peptides capability to enhance zinc absorption in the small intestine.
ACKNOWLEDGMENTS
This research is made possible by a collaborative effort in the Raw Materials for Targeted Therapeutic Drugs Program Home from Research Organization for Health, The National Research and Innovation Agency Republic of Indonesia, Fiscal Year 2024.
AUTHOR CONTRIBUTIONS
Concept and design: ML, MYP, FF, GS; Data acquisition: GS, NG, YH, HH, OGRS; Data analysis/ interpretation: GS, ML, MYP, FF; Drafting manuscript: GS, ML, YH, NG, HH; Critical revision of manuscript: ML; Statistical analysis: GS, ML; Funding: GS, ML, MYP, FF.
Admin, technical or material support: YH, NG; Supervision: ML, MYP, FF, NMDS; Final approval: ML, MYP, FF.
FINANCIAL SUPPORT
This research is funded by the National Research and Innovation Agency of the Republic of Indonesia for the Fiscal Year 2024.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the Animal Research Facility, Faculty of Medicine, University of Indonesia, with approval number: 22-03-0342.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Katimba HA, Wang R, Cheng C. Current findings support the potential use of bioactive peptides in enhancing zinc absorption in humans. Crit Rev Food Sci Nutr. 2021;0(0):1–21.
2. Chasapis CT, Ntoupa PSA, Spiliopoulou CA, Stefanidou ME. Recent aspects of the effects of zinc on human health. Arch Toxicol. 2020;94(5):1443–60.
3. Gupta S, Brazier AKM, Lowe NM. Zinc deficiency in low- and middle-income countries: prevalence and approaches for mitigation. J Hum Nutr Diet. 2020;33(5):624–43.
4. Prasad AS. Zinc: role in immunity, oxidative stress and chronic inflammation. Curr Opin Clin Nutr Metab Care. 2009;12(6):646–52.
5. Duan M, Li T, Liu B, Yin S, Zang J, Lv C, et al. Zinc nutrition and dietary zinc supplements. Crit Rev Food Sci Nutr. 2021;0(0):1–16.
6. Allen LH. Zinc and micronutrient supplements for children. Am J Clin Nutr. 1998;68(2 SUPPL.):S495–8.
7. Cámara F, Amaro MA. Nutritional aspect of zinc availability. Int J Food Sci Nutr. 2003;54(2):143–51.
8. Zhu KX, Wang XP, Guo XN. Isolation and characterization of zinc-chelating peptides from wheat germ protein hydrolysates. J Funct Foods. 2015;12:23–32.
9. Liu X, Wang Z, Yin F, Liu Y, Qin N, Nakamura Y, et al. Zinc-chelating mechanism of sea cucumber (Stichopus japonicus)-derived synthetic peptides. Mar Drugs. 2019;17(8):1–14.
10. Wang C, Li B, Li H. Zn(II) chelating with peptides found in sesame protein hydrolysates: identification of the binding sites of complexes. Food Chem. 2014;165:594–602.
11. Chen D, Liu Z, Huang W, Zhao Y, Dong S, Zeng M. Purification and characterisation of a zinc-binding peptide from oyster protein hydrolysate. J Funct Foods. 2013;5(2):689–97.
12. Lu D, Peng M, Yu M, Jiang B, Wu H, Chen J. Effect of enzymatic hydrolysis on the zinc binding capacity and in vitro gastrointestinal stability of peptides derived from pumpkin (Cucurbita pepo L.) seeds. Front Nutr. 2021;8:647782.
13. Guo H, Yu Y, Hong Z, Zhang Y, Xie Q, Chen H. Effect of collagen peptide-chelated zinc nanoparticles from pufferfish skin on zinc bioavailability in rats. J Med Food. 2021;24(9):987–96.
14. García-Nebot MJ, Barberá R, Alegría A. Iron and zinc bioavailability in Caco-2 cells: influence of caseinophosphopeptides. Food Chem. 2013;138(2–3):1298–303.
15. Tianxin F, Shu Z, Yanan S, Yuchao F, Yingjun J, Yiwei Z, et al. Isolation and characterization of zinc?binding peptides from mung. Eur Food Res Technol. 2019;246:113–24.
16. Udechukwu MC, Downey B, Udenigwe CC. Influence of structural and surface properties of whey-derived peptides on zinc-chelating capacity, and in vitro gastric stability and bioaccessibility of the zinc-peptide complexes. Food Chem. 2018;240:1227–32.
17. Caetano-Silva ME, Netto FM, Bertoldo-Pacheco MT, Alegría A, Cilla A. Peptide-metal complexes: obtention and role in increasing bioavailability and decreasing the pro-oxidant effect of minerals. Crit Rev Food Sci Nutr. 2021;61(9):1470–89.
18. Indriana LF, Firdaus M. Growth performance of sea cucumber Holothuria scabra juvenile in different initial size of pond culture in Lombok, Indonesia. In: E3S Web of Conferences 2020. EDP Sciences. Vol. 147, p 01003.
19. Wulandari DA, Gustini N, Murniasih T, Bayu A, Sari M, Syahputra G, et al. Nutritional value and biological activities of sea cucumber Holothuria scabra cultured in the open pond system. J Aquat Food Prod Technol. 2022;31(6):599–614.
20. Rasyid A, Murniasih T, Putra MY, Pangestuti R, Harahap IA, Untari F, et al. Evaluation of nutritional value of sea cucumber Holothuria scabra cultured in bali, indonesia. AACL Bioflux. 2020;13(4):2083–93.
21. Syahputra G, Firdaus M, Santoso P, Kusharyoto W, Gustini N. Extraction and characterization of collagen from sand sea cucumber (Holothuria scabra) [Ekstraksi dan Karakterisasi Kolagen dari Teripang Pasir (Holothuria scabra)]. Indones J Agric Sci. 2021;26(3):319–27.
22. Khiari Z, Ndagijimana M, Betti M. Low molecular weight bioactive peptides derived from the enzymatic hydrolysis of collagen after isoelectric solubilization/precipitation process of Turkey by-products. Poult Sci. 2014;93(9):2347–62.
23. Park SH, Jo YJ. Static hydrothermal processing and fractionation for production of a collagen peptide with anti-oxidative and anti-aging properties. Process Biochem. 2019;83:176–82.
24. Xie N, Huang J, Li B, Cheng J, Wang Z, Yin J, et al. Affinity purification and characterisation of zinc chelating peptides from rapeseed protein hydrolysates: possible contribution of characteristic amino acid residues. Food Chem. 2015;173:210–7.
25. Liao W, Chen H, Jin W, Yang Z, Cao Y, Miao J. Three newly isolated calcium-chelating peptides from tilapia bone collagen hydrolysate enhance calcium absorption activity in intestinal caco-2 cells. J Agric Food Chem. 2020 Feb;68(7):2091–8.
26. Wang Z, Sun J, Ma X, Liu X, Yin F, Li D, et al. Characterization of a synthetic zinc-chelating peptide from sea cucumber (Stichopus japonicus) and its gastrointestinal digestion and absorption in vitro. J Sci Food Agric. 2022;102(11):4542–50.
27. Da Silva CF, Severino P, Martins F, Chaud MV, Santana MHA. The intestinal permeation of didanosine from granules containing microspheres using the everted gut sac model. J Microencapsul. 2009;26(6):523–8.
28. Tiffany AS, Gray DL, Woods TJ, Subedi K, Harley BAC. The inclusion of zinc into mineralized collagen scaffolds for craniofacial bone repair applications. Acta Biomater. 2019;93:86–96.
29. Haase H, Mocchegiani E, Rink L. Correlation between zinc status and immune function in the elderly. Biogerontology. 2006;7(5–6):421–8.
30. Fraker PJ, King LE, Laakko T, Vollmer TL. The dynamic link between the integrity of the immune system and zinc status. J Nutr. 2000;130(5 SUPPL.):1399S–406S.
31. Fallah A, Mohammad-Hasani A, Colagar AH. Zinc is an essential element for male fertility: a review of Zn roles in men’s health, germination, sperm quality, and fertilization. J Reprod Infertil. 2018;19(2):69–81.
32. Duncan FE, Que EL, Zhang N, Feinberg EC, O’Halloran TV, Woodruff TK. The zinc spark is an inorganic signature of human egg activation. Sci Rep. 2016;6(1):24737.
33. Uriu-Adams JY, Keen CL. Zinc and reproduction: effects of zinc deficiency on prenatal and early postnatal development. Birth Defects Res Part B. 2010;89(4):313–25.
34. Skrovanek S. Zinc and gastrointestinal disease. World J Gastrointest Pathophysiol. 2014;5(4):496.
35. Krebs NF, Miller LV, Michael Hambidge K. Zinc deficiency in infants and children: a review of its complex and synergistic interactions. Paediatr Int Child Health. 2014;34(4):279–88.
36. Salgueiro MJ, Zubillaga MB, Lysionek AE, Caro RA, Weill R, Boccio JR. The role of zinc in the growth and development of children. Nutrition. 2002;18(6):510–9.
37. Kil MS, Kim CW, Kim SS. Analysis of serum zinc and copper concentrations in hair loss. Ann Dermatol. 2013;25(4):405–9.
38. Suzuki T, Kajita Y, Katsumata SI, Matsuzaki H, Suzuki K. Zinc deficiency increases serum concentrations of parathyroid hormone through a decrease in serum calcium and induces bone fragility in rats. J Nutr Sci Vitaminol (Tokyo). 2015;61(5):382–90.
39. Akbar B, Ali K, Lofollah S, Niloufar N, Soheyla V, Abolfazl M. Evaluation and comparison of zinc absorption level from 2-alkyle 3-hydroxy pyranon-zinc complexes and zinc sulfate in rat in vivo. Adv Biomed Res. 2013;2(1):77.
40. Hunt JR. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am J Clin Nutr. 2003;78(3 SUPPL.):633S–9S.
41. Selamassakul O, Laohakunjit N, Kerdchoechuen O, Yang L, Maier CS. Isolation and characterisation of antioxidative peptides from bromelain-hydrolysed brown rice protein by proteomic technique. Process Biochem. 2018;70:179–87.
42. Haider SR, Reid HJ, Sharp BL. Tricine-SDS-PAGE. In: Kurien B, Scofield R, editors. Protein electrophoresis. Methods in molecular biology, Vol. 869. Totowa, NJ: Humana Press; 2012.
43. León-López A, Morales-Peñaloza A, Martínez-Juárez VM, Vargas-Torres A, Zeugolis DI, Aguirre-Álvarez G. Hydrolyzed collagen—sources and applications. Molecules. 2019;24(22):4031.
44. Guo L, Harnedy PA, O’Keeffe MB, Zhang L, Li B, Hou H, et al. Fractionation and identification of Alaska pollock skin collagen-derived mineral chelating peptides. Food Chem. 2015;173:536–42.
45. Hu Z, Yang P, Zhou C, Li S, Hong P. Marine collagen peptides from the skin of Nile Tilapia (Oreochromis niloticus): characterization and wound healing evaluation. Mar Drugs. 2017;15(4):102.
46. Bao X, Lv Y, Yang B, Ren C, Guo S. A study of the soluble complexes formed during calcium binding by soybean protein hydrolysates. J Food Sci. 2008;73(3):C117–21.
47. Seth A, Mahoney RR. Binding of iron by chicken muscle protein digests: the size of the iron-binding peptides. J Sci Food Agric. 2000;80(11):1595–600.
48. Lv Y, Bao X, Liu H, Ren J, Guo S. Purification and characterization of caclium-binding soybean protein hydrolysates by Ca2+/Fe3+ immobilized metal affinity chromatography (IMAC). Food Chem. 2013;141(3):1645–50.
49. Kuipers BJH, Alting AC, Gruppen H. Comparison of the aggregation behavior of soy and bovine whey protein hydrolysates. Biotechnol Adv. 2007;25(6):606–10.
50. Otte J, Lomholt SB, Ipsen R, Stapelfeldt H, Bukrinsky JT, Qvist KB. Aggregate formation during hydrolysis of β-lactoglobulin with a Glu and Asp specific protease from Bacillus licheniformis. J Agric Food Chem. 1997;45(12):4889–96.
51. Ferraretto A, Gravaghi C, Fiorilli A, Tettamanti G. Casein-derived bioactive phosphopeptides: role of phosphorylation and primary structure in promoting calcium uptake by HT-29 tumor cells. FEBS Lett. 2003;551(1–3):92–8.
52. Arshad ZIM, Amid A, Yusof F, Jaswir I, Ahmad K, Loke SP. Bromelain: an overview of industrial application and purification strategies. Appl Microbiol Biotechnol. 2014;98:7283–97.
53. Hale LP, Greer PK, Trinh CT, James CL. Proteinase activity and stability of natural bromelain preparations. Int Immunopharmacol. 2005;5(4):783–93.
54. Ovissipour M, Rasco B, Shiroodi SG, Modanlow M, Gholami S, Nemati M. Antioxidant activity of protein hydrolysates from whole anchovy sprat (Clupeonella engrauliformis) prepared using endogenous enzymes and commercial proteases. J Sci Food Agric. 2013;93(7):1718–26.
55. Wang Y, Cai M, Zeng H, Zhao H, Zhang M, Yang Z. Preparation, characterization and iron absorption by caco-2 cells of the casein peptides-iron chelate. Int J Pept Res Ther. 2022;28(4):116.
56. Auwal SM, Zainal Abidin N, Zarei M, Tan CP, Saari N. Identification, structure-activity relationship and in silico molecular docking analyses of five novel angiotensin I-converting enzyme (ACE)-inhibitory peptides from stone fish (Actinopyga lecanora) hydrolysates. PLoS One. 2019;14(5):e0197644.
57. Durand E, Beaubier S, Ilic I, Fine F, Kapel R, Villeneuve P. Production and antioxidant capacity of bioactive peptides from plant biomass to counteract lipid oxidation. Curr Res Food Sci. 2021;4:365–97.
58. Kállay C, Várnagy K, Micera G, Sanna D, Sóvágó I. Copper (II) complexes of oligopeptides containing aspartyl and glutamyl residues. Potentiometric and spectroscopic studies. J Inorg Biochem. 2005;99(7):1514–25.
59. Lv M, Sun J, Wang M, Huang W, Fan H, Xu F, et al. GC-MS based metabolomics study of stems and roots of Ephedra sinica. J Pharm Biomed Anal. 2015;114:49–52.
60. de la Hoz L, da Silva VSN, Morgano MA, Pacheco MTB. Small peptides from enzymatic whey hydrolyzates increase dialyzable iron. Int Dairy J. 2014;38(2):145–7.
61. Guo L, A Harnedy PA, Li B, Hou H, Zhang Z, Zhao X, et al. Food protein-derived chelating peptides: biofunctional ingredients for dietary mineral bioavailability enhancement. Trends Food Sci Technol. 2014;37(2):92–105.
62. Jiang L, Wang B, Li B, Wang C, Luo Y. Preparation and identification of peptides and their zinc complexes with antimicrobial activities from silver carp (Hypophthalmichthys molitrix) protein hydrolysates. Food Res Int. 2014;64:91–8.
63. Li D, Zuo F, Zhu R, Zhang C, Zhang D. Two-step enzymatic modification method to enhance the Zn2+-chelating activity and antioxidant activity of zein. Food Sci Technol Res. 2020;26(2):299–311.
64. Guangrong H, Zhangyan R, Jiaxin J. Separation of iron-binding peptides from shrimp processing by-products hydrolysates. Food Bioprocess Technol. 2011;4(8):1527–32.
65. Wang C, Li B, Ao J. Separation and identification of zinc-chelating peptides from sesame protein hydrolysate using IMAC-Zn2+ and LC-MS/MS. Food Chem. 2012;134(2):1231–8.
66. Zhang Z, Zhou F, Liu X, Zhao M. Particulate nanocomposite from oyster (Crassostrea rivularis) hydrolysates via zinc chelation improves zinc solubility and peptide activity. Food Chem. 2018;258:269–77.
67. Foust KD, Kaspar BK. The effect of Cu2+ and Zn2+ on the Aβ42 peptide aggregation and cellular toxicity. Metallomics. 2010;8(24):4017–8.
68. Alam MA, Al-Jenoobi FI, Al-Mohizea AM. Everted gut sac model as a tool in pharmaceutical research limitations and applications. J Pharm Pharmacol. 2011;64:326–36.
69. Maares M, Haase H. A guide to human zinc absorption: general overview and recent advances of in vitro intestinal models. Nutrients. 2020;12(3):762.
70. Steinhardt HJ, Adibi SA. Interaction between transport of zinc and other solutes in human intestine. Am J Physiol. 1984;10(2):G176–82.
71. Lee HH, Prasad AS, Brewer GJ, Owyang C. Zinc absorption in human small intestine. Am J Physiol Liver Physiol. 1989;256(1):G87–91.
72. Krebs NF. Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr. 2000;130(5 SUPPL.):1374S–7S.
73. Kimura T, Kambe T. The functions of metallothionein and ZIP and ZnT transporters: an overview and perspective. Int J Mol Sci. 2016;17(3):10–2.
74. Hennigar SR, Kelley AM, McClung JP. Metallothionein and zinc transporter expression in circulating human blood cells as biomarkers of zinc status: a systematic review. Adv Nutr. 2016;7(4):735–46.
75. Myers SA, Nield A, Myers M. Zinc transporters, mechanisms of action and therapeutic utility: Implications for type 2 diabetes mellitus. J Nutr Metab. 2012;2012.
SUPPLEMENTARY MATERIAL
The supplementary material can be accessed at the journal's website: Link Here [https://japsonline.com/admin/php/uploadss/4335_pdf.pdf].