INTRODUCTION
Menopause refers to the permanent cessation of ovarian function, marking the transition of women from a reproductive phase to a nonreproductive phase in life. This crucial stage involves notable alterations in hormonal and menstrual cycles, alongside various physiological and psychological challenges. Considering the average life expectancy of around 81 years for women in the US, a significant portion of their lives, up to 40%, will be spent in the postmenopausal phase [1].
After menopause, women have a higher susceptibility to developing high blood pressure compared to men. While males were more likely to experience hypertension before the age of 65, data from NHANES 2013–2016 revealed that beyond that age, the likelihood of hypertension increased more in women. Moreover, women over 60 years old are less likely to have their blood pressure under control (49.2%) compared to younger women (40–49 years: 54.2%; 18–39 years: 62.6%) [1].
The presence of hypertension poses a significant risk for cardiovascular disease (CVD). Despite significant declines in CVD mortality in the past 30 years, it continues to be the leading cause of death among women [2]. Throughout the menopausal transition, there is a notable rise in risk factors for CVD that remains independent of age. As women progress through this phase, they become more susceptible to developing coronary heart disease later in life compared to males [1,3].
Endothelial dysfunction is a crucial early stage in the development of CVD. It is suggested that there are considerable reductions in endothelial vasodilator function during the menopausal transition [2–4]. It is believed that improving endothelial function could lead to a decreased risk of cardiovascular events during menopause.
One of the natural substances with the potential to improve endothelial function is the extract of Physalis spp. leaves. There are primarily two types of Physalis in Indonesia, particularly in Java, known as Physalis angulata L. and Physalis minima L. [5]. These species have been extensively studied for their phytochemical and medicinal properties, and both have a long history of traditional usage [6].
Previous studies have demonstrated that the extract of P. minima leaves has beneficial effects in hypertensive rat models induced with deoxycorticosterone acetate (DOCA)-salt. These effects include the promotion of re-endothelialization and reduction in blood pressure [7]. Additionally, the extract of P. minima leaves has been shown to reduce anxiety [8] and cardiac fibrosis [9] in ovariectomized (OVX) rats. Given these findings, this is the first study to investigate the effects of the methanol extract of P. minima leaves (MEP) on endothelium-dependent vascular relaxation and blood pressure in OVX rats.
MATERIAL AND METHODS
Ethical considerations
Ethical standards were followed, adhering to EU Directive 2010/63/EU for animal experiments. The study protocol was approved by the ethics committee of the Faculty of Medicine, Universitas Brawijaya (No. 359/EC/KEPK-S2/06/2014). Every effort was made to alleviate any animal distress. Competent researchers carried out all procedures, which included tasks such as injections, surgeries, and the administration of the extract using an oral gavage feeding tube.
Plant material and extraction
Physalis minima plants were collected from Materia Medica, Batu, East Java, Indonesia (GPS coordinates: −7.867432426003079, 112.5192695810684). The fresh leaves were thoroughly washed with distilled water and then dried at 40°C in a dark condition for three days. Subsequently, they were ground into a fine powder using a miller.
The dried powder was subjected to maceration with 95% methanol (100 g dried powder/1,000 ml of 95% methanol) for 24 hours (x3) at room temperature (RT) with continuous shaking. Afterward, the filtrates were collected, and the solvent was removed under vacuum conditions at 45°C using a rotary evaporator (Janke and Kunkel, IKA-Labortechnik, Germany). The obtained crude extracts were stored at −20°C in airtight containers until further application.
Animals
Female Wistar rats were obtained from Institut Teknologi Bandung (ITB), Bandung, West Java, Indonesia. They were housed in conventional cages with six rats in each cage, maintained at a room temperature of 21°C ±1°C, and subjected to a 12-hours light/dark cycle. The rats had access to standard pellets and tap water ad libitum.
Thirty female rats, 12 weeks old, weighing between 180 and 220 g, were anesthetized using intraperitoneal ketamine (40 mg/kg BW). Through a transabdominal incision, both ovaries were removed, and the rats were allowed a recovery period of five weeks after ovariectomy (OVX). Following the recovery period, the rats were randomly divided into five groups: 5-week OVX rats, 9-week OVX rats, and 5-week OVX rats treated with the MEP at doses of 500, 1,500, and 2,500 mg/kg BW for four weeks, respectively. The control group consisted of six sham-operated rats without any treatment. At the end of the four-week experimental period, rats were euthanized using a lethal dose of diethyl ether, and the aorta was isolated from the surrounding tissue for vascular relaxation analysis and histological sample preparation [9].
Measurement of the vascular relaxation response
The descending thoracic aorta was carefully isolated, and any adherent fat and connective tissue were thoroughly cleaned. Subsequently, the aorta was cut into 4 mm segments. These aortic segments were placed in an organ bath filled with Krebs solution (pH 7.4) at a constant temperature of 37°C and continuously gassed with carbogen (95% O2, 5% CO2). Each aortic ring was then mounted on a wire connected to an isometric transducer. Afterward, the aortic rings were equilibrated for 60 minutes at a resting tension of 1 g [10].
To assess the function of the endothelial cells, the aorta was pre-contracted using 10−6 M phenylephrine (Sigma-Aldrich, St. Louis, MO). Subsequently, a cumulative dose of methacholine (10−6 to 10−4 M, Sigma-Aldrich) was administered [10]. The response of the aorta was recorded using the PowerLab data acquisition system (ADInstruments Pty Ltd., Bella Vista, NSW, Australia). The relaxation response to methacholine was determined by calculating the percentage (%) reduction of the aortic constriction.
Counting the number of thoracic aorta endothelial cells
One segment of the thoracic aorta (5 mm) was excised and fixed in 10% buffered formalin. After fixation, it was dehydrated in ethanol, embedded in paraffin, cross-sectioned at a thickness of 3–4 μm, and stained with hematoxylin-eosin (HE). The number of thoracic aorta endothelial cells was quantified as the mean from ten microscope fields (magnification x100).
Analysis of systolic blood pressure (SBP)
SBP was measured in un-anesthetized rats using an indirect tail-cuff method with an animal blood pressure analyzer from IITC Life Science (Woodland Hills, CA).
Statistical analysis
The data were analyzed using the Shapiro-Wilk test, a one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparisons test. A significance level of p < 0.05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism for Windows, Version 9.3.0, San Diego, CA.
RESULTS
Endothelium-dependent vascular relaxation of the thoracic aortic ring
The study revealed a significant (p < 0.01) reduction in endothelium-dependent vascular relaxation of the thoracic aortic ring in 9-week OVX rats (0.260% ± 4.160%) when contrasted with the sham group (12.55% ± 2.225%). Treatment with MEP at doses of 1,500 and 2,500 mg/kg BW (12.24% ± 3.064% and 12.03% ± 2.981%, respectively) significantly (p < 0.01) enhanced aortic ring dilation in OVX rats in comparison to 9-week OVX rats, reaching levels observed in the sham group (p > 0.999), as shown in Fig. 1.
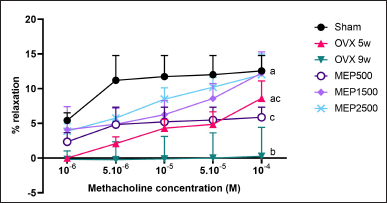 | Figure 1. Relaxation response of the isolated thoracic aortic rings to methacholine. Data are presented as mean ± SD (n = 5). The different notations indicate significant differences from all other groups, determined by ANOVA followed by Tukey’s multiple comparisons test (p < 0.05). OVX 5w: 5-week ovariectomized rats; OVX 9w: 9-week ovariectomized rats; MEP500, 1,500, 2,500: 5-week ovariectomized rats treated with the methanol extract of Physalis minima leaves at doses of 500, 1,500, and 2,500 mg/kg BW, respectively, for 4 weeks. [Click here to view] |
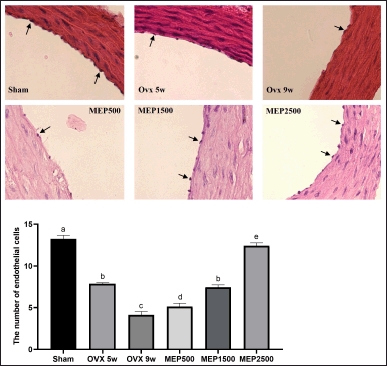 | Figure 2. Thoracic aorta endothelial cells. A. The photomicrograph displays the representative HE-stained thoracic aorta (magnification × 100). The black arrows indicate representative endothelial cells in the tunica intima. B. The bar graph represents the number of thoracic aorta endothelial cells. Data are expressed as mean ± SD (n = 5). The different notations indicate significant differences from all other groups, determined by ANOVA followed by Tukey’s multiple comparisons test (p < 0.05). OVX 5w: 5-week ovariectomized rats; OVX 9w: 9-week ovariectomized rats; MEP500, 1,500, 2,500: 5-week ovariectomized rats treated with the methanol extract of Physalis minima leaves at doses of 500, 1,500, and 2,500 mg/kg BW, respectively, for 4 weeks. [Click here to view] |
Thoracic aorta endothelial cells
As depicted in Fig. 2, numerous endothelial cells were observed to be detached from the thoracic aortic tunica intima in OVX rats. A significant (p < 0.0001) decrease in the number of endothelial cells was noted in 5-week (7.875 ± 0.126) and 9-week OVX rats (4.150 ± 0.379) compared to the sham group (13.23 ± 0.435). Treatment with the extract at a 2,500 mg/kg BW dose significantly (p < 0.0001) increased endothelial cell number in OVX rats (12.43 ± 0.330) compared to both 5-week and 9-week OVX rats, although not fully recovering to sham levels (p < 0.05).
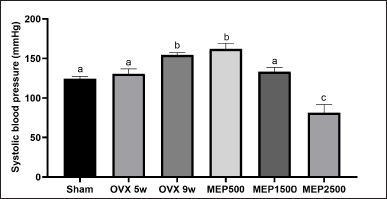 | Figure 3. SBP. Data are expressed as mean ± SD (n = 5). The different notations indicate significant differences from all other groups, as determined by ANOVA followed by Tukey’s multiple comparisons test (p < 0.05). OVX 5w: 5-week ovariectomized rats; OVX 9w: 9-week ovariectomized rats; MEP500, 1,500, 2,500: 5-week ovariectomized rats treated with the methanol extract of Physalis minima leaves at doses of 500, 1,500, and 2,500 mg/kg BW, respectively, for 4 weeks. [Click here to view] |
SBP
The study indicated a significant (p < 0.0001) increase in SBP in 9-week OVX rats (154.5 ± 2.887 mmHg) compared to the sham group (124.3 ± 3.096 mmHg). Treatment with the 1,500 mg/kg BW extract significantly (p < 0.01) reduced SBP (133.3 ± 5.252 mmHg) compared to 9-week OVX rats, nearing the sham level (p = 0.395). Moreover, the 2,500 mg/kg BW extract further lowered SBP (81.25 ± 10.66 mmHg), even below the sham group (p < 0.0001), as illustrated in Fig. 3.
DISCUSSION
Since the 20th century, the study of endothelial health has been a prominent subject of research. The endothelium, a single layer of cells that lines the innermost layer of blood vessels (intima), regulates vascular tone by producing endothelium-derived relaxing factors as well as endothelium-derived contracting factors. Substances that induce vasodilation include nitric oxide (NO), prostacyclin (PGI2), and endothelium-derived hyperpolarizing factor. On the other hand, substances that induce vasoconstriction include endothelin-1 (ET-1), thromboxane A2 (TXA2), and angiotensin II (Ang II). These substances are released depending on the specific cell type that responds to the stimulus, whether it is endothelial cells or vascular smooth muscle cells (VSMCs) [2,10].
Endothelial dysfunction plays a crucial role in the development of CVD, with considerable reductions in endothelial vasodilator function believed to occur during the menopausal transition. The degradation of endothelial function is linked to estrogen insufficiency and is not solely dependent on chronological age. The onset of menopause is associated with accelerated vascular aging, which seems distinct from the gradual decline in vascular function that accompanies chronological aging. This condition creates a favorable environment for the development of vascular diseases such as hypertension and atherosclerosis [2]. These facts highlight the significance of conducting vascular studies that include endothelial cells to analyze the risk of cardiovascular disorders during menopause.
To investigate the effects of sustained reductions in steroid hormone levels, bilateral ovariectomy in mice and rats serves as a valuable surgical menopausal model in preclinical research [11,12]. In our study, we employed the ovariectomy model in rats and conducted isolated rat aorta experiments to evaluate the functional changes in endothelial regulation of vasodilation. The endothelium-dependent relaxation induced by acetylcholine (ACh) in phenylephrine (PHE)-precontracted rings is a suitable method for testing endothelial functional integrity [10]. Phenylephrine primarily acts as an α1-adrenergic receptor agonist and exhibits similar potency to norepinephrine, but it has a slightly extended duration of action. When α1-receptors are activated by phenylephrine on the arterial vasculature, it results in elevations in arterial pressure, systemic vascular resistance (SVR), and ventricular afterload [13]. Whereas, ACh serves as the predominant neurotransmitter in the parasympathetic branch of the autonomic nervous system [14]. However, in this study, we used methacholine.
The main differences in the pharmacological effects of methacholine and ACh lie in their duration of action and selectivity. Unlike ACh, methacholine is broken down exclusively by acetylcholinesterase at a considerably slower rate. Consequently, methacholine’s effects last much longer than those of ACh. Additionally, the presence of a methyl group at the carbon of choline enhances the specificity of methacholine’s action. Methacholine primarily targets muscarinic receptors in smooth muscle, glands, and the heart, with minimal impact on nicotinic receptors in skeletal muscle autonomic ganglia [14].
Blood vessels exhibit relaxation solely in the presence of the endothelium when stimulated by either ACh or methacholine. They indirectly induce the relaxation of VSMCs by triggering the release of established EDRFs. NO is the primary and most effective EDRF that regulates endothelial-dependent relaxation in the majority of blood vessels. In response to ACh, shear pressure, or bradykinin, the calcium-calmodulin complex (CaM) binds to endothelial NO synthase (eNOS), facilitating the interaction of phosphorylated protein kinase B (Akt) with eNOS. This interaction, supported by the presence of tetrahydrobiopterin (BH4) as an essential cofactor, leads to the conversion of the amino acid L-arginine (L-Arg) into NO and L-citrulline [10,15].
The endothelium also uses nicotinamide adenine dinucleotide phosphate (NADPH) oxidase and the ATP-sensitive K+ (KATP) channel to promote endothelial-dependent relaxation and vascular tone. NADPH oxidase serves a fundamental function in creating reactive oxygen species (ROS). The endoplasmic reticulum (ER)-resident NADPH oxidase (NOX4) is well-known for producing hydrogen peroxide (H2O2) and superoxide (O2•–). H2O2 is an important signaling chemical that promotes the activation of KATP channels. When endothelial KATP channels are triggered, an influx of Ca2+ into endothelial cells occurs, triggering the production of CaM. CaM production can activate eNOS via calcium-dependent mechanisms [15].
Estrogen acts on blood vessels through the activation of the estrogen receptor (ER), which consists of two isoforms, namely ERα and ERβ. The stimulation of ER induces NO production via the activation of eNOS (chronic effects/genomics) and NOS-dependent activation of Ca2+ (rapid effects/non-genomics) [16,17]estrogen and progesterone, or estrogen and MPA. Isolated cerebral vessels were also treated in vitro with estrogen in the absence and presence of progesterone, MPA, tamoxifen, and the estrogen receptor antagonist ICI 182 780. Levels of eNOS were measured by Western blot, and NOS activity was measured by [14C]arginine-[14C]citrulline conversion. Results - Chronic hormone treatment in vivo resulted in plasma levels of 17β-estradiol, progesterone, and MPA in the range of values found in humans. Estrogen treatment resulted in higher levels of cerebrovascular NOS activity that paralleled increases in eNOS protein. In vitro estrogen treatment for 18 hours also resulted in a concentration-dependent increase in eNOS protein (EC50 ≈300 pmol/L. Thus, the reduction of estrogen in OVX rats can lead to endothelial dysfunction. A previous study reported a significant correlation between serum estradiol levels and NO in postmenopausal women [18].
The results of this study revealed that the most significant decline in methacholine-mediated dilations in the rat aorta occurred after 9 weeks following ovariectomy. Previous studies have also reported impaired vascular reactivity to ACh in blood vessels isolated from OVX rats [19,20]. Another study documented the maximum loss of ACh-mediated dilations in rat tail arteries occurring after 12 weeks of ovariectomy [11]. These reduced dilations indicate endothelial dysfunction, which is associated with reduced eNOS activity and/or expression, leading to a decrease in NO bioavailability [15].
Endothelial dysfunction can be attributed, in part, to increased oxidative stress. NADPH oxidase-generated O2•– rapidly degrades NO into peroxynitrite (ONOO−), a highly reactive and potentially harmful molecule. This potent oxidant intensifies eNOS uncoupling by oxidizing its cofactor BH4. Moreover, ONOO− induces protein oxidation and nitration, resulting in cellular damage. In addition to its role in scavenging NO, O2•– triggers eNOS uncoupling. The key mechanisms of eNOS uncoupling include oxidative depletion of the crucial eNOS cofactor BH4, eNOS substrate (L-Arg) deficiency, accumulation of L-Arg analog (asymmetrical dimethylarginine/ADMA), and eNOS S-glutathionylation. Uncoupled eNOS generates O2•- rather than NO, becoming a source of damaging free radicals that exacerbate oxidative stress. Uncoupling of eNOS is thought to be a major underlying component in the development of endothelial dysfunction seen in the pathophysiology of vascular disorders [21,22].
Numerous studies have demonstrated the presence of oxidative stress in OVX rats [23–27]. Additionally, some studies have confirmed a higher level of oxidative stress in post-menopausal women [28–30]. After ovariectomy or during postmenopause, the absence of estrogen leads to changes in the redox state. Estrogens, particularly estradiol, have been shown to reduce vascular oxidative stress by regulating the expression and activity of NADPH oxidases and antioxidant enzymes (superoxide dismutase/SOD, glutathione peroxidase/GPx, catalase). This modulation offers protection against oxidative stress during the reproductive stage. Estradiol molecules possess a chemical structure that allows them to function as scavengers for free radicals, thereby protecting against oxidative damage. The crucial component responsible for their antioxidant effect is the phenolic ring located in the A position of the estradiol molecules [30].
In this study, treatment with P. minima methanol extract at doses of 1,500 and 2,500 mg/kg BW significantly improved methacholine-mediated dilations in the aorta isolated from OVX rats. These enhanced dilations indicate an improvement in endothelial function. A previous in vitro study found that the extract of P. minima leaves increased the cellular expression of eNOS and the generation of NO in human umbilical vein endothelial cells [31]. A study has shown that the ethanol extract of P. minima leaves at a dose of 500 mg/kg BW significantly increased serum NO levels in DOCA-salt-induced hypertensive rats [7]. Another study using a different species of Physalis demonstrated that the administration of the ethanol extract of P. angulata leaves at a dose of 2,500 mg/kg BW in L-NG-nitro arginine methyl ester (L-NAME)-induced hypertensive rats also increased serum NO levels [32]. Studies on L-NAME-induced preeclampsia rats treated with the extract of P. angulata leaves have also revealed an increase in tail artery eNOS expression and serum NO levels [33,34].
The improvement in endothelial function may be attributed to the antioxidant activity of the extracts. A study confirmed that the ethanol extract of P. minima leaves exhibits strong antioxidant activity, as evidenced by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay, Fe2+ chelating activity assay, and Fe3+ reducing power assay [35]. A previous study has demonstrated that extracts of Physalis leaves can alleviate oxidative stress, as indicated by reduced serum malondialdehyde (MDA) levels and increased serum SOD activity [32–34].
The ethanol extract of Physalis leaves contains various bioactive compounds, including trigonelline, DL-stachydrine (alkaloid), chlorogenic acid (polyphenol), quercetin, rutin, kaempferol (flavonoid), and withanolides (steroid lactones) [34]. Numerous studies have demonstrated the antioxidant activities of these bioactive compounds [36–43]cardiovascular disease and cancer. These beneficial effects have partly been attributed to the antioxidant activity of coffee. We determined composition and antioxidant potential of differentially roasted coffee extracts and investigated the impact of selected original constituents and roast products.Methods and results: Parameters studied were direct antioxidant activity (trolox equivalent antioxidant capacity/oxygen radical absorbing capacity. The inherent antioxidant capabilities within the compounds present in the P. minima methanol extract demonstrate proficiency in scavenging superoxide, consequently alleviating eNOS uncoupling and preserving the bioavailability of NO. This dual action significantly contributes to the enhancement of endothelial function [21,22]. Furthermore, studies have highlighted the positive impact of specific compounds, including stachydrine [44], chlorogenic acid [39,45,46], quercetin [47–50], rutin [51], and also kaempferol [52], on the improvement of the eNOS/NO signaling pathway. Additionally, trigonelline has been shown to enhance the Ca2+-dependent eNOS/NO signaling pathway [53]. In this study, the deliberate choice of crude extract was made to preserve potential synergistic effects among its various components, ensuring a comprehensive and effective intervention.
However, there was a limitation in using the isolated rat aorta to evaluate functional changes in endothelial regulation of vasodilation instead of the rat tail artery, which was chosen due to limitations in transducer sensitivity. It is worth noting that for evaluating the effects of substances on blood pressure and considering SVR, using the rat tail artery may be more relevant. Some studies consider the tail artery a resistance artery. Resistance vessels, comprising arterioles and small arteries, play a significant role in SVR, with approximately 40 to 55% of the resistance residing in vessels with diameters >100 μm up to a limit of 400 μm [54]. Additionally, although the isolated organ bath has been widely used to assess vascular function in animal models, it only evaluates biological activities that occur within endothelial cells and VSMCs [55]. It falls short of explaining the intricate pathophysiology of blood pressure.
In this study, we also observed histopathological changes in the vascular endothelial layer of OVX rats, which exhibited arterial denudations. This result is consistent with a previous study that showed impairment of the integrity of the vascular endothelium in OVX rats [20]. The endothelial dysfunction, apoptosis, and pyroptosis of endothelial cells, along with alterations in tight junctions, may contribute to the detachment of endothelial cells [56,57]. In certain situations, endothelial cells may not detach as entire cells but as apoptotic endothelial microparticles. Arterial denudation may trigger important atherosclerotic processes, such as smooth muscle cell proliferation, migration, and matrix secretion [58].
The administration of P. minima methanol extract at doses of 1,500 and 2,500 mg/kg BW significantly increased the number of thoracic aorta endothelial cells in OVX rats. An in vitro study demonstrated that withaferin A, a type of withanolide contained in the extract of Physalis leaves, can dose-dependently increase VEGF secretion in endothelial cells, thereby enhancing endothelial cell proliferation and migration [59]. However, in this study, the extract did not fully restore the number of thoracic aorta endothelial cells to the levels observed in the sham group. Nevertheless, the methacholine-mediated dilations in the aorta isolated from OVX rats treated with the extract at doses of 1,500 and 2,500 mg/kg BW were significantly enhanced, almost approaching the levels observed in the sham group. This suggests a limitation in using endothelial cell counts in histopathological specimens within a predetermined fixed frame, as the frame size may significantly affect accuracy [60]. To validate these histopathological findings, it is recommended to use flow cytometry and a combination of magnetic bead selection and fluorescent microscopy to measure circulating markers of endothelial cell damage, such as endothelial microparticles derived from activated or apoptotic cells, as well as whole endothelial cells [61].
The consequence of endothelial dysfunction is an increase in blood pressure. Following this phenomenon, our study found that the SBP in 9-week OVX rats was significantly higher than that of sham rats and 5-week OVX rats. In contrast, treatment with the methanol extract of P. minima at a dose of 1500 mg/kg BW significantly reduced SBP in OVX rats due to the improvement in endothelial function, as proven by methacholine-mediated dilations. Previous studies on hypertensive and preeclampsia rats also revealed the effect of Physalis extract in lowering blood pressure [32–34].
NO is a potent vasodilator. It activates soluble guanylate cyclase (sGC), which converts guanosine-5’-triphosphate (GTP) into cyclic guanosine-3’,5’-monophosphate (cGMP), leading to the activation of protein kinase A and protein kinase G. The activated protein kinases induce VSMCs relaxation by reducing the activity of myosin light-chain kinase and enhancing the activity of myosin light-chain phosphatase, resulting in the dephosphorylation of 20-kDa myosin light-chain [62]superoxide (O2−. Moreover, protein kinase G increases the phosphorylation of sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) as well as Ca2+-ATPase and Na+/Ca2+ exchanger on the cell membrane. This leads to a reduction in intracellular Ca2+ levels, triggering vasorelaxation and decreasing peripheral blood vessel resistance. Vasorelaxation mediated by NO can also occur through cGMP-independent mechanisms, such as direct activation of K+ channels [63].
The ethanol extract of Physalis leaves contains various bioactive compounds that can lower blood pressure. Ca2+-dependent phosphodiesterases can be inhibited by quercetin and kaempferol [64,65]each was found to be approximately equipotent in inhibiting the calcium-dependent hydrolysis of either cyclic AMP or cyclic GMP. In contrast, the inhibitors displayed a marked substrate specificity for the calcium-independent enzyme with ratios of IC50 values for inhibition of cyclic GMP hydrolysis when compared to cyclic AMP hydrolysis in decreasing order being: ZK 62711 (> 100. Furthermore, kaempferol has been shown to inhibit myosin light-chain kinase activity [66], resulting in myosin light-chain dephosphorylation and vasorelaxation [63]. In hypertensive individuals, both chlorogenic acid [67] and quercetin [68,69] have been shown to increase endothelium-dependent vasodilation and lower blood pressure. Furthermore, chlorogenic acid and quercetin have shown their potential to inhibit angiotensin-converting enzyme (ACE) in endothelial cells [70]. Additionally, research highlights the potential of the groundcherry extract to alleviate anxiety [8] and improve cardiac fibrosis [9] in OVX rats. As a result, the significance of the groundcherry extract for addressing postmenopausal conditions is noteworthy.
In this study, treatment of methanol extract of P. minima at a dose of 2,500 mg/kg BW significantly decreased SBP in OVX rats below the level observed in the sham group, despite the methacholine-mediated dilations being at the same level as the sham group. This suggests that there might be other factors influencing blood pressure. Physalis leaves are known to be used as diuretics by the Mestizo population in Latin America, residents of Nigeria, Thailand, and the Malay Peninsula [6,71,72]. Additionally, the methanol extract of Physalis leaves has been proven to increase urine volume and enhance sodium excretion in the urine output of rats [73]. Diuretics induce a reduction in plasma volume and cardiac output, thus promoting the initial decrease in blood pressure. This initial reduction in blood pressure is followed by a subsequent decrease in vascular resistance, contributing to sustained lower blood pressure. In individuals with hypertension, their blood vessels become “waterlogged” with excessive amounts of sodium and water, making them more responsive to sympathetic nervous system stimuli. Diuretics, however, counteract this effect on the vessels, making them less sensitive to vasoconstrictive activity [74].
The hypotensive effect observed with the highest dose of methanol extract of P. minima could serve as a cautionary signal for determining the appropriate dosage to treat hypertension in postmenopausal conditions. We should be mindful of the potential side effects, such as postural hypotension, electrolyte imbalance, and the possibility of dehydration when using the extract. Further studies are necessary to validate and investigate these potential side effects in greater detail.
Another limitation of our study is the absence of a direct comparison with controls, such as standard hypertension medication. Future research is essential to assess the efficacy of P. minima leaf extract compared to standard medication in improving endothelium-dependent vasodilation and reducing blood pressure in OVX rats. This comparison is crucial for determining the extract’s relative effectiveness, guiding potential alternative or complementary treatment strategies, and enhancing our understanding of its role in addressing postmenopausal vascular issues and hypertension.
CONCLUSION
Our findings revealed that 9-week OVX rats exhibited diminished aortic relaxation and detachment of endothelial cells, paralleled by increased SBP compared to sham-operated rats. Notably, treatment with P. minima methanol extract at 1,500 and 2,500 mg/kg BW significantly restored aortic dilation, while the 2,500 mg/kg BW dose remarkably lowered SBP below even the sham level.
These results suggest the potential of P. minima methanol extract as a therapeutic agent to address vascular dysfunction and hypertension associated with postmenopausal conditions. Nevertheless, to fully understand the underlying mechanisms and assess their applicability in human subjects, further investigations are warranted. The notable hypotensive effect at the highest dose underscores the importance of dosage determination, warranting further investigation into potential side effects.
ACKNOWLEDGMENT
This study received a research grant (BOPTN year 2013) from the Ministry of Research, Technology, and Higher Education of the Republic of Indonesia. The authors thank the Laboratory of Pharmacology and Laboratory of Anatomic Pathology, Faculty of Medicine, Universitas Brawijaya for the technical support.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The study protocol was approved by the ethics committee of the Faculty of Medicine, Universitas Brawijaya (No. 359/EC/KEPK-S2/06/2014).
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. El Khoudary SR, Aggarwal B, Beckie TM, Hodis HN, Johnson AE, Langer RD et al. Menopause transition and cardiovascular disease risk: implications for timing of early prevention: a scientific statement from the American Heart Association. Circulation. 2020;142(25):E506–E532. CrossRef
2. Somani YB, Pawelczyk JA, De Souza MJ, Kris-Etherton PM, Proctor DN. Aging women and their endothelium: probing the relative role of estrogen on vasodilator function. Am J Physiol Hear Circ Physiol. 2019;317(2):H395–H404. CrossRef
3. Witkowski S, Serviente C. Endothelial dysfunction and menopause: is exercise an effective countermeasure? Climacteric. 2018;21(3):267–75. CrossRef
4. Higashi Y, Kihara Y, Noma K. Endothelial dysfunction and hypertension in aging. Hypertens Res. 2012;35(11):1039–47. CrossRef
5. Nadhifah A, Suratman S, Pitoyo A. Kekerabatan fenetik ciplukan (Physalis angulata L.) di Wilayah Eks-Karesidenan Surakarta Berdasarkan Karakter Morfologis, Palinologis dan Pola Pita Isozim. J Tumbuh Obat Indones. 2016;9(1):1–10. CrossRef
6. Salgado ER, Arana GV. Physalis angulata L. (bolsa mullaca): usos tradicionais, fitoquímica e farmacologia. Rev Fitoter. 14(1):49–64. 2014.
7. Nugrahenny D, Permatasari N, Saifur Rohman M. Physalis minima leaves extract induces re-endothelialization in deoxycorticosterone acetate-salt-induced endothelial dysfunction in rats. Res J Life Sci. 2017;4(3):199–208. CrossRef
8. Nurfitria S, Permatasari N, Ratnawati R. evaluation anxiolytic effect of methanol extract of ceplukan leaves (Physalis minima L.) in the Elevated Plus Maze Test through IL-6 level changes in ovariectomized rats. J Trop Life Sci. 2015;5(1):8–13. CrossRef
9. Lestari B, Permatasari N, Rohman MS. Methanolic extract of ceplukan leaf (Physalis minima L.) attenuates ventricular fibrosis through inhibition of TNF-α in ovariectomized rats. Adv Pharmacol Sci. 2016;2016:2428052. CrossRef
10. Knox M, Vinet R, Fuentes L, Morales B, Martínez JL. A review of endothelium-dependent and -independent vasodilation induced by phytochemicals in isolated rat aorta. Anim Open Access J MDPI. 2019;9(9):623. CrossRef
11. Moien-Afshari F, Kenyon E, Choy JC, Battistini B, McManus BM, Laher I. Long-term effects of ovariectomy and estrogen replacement treatment on endothelial function in mature rats. Maturitas. 2003;45(3):213–23. CrossRef
12. Rodríguez-Landa JF. Considerations of timing post-ovariectomy in mice and rats in studying anxiety- and depression-like behaviors associated with surgical menopause in women. Front Behav Neurosci. 2022;16:829274. CrossRef
13. Nguyen LP, Gerstein NS. Cardiovascular pharmacology in noncardiac surgery. Essentials of cardiac anesthesia for noncardiac surgery: a companion to Kaplan’s Cardiac Anesthesia. Amsterdam, The Netherlands: Elsevier; 2018, pp. 247–88. CrossRef
14. Vardanyan RS, Hruby VJ. Cholinomimetics. Synthesis of essential drugs. Amsterdam, The Netherlands: Elsevier; 2006, pp. 179–93. CrossRef
15. Mustapha S, Azemi AK, Wan Ahmad WAN, Rasool AHG, Mustafa MR, Mokhtar SS. Inhibition of Endoplasmic reticulum stress improves acetylcholine-mediated relaxation in the aorta of type-2 diabetic rats. Molecules. 2022;27(16):5107. CrossRef
16. McNeill AM, Zhang C, Stanczyk FZ, Duckles SP, Krause DN. Estrogen increases endothelial nitric oxide synthase via estrogen receptors in rat cerebral blood vessels: effect preserved after concurrent treatment with medroxyprogesterone acetate or progesterone. Stroke. 2002;33(6):1685–91. CrossRef
17. Simoncini T, Mannella P, Fornari L, Caruso A, Varone G, Genazzani AR. Genomic and non-genomic effects of estrogens on endothelial cells. Steroids. 2004;69(8–9):537–42. CrossRef
18. Bednarek-Tupikowska G, Tworowska-Bardzi?ska U, Tupikowski K. Effects of estrogen and estrogen-progesteron on serum nitric oxide metabolite concentrations in post-menopausal women. J Endocrinol Invest. 2008;31(10):877–81. CrossRef
19. Caliman IF, Lamas AZ, Dalpiaz PLM, Medeiros ARS, Abreu GR, Gomes Figueiredo S et al. Endothelial relaxation mechanisms and oxidative stress are restored by atorvastatin therapy in ovariectomized rats. PLoS One. 2013;8(11):e80892. CrossRef
20. Sharma S, Singh M, Sharma PL. Ameliorative effect of daidzein: a caveolin-1 inhibitor in vascular endothelium dysfunction induced by ovariectomy. Indian J Exp Biol. 2012;50(1):28–34. [cited 2023 Aug 7]. Available from: https://pubmed.ncbi.nlm.nih.gov/22279937/
21. Janaszak-Jasiecka A, P?oska A, Wiero?ska JM, Dobrucki LW, Kalinowski L. Endothelial dysfunction due to eNOS uncoupling: molecular mechanisms as potential therapeutic targets. Cell Mol Biol Lett. 2023;28(1):1–28. CrossRef
22. Pérez de la Lastra JM, Juan CA, Plou FJ, Pérez-Lebeña E. The nitration of proteins, lipids and DNA by peroxynitrite derivatives-chemistry involved and biological relevance. Stresses. 2022;2(1):53–64. CrossRef
23. Muthusami S, Ramachandran I, Muthusamy B, Vasudevan G, Prabhu V, Subramaniam V. Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta. 2005;360(1–2):81–6. CrossRef
24. Arslan A, Orkun S, Aydin G, Keles I, Tosun A, Arslan M. Effects of ovariectomy and ascorbic acid supplement on oxidative stress parameters and bone mineral density in rats. Libyan J Med. 2011;6(1):1–9. CrossRef
25. Al-Rahbi B, Zakaria R, Othman Z, Hassan A, Ahmad AH. Protective effects of tualang honey against oxidative stress and anxiety-like behaviour in stressed ovariectomized rats. Int Sch Res Not. 2014;2014:1–10. CrossRef
26. Nurdiana N, Mariati N, Noorhamdani N, Setiawan B, Budhiparama N, Noor Z. Effects of Labisia pumila on oxidative stress in rat model of post-menopausal osteoporosis. Asian Pacific J Reprod. 2016;5(5):391–4. CrossRef
27. Wei L, Chai S, Yue C, Zhang H, Li J, Qin N. Resveratrol protects osteocytes against oxidative stress in ovariectomized rats through AMPK/JNK1-dependent pathway leading to promotion of autophagy and inhibition of apoptosis. Cell Death Discov. 2023;9(1):1–12. CrossRef
28. Sánchez-Rodríguez MA, Castrejón-Delgado L, Zacarías-Flores M, Arronte-Rosales A, Mendoza-Núñez VM. Quality of life among post-menopausal women due to oxidative stress boosted by dysthymia and anxiety. BMC Womens Health. 2017;17(1):1–9. CrossRef
29. Sánchez-Rodríguez MA, Zacarías-Flores M, Arronte-Rosales A, Mendoza-Núnez VM. Association between hot flashes severity and oxidative stress among mexican postmenopausal women: a cross-sectional study. PLoS One. 2019;14(9):e0214264. CrossRef
30. Sánchez-Rodríguez MA, Zacarías-Flores M, Arronte-Rosales A, Correa-Muñoz E, Mendoza-Núñez VM. Menopause as risk factor for oxidative stress. Menopause. 2012;19(3):361–7. CrossRef
31. Permatasari N, Nurdiana N, Karyono S. Efek non genomik dan genomik ekstrak daun ceplukan (Physalis minima L.) pada kultur sel endotel manusia (HUVECs). J Ilmu-Ilmu Hayati Life Sci. 2010;22:14–9.
32. Nugrahenny D, Permatasari N, Soeharto S, Rahayu ID, Widodo E, Mintaroem K. Physalis angulata leaf ethanol extract reduces oxidative stress and improves endothelial progenitor cells in L-NAME-induced hypertensive rats. HAYATI J Biosci. 2023;30(1):81–7. CrossRef
33. Nugrahenny D, Soeharto S, Permatasari N, Rudijanto A, Wiyasa IWA, Widjajanto E. Physalis angulata leaf extract protects against oxidative stress and antiangiogenic factor in LNAME-induced preeclampsia rats. Int J Pharm Res. 2021;13(01):5092–102. CrossRef
34. Nugrahenny D, Rudijanto A, Permatasari N, Wiyasa IWA, Widodo MA, Mintaroem K. Physalis angulata leaf extract ameliorates L-N G-nitroarginine methyl ester (L-NAME)-induced preeclampsia symptoms in rats through improved endothelial progenitor cells and endothelial cells due to reduced antiangiogenic factor and oxidative stress. F1000Research. 2022;11:780. CrossRef
35. Karpagasundari C, Kulothungan S. Free radical scavenging activity of Physalis minima Linn. leaf extract (PMLE). J Med Plants Stud. 2014;2(4):59–64.
36. Bakuradze T, Lang R, Hofmann T, Stiebitz H, Bytof G, Lantz I. Antioxidant effectiveness of coffee extracts and selected constituents in cell-free systems and human colon cell lines. Mol Nutr Food Res. 2010;54(12):1734–43. CrossRef
37. Crespo I, García-Mediavilla MV, Gutiérrez B, Sánchez-Campos S, Tuñón MJ, González-Gallego J. A comparison of the effects of kaempferol and quercetin on cytokine-induced pro-inflammatory status of cultured human endothelial cells. Br J Nutr. 2008;100(5):968–76. CrossRef
38. Devkar ST, Kandhare AD, Zanwar AA, Jagtap SD, Katyare SS, Bodhankar SL. Hepatoprotective effect of withanolide-rich fraction in acetaminophen-intoxicated rat: decisive role of TNF-α, IL-1β, COX-II and iNOS. Pharm Biol. 2016;54(11):2394–403. CrossRef
39. Jiang R, Hodgson JM, Mas E, Croft KD, Ward NC. Chlorogenic acid improves ex vivo vessel function and protects endothelial cells against HOCl-induced oxidative damage, via increased production of nitric oxide and induction of Hmox-1. J Nutr Biochem. 2016;27:53–60. CrossRef
40. Wang W, Wu QH, Sui Y, Wang Y, Qiu X. Rutin protects endothelial dysfunction by disturbing Nox4 and ROS-sensitive NLRP3 inflammasome. Biomed Pharmacother. 2017;86:32–40. CrossRef
41. Yan Z, Guo R, Gan L, Lau WB, Cao X, Zhao J. Withaferin A inhibits apoptosis via activated Akt-mediated inhibition of oxidative stress. Life Sci. 2018;211:91–101. CrossRef
42. Yin J, Zhang ZW, Yu WJ, Liao JY, Luo XG, Shen YJ. Stachydrine, a major constituent of the Chinese herb leonurus heterophyllus sweet, ameliorates human umbilical vein endothelial cells injury induced by anoxia-reoxygenation. Am J Chin Med. 2010;38(1):157–71. CrossRef
43. Zhou J, Zhou S, Zeng S. Experimental diabetes treated with trigonelline: effect on β cell and pancreatic oxidative parameters. Fundam Clin Pharmacol. 2013;27(3):279–87. CrossRef
44. Xie X, Yang C, Cui Q, Ma W, Liu J, Yao Q. Stachydrine mediates rapid vascular relaxation: activation of endothelial nitric oxide synthase involving AMP-activated protein kinase and akt phosphorylation in vascular endothelial cells. J Agric Food Chem. 2019;67(35):9942–9. CrossRef
45. Suzuki A, Yamamoto N, Jokura H, Yamamoto M, Fujii A, Tokimitsu I. Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J Hypertens. 2006;24(6):1065–1073. CrossRef
46. Tsai KL, Hung CH, Chan SH, Hsieh PL, Ou HC, Cheng YH. Chlorogenic acid protects against oxLDL-induced oxidative damage and mitochondrial dysfunction by modulating SIRT1 in endothelial cells. Mol Nutr Food Res. 2018;62(11):1700928. CrossRef
47. Dagher O, Mury P, Thorin-Trescases N, Noly PE, Thorin E, Carrier M. Therapeutic potential of quercetin to alleviate endothelial dysfunction in age-related cardiovascular diseases. Front Cardiovasc Med. 2021;8:220. CrossRef
48. Li PG, Sun L, Han X, Ling S, Gan WT, Xu JW. Quercetin induces rapid eNOS phosphorylation and vasodilation by an Akt-independent and PKA-dependent mechanism. Pharmacology. 2012;89(3–4):220–8. CrossRef
49. Qian Y, Babu PVA, Symons JD, Jalili T. Metabolites of flavonoid compounds preserve indices of endothelial cell nitric oxide bioavailability under glucotoxic conditions. Nutr Diabetes. 2017;7(9):e286. CrossRef
50. Shen Y, Croft KD, Hodgson JM, Kyle R, Lee ILE, Wang Y. Quercetin and its metabolites improve vessel function by inducing eNOS activity via phosphorylation of AMPK. Biochem Pharmacol. 2012;84(8):1036–44. CrossRef
51. Ugusman A, Zakaria Z, Chua KH, Megat Mohd Nordin NA, Abdullah Mahdy Z. Role of rutin on nitric oxide synthesis in human umbilical vein endothelial cells. Sci World J. 2014;2014:169370. CrossRef
52. Xiao HB, Jun-Fang, Lu XY, Chen X jun, Chao-Tan, Sun ZL. Protective effects of kaempferol against endothelial damage by an improvement in nitric oxide production and a decrease in asymmetric dimethylarginine level. Eur J Pharmacol. 2009;616(1–3):213–22. CrossRef
53. Kuroda R, Kazumura K, Ushikata M, Minami Y, Kajiya K. Elucidating the Improvement in vascular endothelial function from sakurajima daikon and its mechanism of action: a comparative study with Raphanus sativus. J Agric Food Chem. 2018;66(33):8714–21. CrossRef
54. Souza FM, Padilha AS, Stefanon I, Vassallo DV. Differences in functional and structural properties of segments of the rat tail artery. Brazilian J Med Biol Res. 2008;41(5):416–23. CrossRef
55. Storch AS, Dario De Mattos J, Alves R, Galdino IDS, Rocha HNM. Methods of endothelial function assessment: description and applications. Int J Cardiovasc Sci. 2017;30(3):262–73. CrossRef
56. Segal MS, Baylis C, Johnson RJ. Endothelial health and diversity in the kidney. J Am Soc Nephrol. 2006;17(2):323–4. CrossRef
57. Six I, Guillaume N, Jacob V, Mentaverri R, Kamel S, Boullier A. The endothelium and COVID-19: an increasingly clear link brief title: endotheliopathy in COVID-19. Int J Mol Sci. 2022;23(11):6196. CrossRef
58. Avogaro A, Albiero M, Menegazzo L, De Kreutzenberg S, Fadini GP. Endothelial dysfunction in diabetes the role of reparatory mechanisms. Diabetes Care. 2011;34(Suppl 2):S285–S290. CrossRef
59. Li T, Jiang S, Wang ZJ, Huang LA. Withaferin a promotes proliferation and migration of brain endothelial cells. Trop J Pharm Res. 2016;15(7):1487–92. CrossRef
60. McCarey BE, Edelhauser HF, Lynn MJ. Review of corneal endothelial specular microscopy for FDA clinical trials of refractive procedures, surgical devices, and new intraocular drugs and solutions. Cornea. 2008;27(1):1–16. CrossRef
61. Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115(10):1285–95. CrossRef
62. Matsubara K, Higaki T, Matsubara Y, Nawa A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int J Mol Sci. 2015;16(3):4600–14. CrossRef
63. França-Silva MS, Balarini CM, Cruz JC, Khan BA, Rampelotto PH, Braga VA. Organic nitrates: past, present and future. Molecules. 2014;19(9):15314–23. CrossRef
64. Davis CW. Assessment of selective inhibition of rat cerebral cortical calcium-independent and calcium-dependent phosphodiesterases in crude extracts using deoxycyclic AMP and potassium ions. BBA Gen Subj. 1984;797(3):354–62. CrossRef
65. Duarte J, Pérez Vizcaíno F, Utrilla P, Jiménez J, Tamargo J, Zarzuelo A. Vasodilatory effects of flavonoids in rat aortic smooth muscle. Structure-activity relationships. Gen Pharmacol. 1993;24(4):857–62. CrossRef
66. Rogers JC, Williams DL. Kaempferol inhibits myosin light chain kinase. Biochem Biophys Res Commun. 1989;164(1):419–25. CrossRef
67. Kajikawa M, Maruhashi T, Hidaka T, Nakano Y, Kurisu S, Matsumoto T et al. Coffee with a high content of chlorogenic acids and low content of hydroxyhydroquinone improves postprandial endothelial dysfunction in patients with borderline and stage 1 hypertension. Eur J Nutr. 2019;58(3):989–96. CrossRef
68. Edwards RL, Lyon T, Litwin SE, Rabovsky A, Symons JD, Jalili T. Quercetin reduces blood pressure in hypertensive subjects. J Nutr. 2007;137(11):2405–11. CrossRef
69. Larson A, Witman MAH, Guo Y, Ives S, Richardson RS, Bruno RS et al. Acute, quercetin-induced reductions in blood pressure in hypertensive individuals are not secondary to lower plasma angiotensin-converting enzyme activity or endothelin-1: Nitric oxide. Nutr Res. 2012;32(8):557–64. CrossRef
70. Huang WY, Fu L, Li CY, Xu LP, Zhang LX, Zhang WM. Quercetin, hyperin, and chlorogenic acid improve endothelial function by antioxidant, antiinflammatory, and ACE inhibitory effects. J Food Sci. 2017;82(5):1239–46. CrossRef
71. Lawal IO, Uzokwe NE, Igboanugo ABI, Adio AF, Awosan EA, Nwogwugwu JOet al. Ethno medicinal information on collation and identification of some medicinal plants in Research Institutes of South-west Nigeria. Afr J Pharm Pharmacol. 2010;4(1):001–007 [cited 2022 June 22]. Available from: http://www.academicjournals.org/ajpp
72. Lim TK. Edible medicinal and non-medicinal plants. Dordrecht, The Netherlands: Springer Netherlands; 2016. Vol. 10. CrossRef
73. Nanumala S, Gunda K, Runja C, Chandra M. Evaluations of diuretic activity of methanolic extract of Physalis angulata L. leaves. Int J Pharma Sci Rev Res. 2012;16(2):40–2. [cited 2022 June 22]. Available from: https://globalresearchonline.net/journalcontents/v16-2/09.pdf
74. Moser M, Feig PU. Fifty years of thiazide diuretic therapy for hypertension. Arch Intern Med. 2009;169(20):1851–6. CrossRef