INTRODUCTION
Nearly 2 million people succumb to liver diseases every year, among which complications associated with cirrhosis alone account for 1 million mortalities and the remaining deaths occur due to viral hepatitis and hepatocellular cancer [1]. Almost every organ in the human body has a limited ability to regenerate; however, the liver being an exception, possesses remarkable regeneration capacity after being exposed to injury or trauma and surgical resection [2]. A vast number of animal models are available for carrying out experimental studies regarding the study of liver regeneration, which can be broadly classified into three categories including toxin-mediated models, surgical models, and progressive hepatic damage models [3]. The partial hepatectomy (PH) can be regarded as one of the pre-eminent surgical models for carrying out liver regeneration studies and their substantial impact can be associated with many forms of hepatocyte injury which play a key role in liver-related disorders [4].
The primary management of chronic liver disease (CLD) involves numerous dietary modifications and pharmacological approaches, including abatement in fat-rich food product consumption and reduction in the use of immune-suppressive and antiviral drugs [5]. However, the CLD can progress to the end-stage liver disease, which is characterized by substantial hepatocyte destruction and further accompanied by degradation of the physiological functions. Clinical repercussions in cirrhosis can be attributed to the damage of functional hepatocytes and notable scarring. In such compromised situations, transplantation is the sole alternative for treating severe cirrhosis, but the clinical necessity supersedes the availability of suitable donor organs. Moreover, there are diverse challenges concerned with liver transplantation including rejection of the transplant, donor shortages, complications with long-term use of immunosuppressive medicines, and high cost of the procedure [6]. Thereby, the molecular and cellular mechanisms underlying liver regeneration could provide the path for potential treatment strategies in treating such inimical liver conditions and hence, abolish the need for liver transplantation [7]..
The current scenario for treating cirrhosis emphasizes the necessity for rapid initiation of the regeneration process as an alternative to liver transplantation. Notably, in early phases of liver conditions such as cirrhosis, the regeneration process is enhanced by several regenerative pathways via the use of various pharmacological interventions, which can be regarded as potential treatment options.
Several shreds of evidence have reported the initiation of the Wnt/β catenin signaling pathway via cyclin D1 cascade to be a notable factor for hepatocyte recovery. The TNF-α has an affinity for binding to the receptors on sinusoidal epithelial cells, Kupfer cells, and hepatic stellate cells. Furthermore, this results in the release of the Wnt ligand into the cytoplasm and eventually into the endoplasmic reticulum (ER), where it is activated by the porcupine enzyme [8,9]. The active Wnt protein is considered a crucial stimulator of the typical Wnt/β-catenin signaling pathway that occurs downstream, which further binds to the frizzled receptor present on hepatocytes and then activates the disheveled protein, which inhibits the activity of GSK-3 [10]. Notably, GSK-3β, along with other components such as β-catenin, casein kinase 1, and adenomatous polyposis coli, is engaged in the production of the destruction complex. The phosphorylation of β-catenin at ser45 occurs via casein kinase 1 and furthermore, the GSK-3 enzyme causes the subsequent phosphorylation of serine/threonine residue. All these events result in the creation of the binding site intended for β-transducin repeat-containing protein, β-propeller, and hence, inducing the proteasome-mediated deprivation of β-catenin. Moreover, the cytoplasmic levels of β-catenin will be reduced in the same pathway, acting as an inhibitory factor for Wnt/β-catenin signaling [11]. Khurana and Bedi [12] have hypothesized that GSK?3 β inhibition could promote the proliferation of hepatocytes by stimulating the Wnt/β?catenin signaling pathway, which could further trigger the process of liver regeneration and thereby eliminate the need for liver transplantation and its associated complications.
MATERIALS AND METHODS
Drugs and chemicals
The drugs and chemicals were procured from standard suppliers and were of AR (analytical) grade. CHIR99021 (Purity: >97.0%(HPLC); CAS RN: 252917-06-9; Product number: C2943) was procured from TCI Chemicals (India) Pvt. Ltd., a division of TOKYO chemical industry and prepared its suspension by dissolving in 0.5% CMC-Na/ saline water (solubility: 5 mg/ ml), N followed by ultra-sonication.
Animals and ethics statement
The experiments were conducted following the rules established by the Committee for Control and Supervision of Experiments on Animals (CCSEA), India. Inbred male adult Wistar albino rats (n = 44) weighing 350–450 g were obtained from an animal house facility, Chitkara College of Pharmacy (Chitkara College of Pharmacy Animal Facility Registration Number:1181/PO/ReBi/S/08/CPCSEA) for the employment of current study with ethical approval number IAEC/CCP/23/01/PR-01. The rats were housed in the departmental animal house in polyacrylic cages at 21°C ± 1°C (temperature) and humidity (45%–55%) restricted room maintained on a 12-hour light/dark cycle along with the supply of standard rat chow, drink water ad libitum. The experiments were conducted in a semi-soundproof laboratory.
Surgical procedure
A hepatectomy, removing two-thirds of the liver, was performed following the standard approach. Essentially, the technique included administering ketamine anesthesia to the rat, which was positioned in a fully supine stance with all four limbs secured to the surgical table. The liver was revealed by retracting the abdominal wall via a 3 cm midline laparotomy incision. A silk thread with a diameter of 3–0 was looped around the left lateral and median lobes and then pulled up as much as possible toward their starting point. The lobes were then cut off below the tied thread. This treatment involves the removal of around 70% of the whole liver. All rats had the right and caudate lobes of the liver preserved. Following the repair of the abdominal wall, the rats were monitored until they regained complete consciousness, at which point they were permitted to resume oral feeding after around 3 hours. Following all procedures, the animal was given a dose of a prolonged-acting NSAID and 1.0 ml of isotonic saline by injection. Before the surgery and killing, the weight of each rat was measured to determine that there was no notable difference in the average body weight between the sets. The study included six distinct groups for evaluating the pharmacological intervention. These groups were Control, Sham control, Disease control (PH3 and PH7), and Treatment group (PH3 + CHIR99021 at a dosage of 6.25 mg/kg; PH7 + CHIR99021 at a dosage of 6.25 mg/kg). The details of these groups may be seen in Table 1.
Estimation of serum and histological parameters
The rat blood samples were collected through the retro-orbital plexus to measure different biochemical parameters. These included the serum levels of glucose, high-density lipoprotein (HDL) cholesterol, total cholesterol (TC), and triglyceride as well as the liver enzymatic profile. The measurements were performed by means of kits (diagnostic commercial Methods; Erba Mannheim) and an autoanalyzer chem-5 plus V2 semiautomatic. The amount of blood IL-6 was measured using an ELISA kit obtained from Elabscience (USA). For the last stage of the experiment, histological examination was conducted using H and E staining.
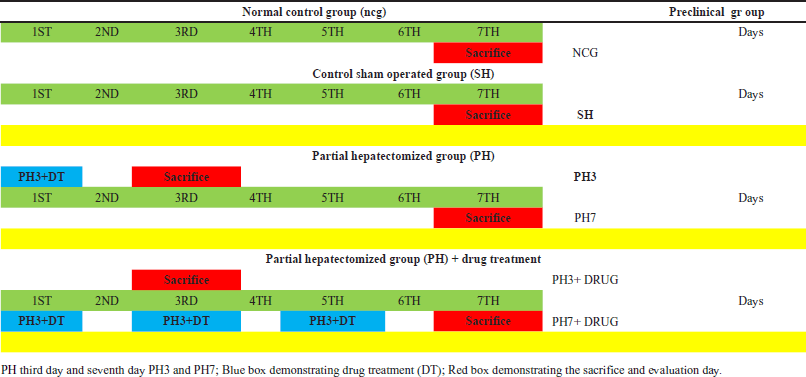 | Table 1. Illustration of detailed preclinical experimentation of partial hepatectomized rats and along with other preclinical rodent groups depicting the partial hepatectomized + Drug Treatment (CHIR99021 6.25mg/kg; i.p) work plan. [Click here to view] |
Assessment of oxidative stress and antioxidant enzyme
The protein content was measured using the modified Lowry method (Standard used: bovine serum albumin), and the reduced glutathione levels were evaluated using Boyne and Ellman’s [13] approach. To investigate the thiobarbituric acid reactive substances (TBARSs), the Ohkawa et al. [14] method was applied. Cold phosphate buffer saline (pH 7.4; 805 g; 50 mM; 4 C) was used to prepare homogenate of liver tissue for 10 minutes. The top layer of supernatants was then collected for glutathione (GSH) and TBARS measurements [13–15].
In silico studies experimentation
The molecular 3D structures were drawn and refined in the DS4.1 workspace. The current work obtained “X-ray crystal structures of” GSK-3β complexed with AMP-PNP at a resolution of 2.40 Å from the protein data bank (PDB ID: 1PYX) [16]. The proteins were synthesized to determine the protonation state using the “Prepare Ligands” routine in DS 4.1 at a pH of 7.0 ± 0.5. Afterward, the resultant compound’s molecular geometry was computed. Molecular docking investigations were conducted using the “CDOCKER module of DS 4.1,” which is as per the “CHARMm force field” [17]. The “define and edit binding site” tool was used to precisely determine the active site, and the ligand that was synthesized was positioned in the active site of AMP-PNP by docking.
Statistical investigation
The statistical program “Graphpad Prism” was used to compare the statistical differences between several rodent investigational groups using “analysis of variance” (“one-way and two-way ANOVA”). The investigational data was presented as the standard error of the mean (mean ± S.E.M) and analyzed using p-values p < 0.05.
RESULT
Effect of GSK-3 β inhibitor (CHIR99021) on body weight in partially hepatectomized rats
It was found that there was no statistically notable difference between all the groups concerned with the body weight on the 0th day, third day, and seventh day (Fig. 1).
Effect of GSK-3 β inhibitor (CHIR99021) on TBARS and reduced glutathione in partially hepatectomized rats
The liver’s lipid peroxidation levels were assessed using the liver homogenate technique, which included determining the concentration of TBARS. There was a large (p < 0.05) increase in the levels of TBARS in the PH3 group when compared with the control group (Fig. 2A). Additionally, it was noted that the PH7 group exhibited a statistically notable (p < 0.05) reduction in TBARS levels compared to the PH3 group, depicting that on postoperative day 7 liver partially healed naturally. Also, the drug-treated group, PH3 + CHIR99021 (6.25 mg/kg) showed a notable (p < 0.05) decrease in the levels of TBARS when compared with thePH3. Moreover, the PH7 + CHIR99021 (6.25 mg/kg) treated group showed a notable (p < 0.05) decrease in the TBARS levels in comparison with the PH3 and PH7 groups. It was also found that PH7 + CHIR99021 (6.25 mg/kg) group showed notably (p < 0.05) dose frequency dependent decreased in the TBARS levels in comparison with PH3 + CHIR99021 (6.25 mg/kg) group.
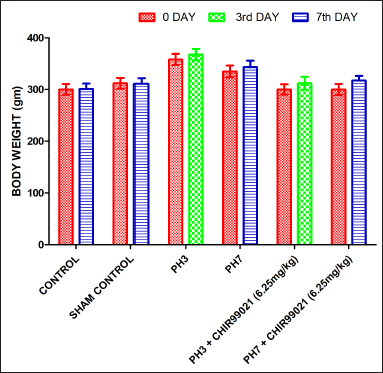 | Figure 1. Effect of GSK-3 β inhibitor (CHIR99021) on body weight in partially hepatectomized rats. [Click here to view] |
It was observed that upon PH, levels of reduced GSH decreased notably (p < 0.05) in the PH3 group when compared with the control group (Fig. 2B). On postoperative day 7 (PH7 group), levels of GSH notably (p <0.05) increased when compared with the PH3 group, which signifies that liver partially healed naturally. The PH3 + CHIR99021 (6.25 mg/kg) group showed a notable (p< 0.05) increase in the GSH levels when compared with the PH3 group. Also, the PH7 + CHIR99021 (6.25 mg/kg) group showed a notable (p < 0.05) increase in the GSH levels when compared with the PH3 and PH7 groups. On comparing both the drug-treated groups, it was found that PH7 + CHIR99021 (6.25 mg/kg) group showed a notable (p < 0.05) increase in the GSH levels as of PH3 + CHIR99021 (6.25 mg/kg) group, indicating that increased frequency of dose triggered the liver regeneration more notably.
Effect of GSK-3 β inhibitor (CHIR99021) on liver profile in partially hepatectomized rats
It was found that post PH the levels of total bilirubin (TB), serum glutamic pyruvic transaminase (SGPT), and serum glutamic-oxaloacetic transaminase (SGOT)were considerably increased (p < 0.05) in the PH3 set compared to the control set in the mouse model of liver regeneration (Fig. 3A–C). The PH7 group shows a notable decrease (p < 0.05) in SGPT, SGOT, and TB levels when compared with the PH3 group which depicted that naturally liver gets partially healed on postoperative day 7. Also, PH3 +CHIR99021 (6.25 mg/kg) treated group notably attenuated (p < 0.05) the levels of SGPT, SGOT, and TB when compared with PH3 set (Fig. 3A–C). It was also observed that the PH7 + CHIR99021 group notably lower (p < 0.05) the levels of SGPT, SGOT, and TB when compared with the PH3, PH7, and PH3 + CHIR99021 groups. The PH3 + CHIR99021 (6.25 mg/kg) group shows hepatoprotective consequence but notably less result was noted as compared to dose frequency limits of the PH7 + CHIR99021 (6.25 mg/kg) group. It was also noted that there was a notable decrease in alkaline phosphatase (ALP) levels in PH7 + CHIR99021 (6.25 mg/kg) group as compared to PH7 and PH3 treated groups (Fig. 3D).
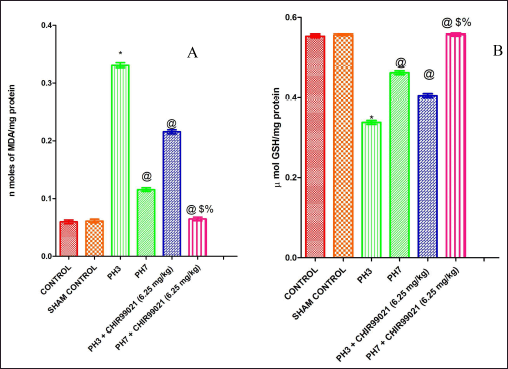 | Figure 2. Effect of GSK-3 β inhibitor (CHIR99021) on TBARS and reduced glutathione in partially hepatectomized rats. [Click here to view] |
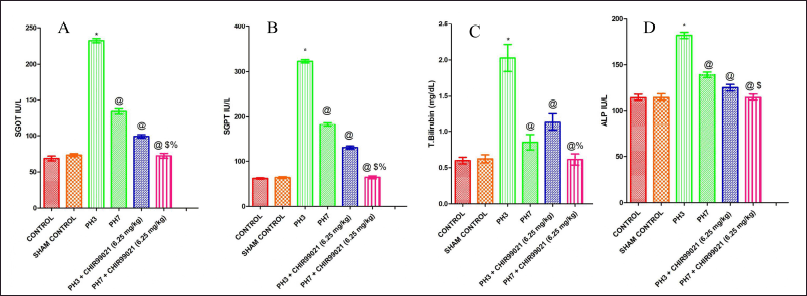 | Figure 3. Effect of GSK-3 β inhibitor (CHIR99021) on liver profile in partially hepatectomized rats. [Click here to view] |
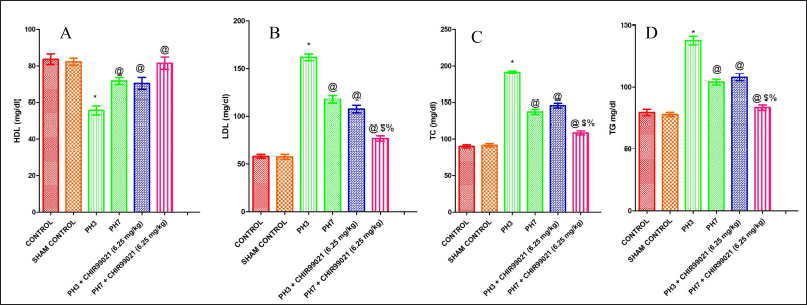 | Figure 4. Effect of GSK-3 β inhibitor (CHIR99021) on lipid profile in partially hepatectomized rats. [Click here to view] |
Effect of GSK-3 β inhibitor (CHIR99021) on lipid profile in partially hepatectomized rats
In terms of HDL, which is regarded as good cholesterol, there was a notable (p < 0.05) decrease in HDL values in the PH3 group as compared to the control group (Fig. 4A). It was observed that the PH7 group has shown notable (p < 0.05) increased in the HDL levels when compared to the PH3 group, which depicted that naturally liver gets partially healed. Both the drug-treated groups (PH3 + CHIR99021 and PH7 +CHIR99021) notably (p < 0.05) increased the levels of HDL when compared with the PH3 group. Also, PH3 + CHIR99021 (6.25 mg.kg) treated group was non-notable with respect to the HDL levels of PH7 group. PH7 + CHIR99021 (6.25 mg/kg) set showed a notable (p < 0.05) increase in the levels of HDL when compared with the PH7 set, possessing CHIR99021 as antihyperlipidemic effect. It was also found that PH7 + CHIR99021 (6.25 mg/kg) group showed notable (p < 0.05) dose frequency-dependent increased in HDL levels as compared to the PH3 + CHIR99021 (6.25 mg/kg) set (Fig. 4A). The low-density lipoprotein (LDL), TC and triglycerides (TG) levels were notably (p < 0.05) increased in the PH3 group when compared with the control set (Fig. 4B–D). It was also found that LDL, TC, and TG levels were notably (p < 0.05) decreased when compared with the PH7 set. There was a statistically notable drop (p < 0.05) in the levels of LDL, TC, and TG in both the drug-treated groups, i.e., PH3 + CHIR99021 (6.25 mg/kg) and PH7 + CHIR99021 (6.25 mg/ kg) in comparison to the PH3 and PH7 group. Also, PH7 + CHIR99021 (6.25 mg/ kg) group showed a notable (p < 0.05) dose frequency-dependent decrease in the levels of LDL, TC, and TG (Fig. 4B–D).
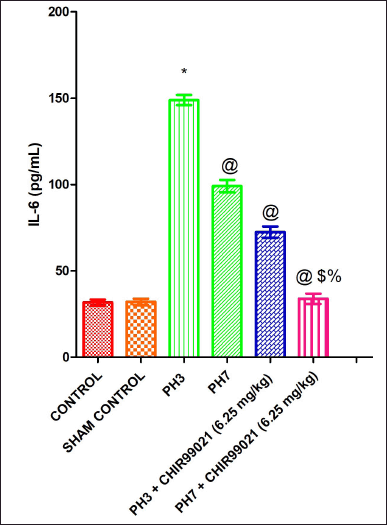 | Figure 5. Effect of GSK-3 β inhibitor (CHIR99021) on IL-6 in partially hepatectomized rats. [Click here to view] |
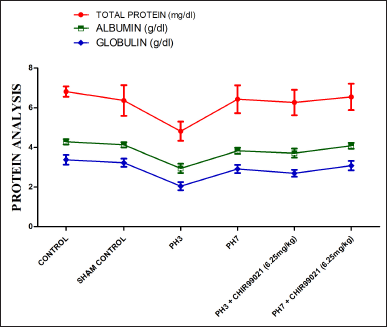 | Figure 6. Effect of GSK-3 β inhibitor (CHIR99021) on total protein, albumin, and globulin in partially hepatectomized rats. [Click here to view] |
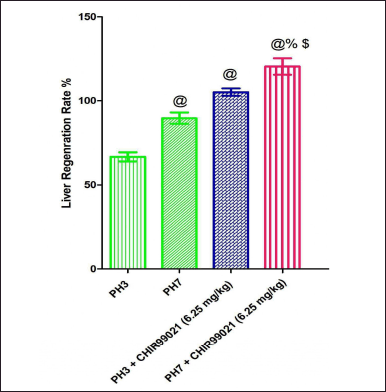 | Figure 7. Effect of GSK-3 β inhibitor (CHIR99021) on liver regeneration rate in partially hepatectomized rats. [Click here to view] |
Effect of GSK-3 β inhibitor (CHIR99021) on IL-6 in partially hepatectomized rats
It was observed that IL-6 levels were notably (p < 0.05) increased in the PH3 group when compared with the control group (Fig. 5). The PH7 group showed notably (p < 0.05) decreased in the IL-6 levels in comparison with the PH3 group. Also, both the drug-treated groups (PH3 + CHIR99021 (6.25 mg/kg) and PH7 + CHIR99021 (6.25 mg/kg) showed notable (p < 0.05) reduced in the levels of IL-6 when contrast with the PH3 group. Moreover, on comparison with the PH3 + CHIR99021 (6.25 mg/kg) and PH7 + CHIR99021 (6.25 mg/kg) group, a notable (p < 0.05) frequency of dose-dependent decrease in the levels of IL-6 was showed by PH7 + CHIR99021 (6.25 mg/kg) group (Fig. 5).
Effect of GSK-3 β inhibitor (CHIR99021) on globulin, total protein, and albumin in partially hepatectomized rats
A statistically notable reduction (p < 0.05) was seen in the range of total protein, albumin, and globulin in the PH3 group in comparison to the normal control set (Fig. 6). It was also noted that levels of the PH7 group were increased notably (p < 0.05) when compared with the PH3 group, which signifies that postoperative day 7 naturally liver gets partially healed. Also, PH3 + CHIR99021 (6.25 mg/kg) and PH7 + CHIR99021 (6.25 mg/kg) group showed notably (p < 0.05) increased in the globulin, total protein, and albumin levels when compared with the PH3 group (Fig. 6). When both the treated groups were compared, PH7 + CHIR99021 (6.25 mg/kg) group showed notably (p < 0.05) increased in the levels of globulin, total protein, and albumin as compared to the PH3 + CHIR99021 (6.25 mg/kg) group, hence, signifies that greater frequency of dose (3 in case of PH7 + CHIR99021 group and 1 in case of PH3 + CHIR99021 group) enhances the serum protein levels and trigger the liver to regenerate fully (Fig. 6).
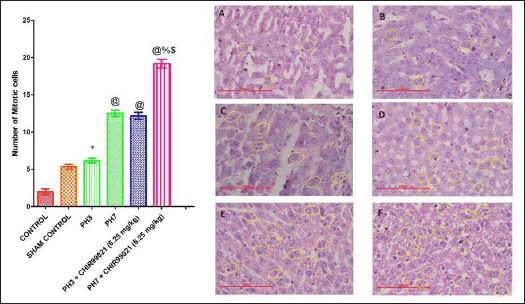 | Figure 8. Effect of GSK-3 β inhibitor (CHIR99021) on mitotic index and illustration of number of mitotic cells with reference to the histopathological description in partially hepatectomized rats [Yellow circles depicted the cells in the mitotic cell phase] (Magnification: 500X). [Click here to view] |
Effect of GSK-3 β inhibitor (CHIR99021) on liver regeneration rate in partially hepatectomized rats
It was observed that post PH, regeneration rate in the PH7 group was notably (p < 0.05) increased when the sets were compared with the PH3 set (Fig. 7A). Also, it was found that both the drug-treated groups [PH3 + CHIR99021 (6.25 mg/kg) and PH7 +CHIR99021 (6.25 mg/ kg)] showed notable (p < 0.05) augmented in the regeneration rate when contrast with the PH3 and PH7 group, depicting that CHIR999021 treatment accelerates the regeneration rate. Moreover, the PH7 + CHIR99021 (6.25 mg/kg) administered group showed notable (p < 0.05) dose frequency-dependent augmentation in the liver regeneration rate in comparison to the PH3 + CHIR99021 (6.25 mg/kg) group (Fig. 7).
Effect of GSK-3 β inhibitor (CHIR99021) on the mitotic index in partially hepatectomized rats
It was observed that microscopic evaluation (Magnification:500X) hepatocytes show regenerative appearance in all the experimental groups but with notable differences (Fig. 8). The hepatocyte regeneration was observed with the help of the mitosis phase of the cells which is characterized by higher karyomegali, binuclear regenerative, swollen nucleus (Fig. 8). Mitosis also observed higher number in the periphery and central region. Also, it was found that the PH3 group showed a notable (p < 0.05) increase in the mitotic index when compared with the control group (Fig. 8). PH7 + CHIR99021 (6.25 mg/kg) group depicted notably (p < 0.05) augmentation in the mitotic index in contrast to the PH3, PH7, and PH3 + CHIR99021 (6.25 mg/kg) group. Moreover, in comparison with both the drug-treated group, it was observed that the PH7 + CHIR99021 (6.25 mg/kg) group showed notable (p < 0.05) dose frequency-dependent increase in the mitotic index (Fig. 8).
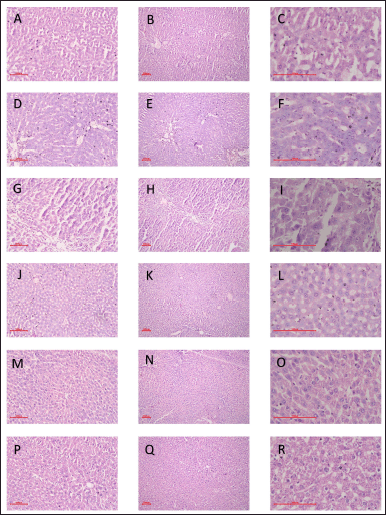 | Figure 9. Effect of GSK-3 β inhibitor (CHIR99021) on liver histological changes in partially hepatectomized rats. [A-C: Control Group; D-F: Sham Control Group; G-I: PH3 Group; J-L: PH7 Group; M-O: PH3 + CHIR99021 (6.25 mg/kg); P-R: PH7 + CHIR99021 (6.25 mg/kg)] (Magnification: 100X;200X;500X). [Click here to view] |
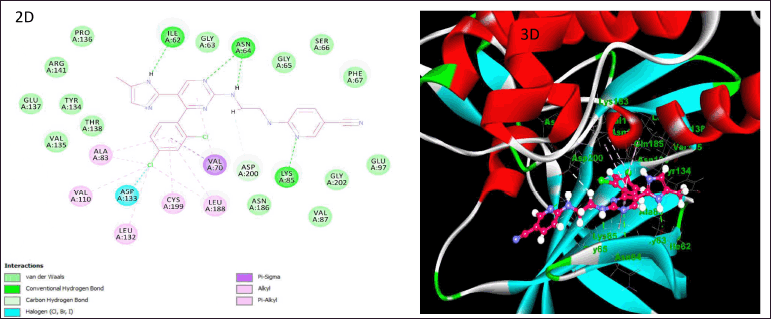 | Figure 10. Illustration of docking studies of CHI99021 and their interactions on the active site of GSK-3β (2D and 3D). [Click here to view] |
Effect of GSK-3 β inhibitor (CHIR99021) on liver histological changes in partially hepatectomized rats.
The histopathological results were shown with three different magnifications (100X, 200X, and 500X) to validate the regeneration process of hepatocytes in all the experimental rodent groups. There notable regeneration was found in the hepatocytes in all the groups of partial hepatectomized groups with and without drug treatment as shown in Figure 9 as compared to the normal control group. The liver’s hepatic lobule, sinusoidal, plate, and hepatic cell structure was seen to be normal in the PH3 + Drug administered, PH7, and PH7 + Drug administered sets, in comparison to the PH3 partly hepatectomized rats. Furthermore, it was noted that the PH7 + Drug and PH3 + Drug treated groups exhibited a notably higher number of mitotic cells compared to both the normal control group and the PH3-treated rats group, as seen in Figure 8. Based on the aforementioned results, it was seen that there was notable regeneration in the PH3 + Drug administered; PH7, and PH7 + Drug administered groups in contrast to the PH3 partly hepatectomized rats.
Illustration of docking studies of CHI99021 and their interactions on the active site of GSK-3β
Docking experiments were performed on GSK-3β using the “CDOCKER module of DS4.1,” which is as per the CHARMm force field. The findings demonstrated notable docking connections between the compounds and the amino acid residues of the enzyme. When CHI99021 was docked into the enzyme’s active site, it notably interacted with the important residues of amino acid along their active site area. The docking interactions of CHI99021 are shown in Figure 10 A and B, using 2D and 3D schematics, respectively. CHI99021 demonstrated substantial aromatic π-π stacking and hydrogen bonding interactions with the crucial amino acid residues located in the active area of GSK-3β. This indicates a beneficial location of the ligand inside the pocket of the GSK 3β enzyme where binding is observed. The primary interactions of significance are conventional hydrogen bonding, hydrophobic interactions (alkyl and π-alkyl), and halogen interactions (chlorine). Compound CHI99021 was resolved by the formation of hydrogen bonds with Ile62, Asn64, and Lys85, halogen bond with Asp133, and finally, hydrophobic interactions with Ala83, Val110, Leu132, Leu188, and Val70. According to the aforementioned findings, essential amino acids and hydrogen bonds both contribute notably to the stability of the ligand-receptor complexes.
DISCUSSION
Liver failure is a rare and progressive clinical condition that is characterized by the rapid loss of functional viability of hepatocytes [18]. According to etiological evidence, liver failure is caused by various disorders, including liver cirrhosis, fibrosis, hepatitis virus (viral infection), and hepatocellular adenocarcinoma, which at the end stage leads to chronic liver injury. After partial liver resection, remaining quiescent liver cells are capable of entering the mitotic phase of cell division. As per research, each leftover hepatocyte has the potential to divide at least 1.6 times to recover the original liver mass fully [19]. Subjects suffering from liver failure still have the chance for the liver to regenerate, and if we trigger this regeneration process by intervening in the molecular signaling via specific targeted molecular agents, we can easily manage the patient’s health. It was demonstrated that there was a pathological change in the levels of various biochemical markers such as liver profile (SGPT, SGOT, ALP, TB, total protein, albumin, and globulin), cholesterol profile (CH, LDL, VLDL, HDL, and TG), and oxidative stress reported in partially hepatectomized rodents as well as in the liver compromised subjects and similar results were also observed in our investigation [20].
As per the literature survey, it was proposed that treatment with CHIR99021, a GSK-3β inhibitor after 1 hour and with consecutive intervals of PH, leads to elevated levels of β-catenin which is involved in the Wnt signaling cascade for triggering the liver regeneration process. The previous studies reported that CHIR99021 is involved in the self-renewal and differentiation of stem cells/ progenitor cells such as embryonic stem cells, and mesenchymal stem cells [12,20,21]. Moreover, CHIR99021 is also involved in the proliferation and regeneration of various cells such as non-small-cell lung cancer cell lines [22], cardio myocytes [23], neuronal progenitor cells [24], bone tissues, hair follicle formation by stimulating human dermal papilla spheroids [25], alveolar epithelial cells and promote lungs regeneration, human corneal endothelial cell proliferation and promotes feather follicle growth and development [26]. It was also demonstrated that the aid of the CHIR99021 drug along with the cocktail of other molecules enhances the differentiation of bipotent progenitor cells into matured hepatocytes and functionally biliary epithelial cells [27]. From this, we get a lead to choose this drug to trigger the Wnt-β catenin pathway for accelerating the liver regeneration process. However, to the best of our knowledge, no in vivo work has been done with respect to liver regeneration via stimulating this signaling cascade in particularly liver-compromised subjects. According to earlier research evidence, it was revealed that there was notable restoration of the liver after 7 days of PH in rodents without the aid of pharmacological intervention [28]. Several reports are there that showed the stimulation of the liver regeneration process other than the Wnt-β catenin pathway in the PH rodents. Therefore, in the current study, the novel signaling pathway was explored to validate the regeneration potential of selected pharmacological intervention.
The current research assessed the considerable reduction in liver function test levels by the CHIR99021 medication (SGOT, SGPT, and ALP) and lipid profile (CH, TG, VLDL, HDL, and LDL) in comparison to the partially hepatectomized group. Similarly, Ohana et al. [29] reported that CF102-treated rats post PH decreased the SGOT and SGPT levels, which are crucial markers for the determination of liver failure. Recently, results related to the decreased lipid profile levels after 70% PH were reported in studies conducted by Jo et al., 2020, as well as similar results were found in present studies. In the present study, the oxidative stress levels were also notably increased in PH rats as compared to the drug-treated rodent groups. There was a notable improvement in the antioxidant levels in the PH + drug-treated rats as compared to the PH rodents group alone.
Molecular docking studies play an important role in the drug design process by predicting the binding location of ligands to their active sites and determining the binding affinity of ligands [30]. GSK-3β is a regulatory serine/threonine kinase that has numerous biological substrates. The GSK-3β protein is comprised of well-defined three nucleotide phosphate groups and two magnesium (mg2+) ions when complexed with AMP-PNP as per their crystal structure. The hydrophobic region of this protein comprised of some important residues Ala83, Val70, Leu132, Val110, Ile62, Tyr134, and Leu188, whereas the hydrogen bonding region consists of some crucial amino acids Asp113, Val135, Glu185, and Arg145 [31]. Therefore, this enzyme is capable of accommodating a variety of different molecular scaffolds. To examine the preferred way in which the test ligand CHI99021 binds to the enzyme GSK-3β, docking research was conducted using the given details.
CONCLUSION
In conclusion, it was concluded that GSK-3β inhibition leads to the progression of the liver regeneration process in partially hepatectomized rats. The selected pharmacological intervention, i.e., CHIR99021 showed a notable hepatoprotective and liver regeneration effect by normalizing the level of liver profile enzymes and by improving the regeneration rate. Moreover, there is an unmet need to validate the in vivo results through different in vitro and in silico studies which is yet to be explored with the help of advanced techniques and biochemical parameters. However, the present evidences give a good insight into CHIR99021 as a better option for preclinical liver regeneration studies for the management of liver cirrhosis and fibrosis.
ACKNOWLEDGMENTS
The authors would like to express their gratitude to the Chitkara University administration for their continued support in the successful completion of this manuscript.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
The experiments were conducted following the guidelines issued by the Committee for Control and Supervision of Experiments on Animals (CCSEA), India. The protocol of this study was approved by IAEC with approval number IAEC/CCP/23/01/PR-01. Inbred male adult Wistar albino rats (n = 44) weighing 350–450 g were obtained from an animal house facility, Chitkara College of Pharmacy (Chitkara College of Pharmacy Animal Facility Registration Number: 1181/PO/ReBi/S/08/CPCSEA).
DATA AVAILABILITY
All the data is available with the authors and shall be provided upon request.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Asrani SK, Devarbhavi H, Eaton J, Kamath PS. Burden of liver diseases in the world. J Hepatol. 2019 1;70(1):151–71. CrossRef
2. Baddour JA, Sousounis K, Tsonis PA. Organ repair and regeneration: an overview. Birth Defects Res C Embryo Today. 2012;96(1):1–29. CrossRef
3. Gijbels E, Pieters A, De Muynck K, Vinken M, Devisscher L. Rodent models of cholestatic liver disease: a practical guide for translational research. Liver Int. 2021;41(4):656–82. CrossRef
4. Mao SA, Glorioso JM, Nyberg SL. Liver regeneration. Transl Res. 2014;163(4):352–62. CrossRef
5. Kumar S, Behl T, Sachdeva M, Sehgal A, Kumari S, Kumar A, et al. Implicating the effect of ketogenic diet as a preventive measure to obesity and diabetes mellitus. Life Sci. 2021;264:118661. CrossRef
6. Behl T, Kumar K, Brisc C, Rus M, Nistor-Cseppento DC, Bustea C, et al. Exploring the multifocal role of phytochemicals as immunomodulators. Biomed Pharmacother. 2021;133:110959. CrossRef
7. Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatol. 2006;43(S1):S45–53. CrossRef
8. Abu Rmilah A, Zhou W, Nelson E, Lin L, Amiot B, Nyberg SL. Understanding the marvels behind liver regeneration. Wiley Interdiscip Rev Dev Biol. 2019;8(3):e340. CrossRef
9. Behl T, Rana T, Alotaibi GH, Shamsuzzaman M, Naqvi M, Sehgal A, et al. Polyphenols inhibiting MAPK signalling pathway mediated oxidative stress and inflammation in depression. Biomed Pharmacother. 2022;146:112545. CrossRef
10. Mohan M, Mannan A, Singh TG. Therapeutic implication of Sonic Hedgehog as a potential modulator in ischemic injury. Pharm Rep. 2023;22:1–23. CrossRef
11. Stamos JL, Weis WI. The β-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5(1):a007898. CrossRef
12. Khurana C, Bedi O. Proposed hypothesis of GSK-3 β inhibition for stimulating Wnt/β-catenin signaling pathway which triggers liver regeneration process. Naunyn Schmiedebergs Arch Pharmacol. 2022;395(3):377–80. CrossRef
13. Boyne AF, Ellman GL. A methodology for analysis of tissue sulfhydryl components. Anal Biochem. 1972;46(2):639–53. CrossRef
14. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95(2):351–8. CrossRef
15. Lowry O, Rosebrough N, Farr AL, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem.1951;193(1):265–75. CrossRef
16. Cheung J, Rudolph MJ, Burshteyn F, Cassidy MS, Gary EN, Love J, et al. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J Med Chem. 2012;55(22):10282–6. CrossRef
17. Wu G, Robertson DH, Brooks III CL, Vieth M. Detailed analysis of grid-based molecular docking: a case study of CDOCKER—A CHARMm-based MD docking algorithm. J Comput Chem. 2003;24(13):1549–62. CrossRef
18. Lee WM. Acute liver failure. Semin Respir Crit Care Med. 2012 Feb;33(1):36–45. CrossRef
19. Furchtgott LA, Chow CC, Periwal V. A model of liver regeneration. Biophys J. 2009;96(10):3926–35. CrossRef
20. Rotariu D, Babes EE, Tit DM, Moisi M, Bustea C, Stoicescu M, et al. Oxidative stress–complex pathological issues concerning the hallmark of cardiovascular and metabolic disorders. Biomed Pharmacother. 2022;152:113238. CrossRef
21. Wu Y, Ai Z, Yao K, Cao L, Du J, Shi X, et al. CHIR99021 promotes self-renewal of mouse embryonic stem cells by modulation of protein-encoding gene and long intergenic non-coding RNA expression. Exp Cell Res. 2013;319(17):2684–99. CrossRef
22. Li C, Zhang S, Lu Y, Zhang Y, Wang E, Cui Z. The roles of Notch3 on the cell proliferation and apoptosis induced by CHIR99021 in NSCLC cell lines: a functional link between Wnt and Notch signaling pathways. PLoS One. 2013;8(12):e84659. CrossRef
23. Fan C, Oduk Y, Zhao M, Lou X, Tang Y, Pretorius D, et al. Myocardial protection by nanomaterials formulated with CHIR99021 and FGF1. JCI insight. 2020;5(12):e132796. CrossRef
24. Pachenari N, Kiani S, Javan M. Inhibition of glycogen synthase kinase 3 increased subventricular zone stem cells proliferation. Biomed Pharmacother. 2017;93:1074–82. CrossRef
25. Yoshida Y, Soma T, Matsuzaki T, Kishimoto J. Wnt activator CHIR99021-stimulated human dermal papilla spheroids contribute to hair follicle formation and production of reconstituted follicle-enriched human skin. BBRC. 2019;516(3):599–605. CrossRef
26. Wang Y, Jin C, Tian H, Xu J, Chen J, Hu S, et al. CHIR99021 balance TGFβ1 induced human corneal endothelial-to-mesenchymal transition to favor corneal endothelial cell proliferation. Exp Eye Res. 2022;219:108939. CrossRef
27. Katsuda T, Kawamata M, Hagiwara K, Takahashi RU, Yamamoto Y, Camargo FD, et al. Conversion of terminally committed hepatocytes to culturable bipotent progenitor cells with regenerative capacity. Cell stem cell. 2017;20(1):41–55. CrossRef
28. Yagi S, Hirata M, Miyachi Y, Uemoto S. Liver regeneration after hepatectomy and partial liver transplantation Int J Mol Sci. 2020;21(21):8414. CrossRef
29. Ohana G, Cohen S, Rath?Wolfson L, Fishman P. A3 adenosine receptor agonist, CF102, protects against hepatic ischemia/reperfusion injury following partial hepatectomy. Mol Med Rep. 2016;14(5):4335–41. CrossRef
30. Francavilla A, Starzl TE, Barone M, Zeng QH, Porter KA, Zeevi A, et al. Studies on mechanisms of augmentation of liver regeneration by cyclosporine and FK 506. Hepatol. 1991;14(1):140–3. CrossRef
31. Bertrand JA, Thieffine S, Vulpetti A, Cristiani C, Valsasina B, Knapp S, et al. Structural characterization of the GSK-3β active site using selective and non-selective ATP-mimetic inhibitors. J Mol Biol. 2003;333(2):393–407. CrossRef