INTRODUCTION
The evergreen Garcinia cowa plant is native to southwestern China but is also found in Asia, Bangladesh, Myanmar, Malaysia, Vietnam, Laos, and Cambodia. The fruit of this plant is known as cowa mangosteen or cowa fruit [1]. In West Sumatra, G. cowa Roxb is referred to as asam kandis. It is extensively dispersed across the Malay peninsula and Indonesia. The sour-tasting fruits are edible and are used as spices in Indonesia, particularly by the Minang tribes [2]. Young leaves of G. cowa are a popular choice as a vegetable, and the plant itself has a long history of use in folk medicine. The bark has been used as an antipyretic and antimicrobial agent [3]. On the other hand, the leaves and fruits are utilized to stimulate blood flow, alleviate indigestion and coughs, and promote laxative and expectorant effects [4]. Found in different parts of the plant, the chemical components known as xanthones and benzophenones have demonstrated anticancer [2], anti-inflammatory [5,6], antioxidant [7], antibacterial [8], and α-glucosidase inhibitory effects [9]. There have been reports that the peel extract of G. cowa fruit can inhibit the growth of certain bacteria, including E. coli, Staphylococcus aureus, Bacillus subtilis, Bacillus cereus, Bacillus coagulan [10] Methicillin-resistant S. aureus (MRSA), and Salmonella typhimurium [11].
Prior studies have demonstrated that endophytic microbes residing in plant tissue can synthesize secondary metabolite compounds found in plants. Secondary metabolite compounds produced by endophytic microbes can resemble those of their host plants. Venieraki (2017) reported that the secondary metabolite compounds produced by endophytic fungi are the same as the metabolites produced by their host plants. Diverse secondary metabolites will be made if the endophytic fungus is isolated and cultured under different conditions; however, these compounds will retain resemblances to those found in the host plant [12].
There is an insufficient number of research reports on endophytic fungi derived from the G. cowa plant, in contrast to the many studies on the bioactivity of secondary metabolic compounds from this plant. The growing issue of antibiotic-resistant bacteria and their control has necessitated the development of novel antibiotics for treating MRSA, which is now most urgent. Hence, this study was to isolate endophytic fungi from G. cowa plants and investigate their capacity to produce antimicrobial substances to impede the proliferation of pathogenic and drug-resistant bacteria, including S. aureus, MRSA, E. coli, and C. albicans.
MATERIALS AND METHODS
Identification of sample material
Garcinia cowa plants were taken from the Medicinal Plant Garden (KTO) of Andalas University, Padang, West Sumatra, during the rainy season in February 2023 (Fig. 1). The plant organs were stem bark, leaves, and roots with a sample weight of 10 grams each. The geographical coordinates of the garden are 0º 54’ 36” South Latitude and 100º 27’ 45” East Longitude. After successful identification, the specimen was stored at the Herbarium of Universitas Andalas in Padang, Indonesia. The voucher number was RIM002011 for plant authentication, and the letter number was 41/K-ID/ANDA/I/2023.
Sample preparation
For samples (leaves, stem bark, and plant roots) that are healthy, not infected with microbes, and have no insect bite wounds, surface sterilization is carried out and planted directly in the growth medium. The samples were washed with running water for 10 minutes and cut into four pieces with a length of approximately 1 cm each. The sample pieces underwent step-by-step sterilization involving immersion in 70% ethanol for 1 minute. They were put in a bleach solution (5.3% NaOCl) for 5 minutes and dipped again in 70% ethanol for 30 seconds. This sterilization process is carried out in laminar airflow. For leaf samples, scraping was carried out on the leaf surface. The sterilized pieces were placed on tissue paper and allowed to stand until the ethanol evaporated. Each part was put on Sabouraud dextrose agar (SDA) media with the cleavage surface attached to the agar medium. Incubation was carried out at 25°C (room temperature) for 3–5 days. On the 5th day, fungal growth was visible around the sample that had been placed on the agar medium. Endophytic fungi grown on SDA isolation media are gradually purified. Each endophytic fungal isolate that had grown was taken from the colonies on the surface of the media with a loop needle and transferred to another SDA medium for growth again. Each pure isolate was made in duplicate on agar slants. Each is a stock culture and culture for research [13].
 | Figure 1. The pictures of Garcinia cowa Roxb. ex Choisy. (A) Leaves. (B) Stems. [Click here to view] |
The cultivation of pure fungi isolates in rice medium and the preparation of extracts
The pure isolate obtained at the purification stage was then cultured on rice media. Each fungus isolate was first cut into 1 × 1 cm slices in a Petri dish. It was then cultured in rice medium and incubated at room temperature for four to six weeks. The fungus isolate reaches its maximum growth potential when it covers the rice entirely. To produce fungus extract, optimally grown pure fungal isolates are macerated with EtOAc in a 1:1 ratio for twenty-four hours before rotary evaporation [13].
Antimicrobial activity screening
The test microbial suspension of S. aureus ATCC 25923, MRSA, E. coli ATCC 25922, and C. albicans (0.5 McFarland) was poured and spread evenly over the surface of the bacterial growth medium nutrient agar (NA), and the fungal growth medium SDA in a Petri disk. Sterile paper discs were dripped with endophytic fungal extract 5% in 10 μL dimethylsulfoxide (DMSO). As positive antibacterial and antifungal controls, we utilized chloramphenicol discs (Oxoid®) with a concentration of 30 μg/disc and nystatin discs (Oxoid®) with a concentration of 100 UI/disc. In addition, we utilized discs containing DMSO as negative controls. Then, each disk was arranged in an orderly on the prepared Petri disk. Petri disks were incubated for 24 hours at 37oC for bacteria and 3–5 days at 25oC for fungi. Based on the clear zone that surrounded the paper disc that contained endophytic fungal extract, antimicrobial activity was observed and determined. A caliper is used to determine the diameter of the clear zone that is produced as a result [14].
Preliminary phytochemical testing of extracts from endophytic fungi
Secondary metabolite and phytochemical screening were performed to identify secondary metabolite groups from the EtOAc extract of endophytic fungi that showed antibacterial activity (inhibition zone more than 10 mm). A typical application of this qualitative chemical approach is determining whether terpenoids, alkaloids, phenols, flavonoids, or steroids are present. The terpenoid test was carried out using Liebermann–Burchard reagent, which changes the color of the extract to green, blue, or violet. The alkaloid test can be performed using the Dragendorff reagent, which causes the extract to turn orange. The phenolic test involves assessing the presence of phenolic compounds, and this can be done using various reagents, such as ferric chloride, which often produces a color change indicating the presence of phenols [15,16].
Molecular identification
Characterizing endophytic fungi entailed a macroscopic examination of each colony, during which its surface, coloration, and margin were evaluated. The molecular identification procedure entailed applying an Internal Transcribed Spacer (ITS) DNA barcode, which was constructed using primer pairs designed explicitly for fungi. The present study was carried out using the methodology that was delineated by Sandrawati et al. [17]. Large subunit ribosomal RNA, ITS 1, 5.8S ribosomal RNA, and ITS 2 are the four distinct components that comprise the ITS region. The sequencing procedure for the polymerase chain reaction product was carried out at First Base, a Malaysian laboratory facility. For species identification, the sequencing data were analyzed following the method described by Tallei and Kolondam [18] and then compared to the NCBI database (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The phylogenetic tree analysis was performed utilizing the neighbor-joining tree technique with 1,000 bootstrap replicates. The investigation was conducted using MEGA 7.0, a software application developed by Kumar et al. [19]which contains many sophisticated methods and tools for phylogenomics and phylomedicine. In this major upgrade, MEGA has been optimized for use on 64-bit computing systems for analyzing bigger datasets. Researchers can now explore and analyze tens of thousands of sequences in MEGA. The new version also provides an advanced wizard for building timetrees and includes a new functionality to automatically predict gene duplication events in gene family trees. The 64-bit MEGA is made available in two interfaces: graphical and command line. The graphical user interface (GUI.
RESULT AND DISCUSSION
Medicinal plants remain to be utilized for their continued health benefits and have been used in traditional medicines for centuries. Presently, medicinal plants are being utilized to extract drugs derived from plants due to their high efficacy and minimal or absent adverse effects. However, because of the low rates at which these products build up in native medicinal plants, access to plant bioactive compounds is hampered. The natural resources of medicinal plants gradually run out [12]. Several studies have demonstrated that endophytic fungi present in medicinal plants can produce secondary metabolites that are pharmacologically active and comparable to those that their host plants produce. Since the identification of the endophytic fungus Taxomyces andreanae in 1993, which synthesizes the bioactive secondary metabolite taxol (paclitaxel) just like its host plant Taxus brevifolia, multiple subsequent investigations have definitively proven that endophytes are capable of producing plant-derived secondary metabolites [20,21]. The study’s findings suggest that endophytic fungi can be an alternative source for discovering new drugs. We have researched the bioactive compounds produced by endophytic fungi found in sea sponges, mangrove trees, and medicinal plants from West Sumatra, Indonesia. This research was based on the original premise [17,22–27].
A substantial amount of research has been carried out to determine the chemical composition and biological properties of various components of G. cowa. Previous studies have investigated the fresh leaves, fruits, and dried rinds of G. cowa and established that the principal constituents are organic acids and their lactones [28].
In the ongoing investigation of bioactive compounds generated by endophytic fungi residing in G. cowa plants, a total of thirteen strains of endophytic fungi were isolated from the leaves, stem bark, and roots of these plants (Fig. 2), which are recognized for their diverse bioactive oxygenated and prenylated xanthones [2].
To obtain endophytic fungal isolates, it was necessary to investigate the characteristics of the various colonies that were obtained from the leaves, stems, and roots of G. cowa. An equal number of endophyte isolates were found in the plant’s stems and leaves. Leaves of endophytic fungi are assigned the GCD code, stems are assigned the GCB code, and roots are given the GCA code. Five fungal strains were found in leaves, more than in other plant organs (Fig. 2). Each isolated fungus was cultivated in rice media. After maximum growth, the secondary metabolite compounds produced by fungi are extracted with EtOAc. This solvent was selected because its characteristics make it semi-polar. This organic solvent attracted all nonpolar to semi-polar secondary metabolite components. The EtOAc extract was then tested for antimicrobial activity.
The results of the antimicrobial activity screening are shown in Table 1 and Figure 3. If the area around the disc shows a clear zone, the content of secondary metabolite compounds inhibits the growth of bacteria and fungi. Out of the endophytic fungi collected, 80% could impede the growth of S. aureus, MRSA, and E. coli in the experiment. However, none of these fungi showed any activity against C. albicans.
At a concentration of 5%, four fungal extracts were observed to be effective against S. aureus and MRSA, while five fungal extracts were used to combat E. coli. Of all the fungi extracts, the fungal isolate known as GCA3 had the highest inhibition diameter, measuring more than 10 mm. The inhibition zone measurements for S. aureus, MRSA, and E. coli were 15.05 ± 0.51 mm, 13.48 ± 0.15 mm, and 14.68 ± 0.5 mm, respectively (Table 2). In addition, the extracts of GCA3 were subjected to phytochemical screening to determine the presence of bioactive compounds, specifically those with strong antimicrobial properties. The presence of phenolic and flavonoid compounds in the GCA3 extract was confirmed by phytochemical screening (Table 3). In addition, it has been demonstrated that the extract of the G. cowa plant contains secondary metabolites. These secondary metabolites include phenolics, triterpenoids, flavonoids, and xanthones within the extract.
The GCA3 fungus has a colony structure that looks like hard skin. The front of the colony is bluish–blue, and the back is yellow to orange. The walls of conidiophores are smooth. Fialid is in the shape of a bottle. The conidia grow in columns that are round to nearly round. The walls are soft, the outside is a little rough, and they are greenish (Fig. 4).
In sequence, molecular identification showed that GCA3 was 100% identical to Penicillium citrinum. The neighbor-joining method with a bootstrap value of 1.000 was used to limit the phylogenetic tree (Fig. 5). The endophytic fungus P. citrinum is known to make several bioactive compounds with different biological effects. Tanzawaic acid is a polyketide compound initially obtained from P. citrinum in Japan in 1997. This compound exhibits antimicrobial activity against a range of bacteria, including S. aureus, Salmonella sp., Klebsiella pneumoniae, E. coli, B. cereus, Proteus mirabilis, Enterococcus faecalis, and C. albicans [29]. Penicitrinine A derived from P. citrinum exhibits anti-proliferative properties on various tumor cells, including HGC-27, SPC-A1, and A-375. Furthermore, this fungus also generates secondary metabolites that, by downregulating Bcl-2 expression and stimulating Bax and SPC-A1 cells [30], can cause apoptosis in A-375 cancer cells. According to Khamthong et al. (2012), the P. citrinum PSU-F51 exhibits bioactivity in the form of cytotoxic and antibacterial effects on KB cells [31]one isochroman (3. According to El-Neketi et al. (2013), cristiquinochroman exhibits cytotoxic activity on L5178Y lymphoma cells [32]. Kumar et al. (2021) published findings on extracting the endophytic fungus P. citrinum from Azadirachta indica. The secondary metabolites these fungi produce exhibit substantial antimicrobial activity against bacteria and fungi harmful to humans. The area where bacterial growth was inhibited ranged from 15 to 20 mm for S. aureus, E. faecalis, and Aeromonas hydrophila. The highest level of inhibition, measuring 29 mm, was observed against Trichophyton mentagrophytes. Through the utilization of thin layer chromatography, gas chromatography–mass spectrometry, proton nuclear magnetic resonance (H NMR), and Carbon-13 (C13) nuclear magnetic resonance (13C NMR) techniques for characterization and structure elucidation, it has been determined that this bioactive compound is identical to milbemycin [33].
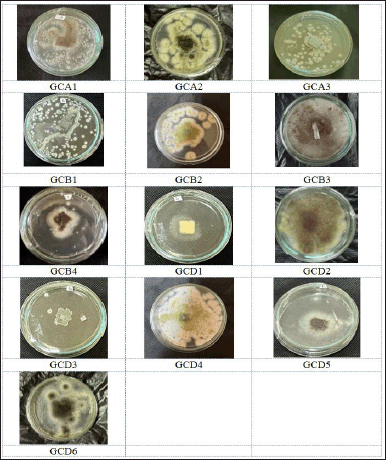 | Figure 2. The pictures of isolated fungal endophytes from Garcinia cowa Roxb. ex Choisy growing on SDA. [Click here to view] |
 | Figure 3. The agar plate pictures of the inhibition zone of fungal isolate extract against the growth of S. aureus (A), MRSA (B), and E. coli (C). [Click here to view] |
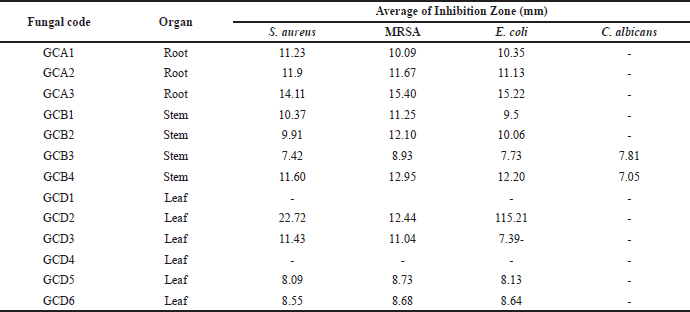 | Table 1. Antimicrobial activity of endophytic fungi from Garcinia cowa Roxb. ex Choisy plant against the pathogenic microbe. [Click here to view] |
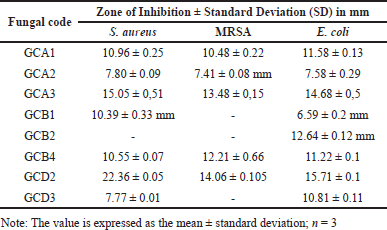 | Table 2. Antimicrobial activity of fungal endophyte. [Click here to view] |
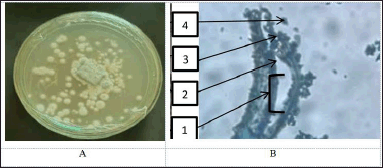 | Figure 4. Macroscopic (A) and microscopic (B) pictures of fungal isolate GCA3. (1). Conidiophore, (2). Metula, (3). Fialide, and (4). Conidia. [Click here to view] |
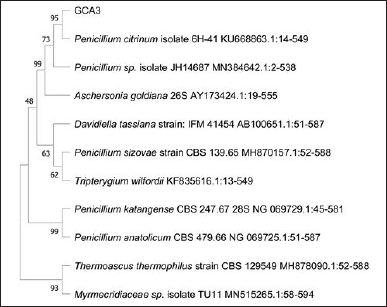 | Figure 5. The phylogenetic tree inferred using the neighbor-joining method of ITS sequence of fungus GCA3 derived from Garcinia cowa Roxb. ex Choisy and its allied taxa. [Click here to view] |
 | Table 3. Phytochemical screening of EtOAc extract of GCA3 from Garcinia cowa Roxb. ex Choisy. [Click here to view] |
As a unique microorganism, P. citrinum can produce bioactive compounds identical or similar to those from its host plant and other bioactive components. The investigation’s findings into the endophytic fungi found on G. cowa plants shed light on the interaction between these microorganisms and their host plants and the variety of naturally occurring bioactive substances these fungi produce. The findings of this research will impact the productivity of several potential candidate compounds by using genetic engineering, microbial fermentation projects, and other effective techniques that can be developed well.
CONCLUSION
The medicinal plant G. cowa offers extensive biological resources in the form of bioactive compounds with significant antibiotic, antioxidant, and anticancer agent potential. This study isolated thirteen endophytic fungi from these plants’ roots, stems, and leaves. The fungal strain GCA3 exhibits significant antibacterial activity, effectively inhibiting the growth of pathogenic bacteria such as S. aureus and MRSA. GCA3 is the same as P. citrinum. A significant obstacle is presented by isolating bioactive chemicals from this fungus to conduct additional research and develop new antibiotic candidates.
ACKNOWLEDGMENT
The authors are grateful to BOPTN of Universitas Andalas, Padang, Indonesia, for their financial support under the project name Indonesian Collaborative Research (RKI) 16 PTNBH Scheme A (Host), 6/UN16.19/PT.01.03/KO-RKI Skema A (Host)/2023.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
CONFLICTS ON INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All of the collected and analyzed data is in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. India Biodiversity Portal. Garcinia cowa Roxb. ex DC. [Internet]. Available from: https://indiabiodiversity.org/species/show/249814
2. Wahyuni FS, Shaari K, Stanslas J, Lajis N, Hamidi D. Cytotoxic compounds from the leaves of Garcinia cowa Roxb. J Appl Pharm Sci. 2015;5(2):006–11.
3. Mahabusarakam W, Chairerk P, Taylor WC. Xanthones from Garcinia cowa Roxb. latex. Phytochemistry. 2005;66(10):1148–53.
4. Lim T. K. Edible medicinal and non-medicinal plants. 1st ed. Australia: Springer Dordrecht; 2012;2, Fruits.
5. Wahyuni FS, Arisanty D, Hayaty NF, Juwita DA, Almahdy. Sub-acute toxicity study of the ethyl acetate fraction of asam kandis rinds (Garcinia cowa Roxb.) on the Liver and renal function in mice. Pharmacogn J. 2017;9(3):345–9.
6. Wahyuni FS, Ali DAI, Lajis NH, Dachriyanus. Anti-inflammatory activity of isolated compounds from the Stem Bark of Garcinia cowa Roxb. Pharmacogn J. 2017;9(1):55–7.
7. Panthong K, Pongcharoen W, Phongpaichit S, Taylor WC. Tetraoxygenated xanthones from the fruits of Garcinia cowa. Phytochemistry. 2006;67(10):999–1004.
8. Sakunpak A, Panichayupakaranant P. Antibacterial activity of Thai edible plants against gastrointestinal pathogenic bacteria and isolation of a new broad spectrum antibacterial polyisoprenylated benzophenone, chamuangone. Food Chem [Internet]. 2012;130(4):826–31. CrossRef
9. Sriyatep T, Siridechakorn I, Maneerat W, Pansanit A, Ritthiwigrom T, Andersen RJ, et al. Bioactive prenylated xanthones from the young fruits and flowers of Garcinia cowa. J Nat Prod. 2015;78(2):265–71.
10. Negi PS, Jayaprakasha GK, Jena BS. Antibacterial activity of the extracts from the fruit rinds of Garcinia cowa and Garcinia pedunculata against food borne pathogens and spoilage bacteria. Lwt. 2008;41(10):1857–61.
11. Siridechakorn I, Phakhodee W, Ritthiwigrom T, Promgool T, Deachathai S, Cheenpracha S, et al. Antibacterial dihydrobenzopyran and xanthone derivatives from Garcinia cowa stem barks. Fitoterapia [Internet]. 2012;83(8):1430–4. CrossRef
12. Venieraki A, Dimou M, Katinakis P. Endophytic fungi residing in medicinal plants have the ability to produce the same or similar pharmacologically active secondary metabolites as their hosts. Hell Plant Prot J. 2017;10(2):51–66.
13. Kjer J, Debbab A, Aly AH, Proksch P. Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. 2010;5(3):479–90.
14. Bauer AW, Perry DM, Kirby WMM. Single-disk antibiotic-sensitivity testing of Staphylococci: an analysis of technique and results. AMA Arch Intern Med. 1959;104(2):208–16.
15. Kishore N, Mishra BB, Tripathi V, Tiwari VK. Alkaloids as potential anti-tubercular agents. Fitoterapia [Internet]. 2009;80(3):149–63. CrossRef
16. Bhardwaj A, Sharma D, Jadon N, Agrawal PK. Antimicrobial and phytochemical screening of endophytic fungi isolated from spikes of Pinus roxburghii abstract screening of bioactive properties of fungal metabolites. Arch Clin Microbiol. 2015;6(3):1–9.
17. Sandrawati N, Hati SP, Yunita F, Putra AE, Ismed F, Tallei TE, et al. Antimicrobial and cytotoxic activities of marine sponge-derived fungal extracts isolated from Dactylospongia sp . J Appl Pharm Sci. 2020;10(04):28–33.
18. Tallei TE, Kolondam BJ. DNA barcoding of sangihe nutmeg (Myristica fragrans) using matK Gene. Hayati J Biosci [Internet]. 2015;22(1):41–7. CrossRef
19. Kumar S, Stecher G, Tamura K. MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33(7).
20. Stierle AA, Stierle DB. Bioactive secondary metabolites produced by the fungal endophytes of conifers. Nat Prod Commun. 2015;10(10):1671–82.
21. Zhao J, Shan T, Mou Y, Zhou L. Plant-derived bioactive compounds produced by endophytic fungi. Mini-Reviews Med Chem. 2011;11(2):159–68.
22. Handayani D, Aminah I, Pontana Putra P, Eka Putra A, Arbain D, Satriawan H, et al. The depsidones from marine sponge-derived fungus Aspergillus unguis IB151 as an anti-MRSA agent: molecular docking, pharmacokinetics analysis, and molecular dynamic simulation studies. Saudi Pharm J [Internet]. 2023;31(9):101744. CrossRef
23. Handayani D, Rivai H, Mulyana R, Suharti N, Rasyid R, Hertiani T. Antimicrobial and cytotoxic activities of endophytic fungi isolated from mangrove plant Sonneratia alba Sm. J Appl Pharm Sci. 2018;8(2).
24. Handayani D, Hafiza H, Rustini R, Putra PP, Syafni N. Isolation of endophytic fungi with antimicrobial activity from medicinal plant Rhodomyrtus tomentosa (Aiton) Hassk. J Appl Pharm Sci. 2023;13(9):190–6.
25. Artasasta MA, Yanwirasti Y, Taher M, Djamaan A, Ariantari NP, Edrada-ebel RA, et al. Apoptotic activity of new oxisterigmatocystin derivatives from the marine-derived fungus Aspergillus nomius NC06. Mar Drugs. 2021;19:631.
26. Handayani D, Rendowati A, Aminah I, Ariantari NP, Proksch P, Biologie P. Bioactive compounds from marine sponge derived fungus Aspergillus unguis WR8. Rasayan J Chem. 2020;13(4):2633–8.
27. Handayani D, Aminah I. Antibacterial and cytotoxic activities of ethyl acetate extract of symbiotic fungi from West Sumatra marine sponge Acanthrongylophora ingens. J Appl Pharm Sci. 2017;7(2):237–40.
28. Jena BS, Jayaprakasha GK, Sakariah KK. Organic acids from leaves, fruits, and rinds of Garcinia cowa. J Agric Food Chem. 2002;50(12):3431–4.
29. Getino M, Fernández-López R, Palencia-Gándara C, Campos-Gómez J, Sánchez-López JM, Martínez M, et al. Tanzawaic acids, a chemically novel set of bacterial conjugation inhibitors. PLoS One. 2016;11(1):1–13.
30. Liu Q, Zhou T, Zhao Y, Chen L, Gong M, Xia Q, et al. Antitumor effects and related mechanisms of penicitrinine A, a Novel Alkaloid with a Unique Spiro Skeleton from the Marine Fungus Penicillium citrinum. 2015;4733–53.
31. Khamthong N, Rukachaisirikul V, Phongpaichit S, Preedanon S, Sakayaroj J. Bioactive polyketides from the sea fan-derived fungus Penicillium citrinum PSU-F51. Tetrahedron [Internet]. 2012;68(39):8245–50. CrossRef
32. El-neketi M, Ebrahim W, Lin W, Gedara S, Badria F, Saad HA, et al. Alkaloids and Polyketides from Penicillium citrinum, an Endophyte Isolated from the Moroccan Plant Ceratonia siliqua. J Nat Prod. 2013;76(6):1099–104.
33. Kumari P, Singh A, Singh DK, Sharma VK, Kumar J, Gupta VK, et al. Isolation and purification of bioactive metabolites from an endophytic fungus Penicillium citrinum of Azadirachta indica. South African J Bot. 2021;139:449–57.