INTRODUCTION
In recent years, the search for environmentally friendly and sustainable extraction techniques for natural products has been intensively studied. Extraction methods using green solvents have attracted much attention because of their potential to reduce harmful environmental impacts and improve the quality of extracted compounds. Various efforts to find innovative and efficient extraction techniques such as minimizing extraction time and selection of selective solvents to obtain higher yield of desired compounds have been conducted [1,2]. The selection of solvent types greatly affects the extraction results. One of the extraction solvent types that is widely being developed is natural deep eutectic solvent (NADES). The use of NADES as an alternative to conventional organic solvents has become a promising green chemistry approach.
NADES is a mixture of compounds that exist in a liquid state at room temperature. They are formed by hydrogen bonding acceptors and donors and are composed of common substances found in everyday food or pharmaceutical excipients. It may be produced rapidly, safely, and in an environmentally sustainable manner [3–5]. Due to the formation of hydrogen bonds between its components, the NADES has a lower melting point than that of the individual component [6,7].
The selection of NADES composition at least should contain components that function as balanced acceptor hydrogen bonds (HBA) and donor hydrogen bonds (HBD) to match the characteristics of the target compound. After mixing, these hydrogen bonds produce a strong interaction, resulting in a eutectic mixture, typically liquid in closed-space conditions. This green solvent has many advantages over conventional organic solvents, such as low toxicity, biocompatibility, and being environmentally friendly. Also, the obtained extracts can be directly used as the final product [8,9].
Microwave-assisted extraction (MAE) is considered efficient in various extraction processes since it offers many benefits including rapid, more selectivity, and improved overall efficiency [10,11]. However, opting for the correct solvent system is crucial to enhance further extraction processes and the results of target compounds. Therefore, combining NADES with MAE is one of the innovative extraction methods that are easy, fast, and effective in extracting secondary metabolite targets of natural products [12].
Eleutherine bulbosa Mill. Urb. is a plant species rich in bioactive compounds such as polyphenols. It has drawn interest from the pharmaceutical, nutraceutical, and cosmetic industries because of its various medical traits or biological activity. Natural polyphenols have been proven to exhibit several beneficial effects in treating diseases including cancer, cardiovascular disease, diabetes, obesity, digestive disease, and neurodegenerative diseases [13].
Eleutherine bulbosa is a member of the Iridaceae family and indigenous to Kalimantan. The local ethnic communities in the area have been using this plant for many generations to treat various illnesses [14]. To optimize its potential, an efficient extraction method is required to get the optimum results for the target bioactive compounds.
Recently, we have developed an extraction method called NADES-based MAE for polyphenol enrichment from E. bulbosa bulbs. Several compositions of NADES have been applied including glucose and citric acid [15], sucrose and lactic acid [16], choline chloride and sorbitol [17], and an optimized extraction method using ethanol as a solvent [18]. Various types of compositions can increase levels of targeted secondary metabolites. In addition, NADES composition is carefully considered to adjust the desired final pharmaceutical products. Therefore, NADES-type exploration is needed to obtain a suitable composition. One of the NADES compositions studied in this research is citric acid and glycerol.
Response surface methodology (RSM) in the design-expert software is often used to observe the reciprocal influence between extraction conditions and responses. The box–behnken design (BBD) of RSM operates at three levels (low, medium, and high) and requires few experiments. RSM is a highly effective technique utilized to optimize intricate extraction processes that involve a multitude of variables [19–21].
In this study, we have formulated and tested citric acid and glycerol as NADES composition for their potential as an alternative solvent with MAE of total polyphenol content (TPC) in E. bulbosa bulbs. Microwave irradiation power and time, the ratio of solid to liquid in the mixture of NADES and MAE, and the NADES (CA-Gly) ratio are a few extraction factors that can be employed as variables. The RSM can be applied to create optimization strategies. By examining the correlations between various factors in the response, RSM is a statistical technique that may determine the best state of MAE based on the type of the target’s secondary metabolites [19]. This study aims to evaluate and improve the performance of the NADES (CA-Gly)-based MAE extraction conditions on the yield value of TPC using RSM with BBD.
MATERIAL AND METHODS
Materials and equipment
The plant material of E. bulbosa bulb (voucher specimen number: 112/RIIM-LP/FFUNMUL/II/2023) was collected from Kutai-Kertanegara District Indonesia and authenticated by Prof. Paulus Matius in the Laboratory of Dendrology, Universitas Mulawarman. The chemicals and equipment used in this study were glycerol and citric acid (Merck); distilled water, ethanol, and methanol (PT SmartLab Indonesia, Indonesia); Na2CO3, gallic acid, and Folin-Ciocalteau reagent (Merck). A UV-VIS spectrophotometer double beam (Shimadzu, Japan), a food dehydrator (Wiratech, Indonesia), and a microwave 900 watts (Modena, Buono-MV 3002, USA), the purity of the chemical material was checked to ensure it met the required standards before the experiment.
Preparation of NADES from citric acid and glycerol
Citric acid and glycerol were combined to generate citric acid-glycerol (CA-Gly) NADES [22]. The mixture of CA and Gly was heated in a water bath at 50°C for 30–40 minutes with continuous stirring. After a viscous CA-Gly NADES was obtained, 30% of distilled water from the total volume of NADES was added to the solution. The CA-Gly NADES liquid was kept at room temperature, until further use [15].
Extraction procedure
The MAE was conducted by following previous studies and using a combination of glycerol and citric acid as NADES composition [15–18]. Briefly, dried E. bulbosa bulb powder (8 g) and a NADES CA-Gly solution were mixed, and then extracted under a certain MAE condition (Table 1). Then, the extract solution was filtered using a Buchner filter, which took around 20 minutes to complete at room temperature. The extract solution obtained was concentrated and dried using a food dehydrator. Condensed or dry extracts are stored in tightly closed containers until the TPC determination is done.
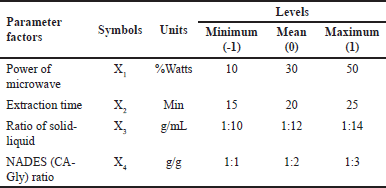 | Table 1. Experimental design of CA-Gly-based NADES-MAE. [Click here to view] |
Determination of yield value of TPC
The TPC value was determined by following previous studies [16], with some modifications. Briefly, 50 ml of aqua demineralization was added to 1 ml (100 g/ml) of the diluted extract along with 5 ml of Folin-Ciocalteau solution. The mixture was incubated for 5 minutes. Subsequently, 2 ml of diluted NaCO3 was added to the preparation, which was re-incubated for 30 minutes. The final absorbances were quantified with a wavelength of 761 nm UV-VIS spectrophotometer. The equation of Y = 0.0046X–0.0075 with R2 = 0.9994 is used to express the yield value of TPC (mg GAE/g DW extracts) at different concentrations [15,17], where X is the yield value of TPC and Y is the absorbance.
Screening of single-factor
The main factors affecting the CA-Gly-based MAE on TPC value including microwave irradiation power, irradiation time, solid–liquid ratio, and NADES CA-Gly ratio were tested by single-factor experiments.
Single-factor screening aims to determine each extraction condition’s level (minimum, mean, and maximum). The screening process was carried out in accordance with our previous studies. The level of investigated factor was varied, while other parameters were kept consistent [16,17]. In detail, the effects of microwave power were screened by setting various percentages of power (%Watts) at 10%, 30%, 50%, 70%, and 90% Watts while keeping the other tested factors constant. As for the variation of irradiation times, it ranged from 10, 15, 20, 25, and 30 minutes. The effects of NADES ratio were screened by varying the ratio of CA-Gly at the ratio of 2:1, 1:1, 1:2, 1:3, and 1:4 g/g. For the ratio of solid–liquid effects, it was observed by modifying the ratio at 1:8, 1:10, 1:12, 1:14, and 1:16 g/ml. When a factor was screened, the other three factors were set constant as the following conditions: CA-Gly ratio of 1:1, extraction time of 15 minutes, microwave power of 30% Watts, and solid-to-liquid ratio of 1:10. Five grams of the dried sample was used in each screening process. Each level of factors was performed in triplicate.
Statistical analysis for optimization
To optimize the condition of CA-Gly-based MAE, statistical analysis of RSM using BBD was performed. Based on the results from screening of single factors, the TPC values were employed as responses or dependent variables to determine the optimum extraction conditions, while each factor (i.e., extraction time, solid–liquid ratio, microwave power, and NADES CA-Gly ratio) was used as an independent variable (Table 1). Using a 4-factor and 3-level design method, BBD of RSM generated 29 runs (each with one block and five center points) for response surface testing created, and the ideal processing conditions were identified. The multilinear quadratic regression model analysis was estimated with the Design Expert v13 program.
RESULT AND DISCUSSION
Single-factor effect
The total polyphenolic compounds from E. bulbosa bulbs were enriched utilizing a mixture of glycerol and citric acid as a NADES composition, and the impact of a single component at the screening stage of numerous extraction conditions at different levels was assessed. Several levels of each factor were used in a single-factor analysis, as indicated in Table 1. Figure 1 displays the impact of a single element on the TPC value in the meantime.
The power of the microwave at 10%–90% affects the yield value of TPC, as demonstrated in Figure 1A. The graph displays the highest TPC value at 30% or 270 watts of microwave power. These results align with our previous studies [15,16]. The temperature rises when the microwave power is increased. A high-temperature increase may cause the sample matrix to deteriorate, which may also change the structure of the target compounds [21,23]. As a result, 30% of the microwave power was employed for the extraction process optimization in this investigation.
The extraction time impacts the yield of the TPC during the extraction process, where the expected condition is to get a high TPC with a short extraction time. Figure 1B illustrates the effect of extraction time as a single factor on TPC value using NADES Ca-Gly-MAE. Overall, 20 minutes was the optimum condition for the extraction time in the single-factor screening. The given chart shows that there was an increase in TPC as the extraction time increased from 10 to 20 minutes. However, the yield value of TPC reduced steadily over a 20 to 30-minute period as time went on. This may be due to the hydrolysis of phenolic compounds at a high temperature for more than 20 minutes. In addition, a longer extraction process may cause the polyphenolic hydroxyl groups of the samples to be oxidized [22,24].
The effectiveness of extraction using different ratios of sample (solid) to solvent (liquid) is shown in Figure 1C. The TPC yield increased as the ratios of solid–liquid decreased from 1:8 to 1:12 g/ml. However, the yield decreased as solid–liquid ratio continued to decrease from 1:14 to 1:16 g/ml. The sample-to-solvent ratio of 1:12 g/ml was found to be the optimum ratio to attract the phenolic compounds in the sample. The driving force, mass transfer rate, and ease of solute dissolution in the solvent all rise with an enormous difference in solute concentration, improving extraction effectiveness. Moreover, as the solvent-to-sample ratio decreases, the surface area between the solvent and the plant matrix rises [2]. On the other hand, the reason for the decline in the TPC yield upon decreasing the solid/solvent ratio from 1:12 to 1:16 g/ml was the insufficient solvent volume to facilitate the extraction of phenolics content from the material matrix [25]. The excessive volume of solvent-dissolving impurities and the targeted solute, where these unwanted impurities/compounds can prevent the solute from being extracted by the solvent. In addition, overly declining the solvent ratio can result in poor extraction (waste of solvent) [6,26,27]. As a result, the ratio between the sample and solvent was 1:12 g/ml, which was chosen for further condition optimization.
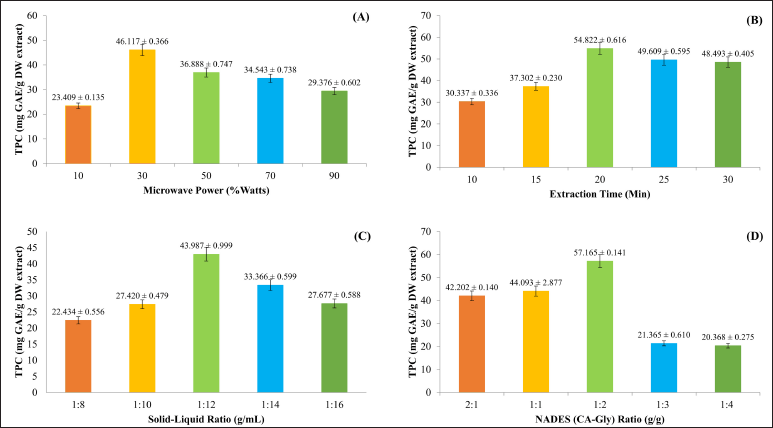 | Figure 1. The effect of a single-factor of NADES-MAE method on the yield value from E. bulbosa bulbs (in triplicate). [Click here to view] |
The ratio of the citric acid and glycerol combination affects the yield of TPC in several different ranges, including 2:1, 1:1, 1:2, 1:3, and 1:4 g/g. Figure 1D shows that the highest TPC yield was obtained at NADES CA-Gly 1:2 g/g ratio. Citric acid and glycerol act as HBA and HBD respectively in the NADES [25,28–30]. The combination of HBA–HBD at a certain concentration will form a stable solution that can affect the physicochemical properties and behavior of the eutectic solvent phase at a certain point. The components will form hydrogen bond interactions leading to an eutectic phenomenon [31].
Statistical analysis and the model fitting
After the single-factor screening was conducted, an investigation into experimental design was applied. To determine the ideal extraction condition and investigate the interactions between each factor, a BBD with 29 trials and four factors with three levels each were used. The 29 trials were conducted in the laboratory to obtain the actual data (TPC yield) after which the data were inserted into the software (Design Expert v13) to generate the best equation based on the statistical parameters such as coefficient correlation (R), predicted R2, adjusted R2, F-value, p-value, and Lack of Fit value.
The data from Table 2 were treated for quadratic regression analysis and ANOVA as suggested by the outcomes of statistical computations (Table 3). Fit Summary gathers crucial data to choose the best model starting circumstances.
According to the ANOVA (Table 4), the p-value of the model (with an F-value of 15.35) was < 0.0001, which indicates that this model is highly significant. These results illustrate that the independent variables of the selected extraction conditions (power, time, solid–liquid, and NADES ratio) have an interaction effect that causes significant variations in the resulting responses. Each coefficient that has p-values not greater than 0.05 (p < 0.05) indicates remarkable influences on TPC values. Four linear coefficients including the power of microwave (X1), extraction time (X2), ratio of solid–liquid (X3), and NADES (CA-Gly) ratio (X4), two interactive coefficients (X1X3 and X1X4), and two quadratic coefficients (X22 and X42). In addition, the coefficients with p-values greater than 0.05 (p > 0.05) are not considered as significant factors in the extraction process. For instance, the interactive coefficients between the microwave power and the extraction time (X1X2); and the interaction between the extraction time and the solid–liquid ratio (X2X3) did not significantly affect the TPC value. Furthermore, the lack-of-fit F-value and p-value were 0.9564 and 0.5759, respectively, indicating that it is not significant in comparison to imply the lack (nonsignificant lack-of-fit is acceptable). Therefore, the regression equation model may adequately explain the outcomes and predict ideal conditions with high precision. The Fit Statistic parameters (namely coefficient correlation (R2), predicted R2, and adjusted R2) suggested by the software (DesignExpert v13) were used to obtain the best coefficient regression model. Based on the p-value and resulting Fit Statistics, the coefficients can be included or excluded as a model to obtain R2 which is close to the value of 1, and the value difference between the predicted and adjusted model is not more than 0.2. These conditions describe the best model to be included. According to the R2 = 0.9201, the model can explain 92.01 % variation. As demonstrated by the data, the model can forecast most of the variability in the extraction results. The Predicted R2 of 0.7153 and the Adjusted R2 differ by less than 0.2, which is relatively consistent [19,32–34].
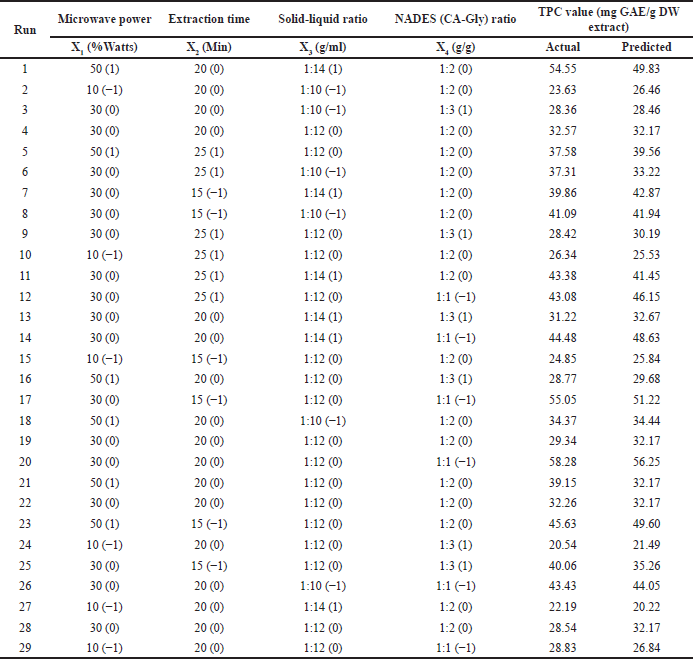 | Table 2. Experimental results of TPC from E. bulbosa bulb. [Click here to view] |
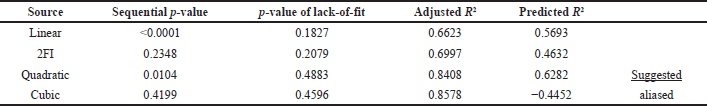 | Table 3. Fit summary. [Click here to view] |
The following equation shows the linear correlation between the four independent factors and the yield value of TPC as a response, Y = 278.778 + 0.172X1 – 10.022X2 – 22.630X3 – 14.057X4 – 0.024X1X2 + 0.135X1X3 – 0.265X1X4 + 0.182X2X3 – 0.005X12 + 0.201X22 + 0.670X32 + 3.509X42, where Y is dependent variable (TPC value) as a response, X1, X2 X3, and X4 are dependent variables or factors (extraction condition).
The estimates of the coefficients, if all other variables remain constant, show the anticipated change in reaction when the factor value changes by one unit (As can be seen in Table 5). The overall average reaction across all trials in an orthogonal design is known as the intercept. Using the factor settings as a guide, the coefficient modifies the average value. The variance inflation factor (VIF), greater than 1, denotes the presence of multicollinearity and will be equal to 1 if the factors are orthogonal. The more substantial the correlation between the components, the higher the VIF score. A VIF score of less than ten is typically considered appropriate [35–37].
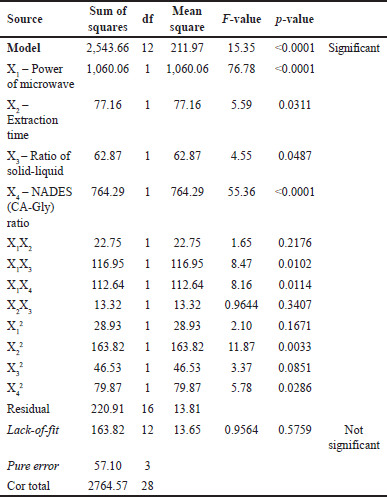 | Table 4. ANOVA for reduced quadratic model. [Click here to view] |
Based on the equation correlating with the actual factors, each element exhibits a specific response level that can be predicted. It is essential to express the levels of each factor in their order of appearance. However, when using this equation to calculate the relative value of each feature, care must be taken because the cutting is not in the middle of the design space. In addition, the obtained coefficients are only adjusted to represent all factors’ units [36,38].
The optimization condition of CA-Gly NADES-based MAE
The interplay between the two variables may impact the extraction efficiency. Simulating and examining the relative significance level of each interaction between these factors is possible, as depicted in Figure 2. The interaction of process variables with response values can be seen using a three-dimensional graph of the response surface, and the slope of the surface corresponds to the apparent interaction effect. The regression equation and this graph are combined to explain the response surface pattern. The relationship between microwave power and the NADES (CA-Gly) ratio (X1X4) is contrasted with the relationship between other parameters in Figure 2. The yield value of TPC increases with the increase in the percentage of microwave power at a low NADES (CA-Gly) ratio, or vice versa, although it is still significantly influenced by the other two factors.
Therefore, the optimal conditions determined by the RSM were used in the validation experiment. These optimal conditions were obtained through RSM analysis, namely 50% microwave power for 15 minutes, 1:14 g/ml solid–liquid ratio, and 1:1 g/g ratio of NADES CA-Gly with a predicted yield value TPC of 74.749 ± 3.716 mg GAE/g DW extract. Confirmation experiments were carried out using this optimal extraction parameter three times, and the resulting TPC value was 84.653 ± 0.918 mg GAE/g DW extract. The model’s accuracy is calculated and reaches 88.3% for the TPC value obtained based on the confirmation results, with values within the low and high 95% confidence interval (CI), indicating that the response model used has good reliability for optimization.
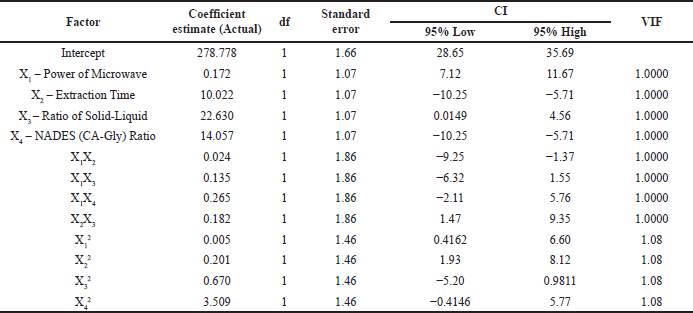 | Table 5. The predicted change in responsiveness for each unit change is shown by the coefficient estimate. [Click here to view] |
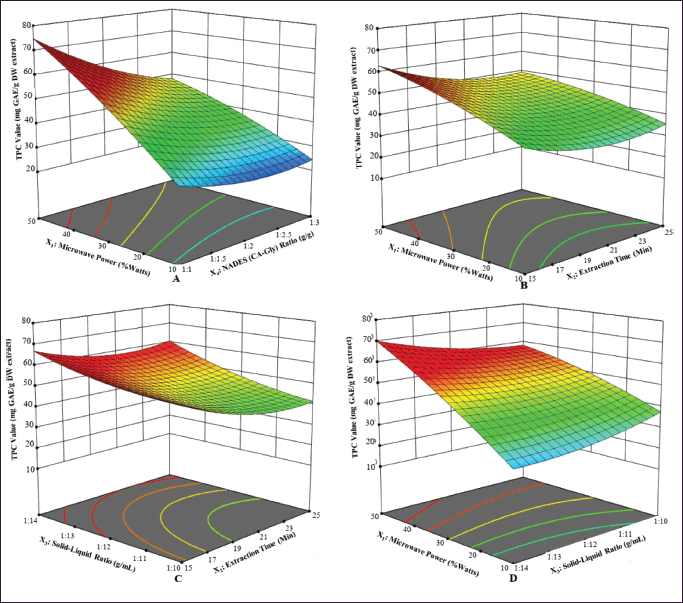 | Figure 2. Contour plot response surface diagrams (three-dimensional) of each factor show the comprehensive effects of experimental factors on the yield of the TPC value of E. bulbosa. A is X1X4, B is X1X2, C is X2X3, and D is X1X3. [Click here to view] |
In the present study, the use of NADES CA-Gly (1:1) with a certain condition resulted in the highest level of TPC compared to other NADES compositions. Based on our previous studies, the extraction of TPC in E. bulbosa bulbs using MAE and NADES with different compositions including choline chloride-sorbitol (1:1) [17], citric acid-glucose (1:1) [15], lactic acid-sucrose (4:1) [16] resulted 39.88 mg GAE/ g, 61.63 mg GAE/g, and 82.43 mg GAE/ g sample, respectively. Moreover, the extraction of the phenolic on the dried powder of E. bulbosa bulbs using maceration with 96% ethanol yielded a TPC value of 2.5 mg GAE/g [39]. These findings show that using a mixture of citric acid and glycerol as a NADES composition has proven to be more effective than the previously reported NADES composition, with successive levels of effectiveness [15–18]. The results of this investigation shed light on the possibility of the NADES CA-Gly solvent system as an efficient and long-lasting method of extracting polyphenols from E. bulbosa bulbs.
The results of this work can contribute to the development of more environmentally friendly and efficient extraction techniques for natural product research, offering application opportunities in various industries, including pharmaceuticals, nutraceuticals, and cosmetics.
CONCLUSION
In this work, we revealed that a mixture of citric acid and glycerol as a NADES composition as an alternative green solvent could extract polyphenols from E. bulbosa bulbs, which is better or the same compared to the NADES composition in our previous studies. A variety of NADES composition options are expected to provide considerations for selecting solvents based on the intended final pharmaceutical dosage form. Based on the RSM analysis results, the ideal parameters were a 1:14 g/ml solid–liquid ratio, a 1:1 g/g NADES (CA-Gly) ratio, and 50% microwave power for 15 minutes. It has now been established that these conditions can result in extracts with higher TPC values. However, given that NADES species are selective in extracting chemicals from the polyphenol group, additional study is required to evaluate each extract from various types of NADES composition for activity.
ACKNOWLEDGMENT
Many Thanks to Deputi Bidang Fasilitas Riset dan Inovasi, National Research and Innovation Agency (BRIN) and Educational Fund Management Institution (LPDP), Ministry of Finance, Republic of Indonesia, provided financial assistance for this study under Program RIIM Gelombang 2 Tahun 2022, with grant number 82/II.7/HK/2022.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Khoddami A, Wilkes M, Roberts T. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18(3):2328–75. doi: 10.3390/molecules18022328
2. Ahmad I, Yanuar A, Mulia K, Mun’im A. Ionic liquid-based microwave-assisted extraction: fast and green extraction method of secondary metabolites on medicinal plant. Pharmacogn Rev. 2018;12(23):20.
3. Janicka P, P?otka-Wasylka J, Jatkowska N, Chabowska A, Fares MY, Andruch V, et al. Trends in the new generation of green solvents in extraction processes. Curr Opin Green Sustain Chem. 2022;37:100670.
4. Hashemi B, Shiri F, Švec F, Nováková L. Green solvents and approaches recently applied for extraction of natural bioactive compounds. Trends Anal Chem. 2022;157:116732.
5. Chemat F, Vian MA, Cravotto G. Green extraction of natural products: concept and principles. Int J Mol Sci. 2012;13(7):8615–27.
6. Shishov A, Pochivalov A, Nugbienyo L, Andruch V, Bulatov A. Deep eutectic solvents are not only effective extractants. Trends Anal Chem. 2020;129:115956.
7. Liu Y, Friesen JB, McAlpine JB, Lankin DC, Chen SN, Pauli GF. Natural deep eutectic solvents: properties, applications, and perspectives. J Nat Prod. 2018;81(3):679–90.
8. Espino M, Fernández M de los Á, Gomez FJV, Boiteux J, Silva MF. Green analytical chemistry metrics: towards a sustainable phenolics extraction from medicinal plants. Microchem J. 2018;141:438–43.
9. Kua YL, Gan S. Natural deep eutectic solvent (NADES) as a greener alternative for the extraction of hydrophilic (Polar) and lipophilic (Non-Polar) phytonutrients. Key Eng Mater. 2019;797(4):20–8.
10. Zhang HF, Yang XH, Wang Y. Microwave assisted extraction of secondary metabolites from plants: current status and future directions. Trends Food Sci Technol. 2011;22(12):672–88.
11. Sagarika N, Prince MV, Sreeja R. Review on microwave assisted extraction technique. Int J Pure Appl Biosci. 2017;5(3):1065–74.
12. Ahmad I, Hikmawan BD, Sulistiarini R, Mun’im A. Peperomia pellucida (L.) Kunth herbs: a comprehensive review on phytochemical, pharmacological, extraction engineering development, and economic promising perspective. J Appl Pharm Sci. 2023;13(1):001–9.
13. Stromsnes K, Lagzdina R, Olaso-gonzalez G, Gimeno-mallench L, Gambini J. Pharmacological properties of polyphenols: bioavailability, mechanisms of action and biological effects in in vitro studies, animal models and humans. Biomedicines. 2021;9(8):1074.
14. Naspiah N, Iskandar Y, Moelyono MW. Review article: Tiwai Onion (Eleutherine americana Merr.), multifunction plant. Indones J Appl Sci. 2014;4(2):18–30. doi: https://doi.org/10.24198/.v4i2.1680
15. Yusuf B, Astati SJ, Ardana M, Herman H, Ibrahim A, Rijai L, et al. Optimizing natural deep eutectic solvent citric acid-glucose based microwave-assisted extraction of total polyphenols content from Eleutherine bulbosa (Mill.) bulb. Indones J Chem. 2021;21(4):797–805.
16. Ahmad I, Kurnya, Iswahyudi I, Suhardi H, Hikwaman BD, Putra AR, et al. The optimum condition design of microwave-assisted extraction combined lactic acid-sucrose based natural deep eutectic solvent on polyphenols enrichment from Eleutherine bulbosa Mill. Urb. Bulbs. J Res Pharm. 2023;27(3):1086–95.
17. Rijai L, Tang ST, Priastomo M, Siska S, Indriyanti N, Ambarwati NS, et al. Microwave-assisted extraction of polyphenols from Eleutherine bulbosa Mill. Urb. bulbs using choline chloride-sorbitol based natural deep eutectic solvent. J Appl Pharm Sci. 2023;13(6):217–24.
18. Ahmad I, Narsa AC, Ramadhani MR, Zamruddin NM, Iswahyudi I, Hajrah H, et al. Optimization of microwave-assisted extraction on polyphenol metabolite from Eleutherine bulbosa (Mill.) urb. bulbs using response surface methodology. J Adv Pharm Technol Res. 2023;14(2):113–8.
19. Alam P, Siddiqui NA, Rehman MT, Hussain A, Akhtar A, Mir SR, et al. Box-Behnken design (BBD)-based optimization of microwave-assisted extraction of parthenolide from the stems of Tarconanthus camphoratus and cytotoxic analysis. Molecules. 2021;26(7):1876.
20. Alam ST, Amin MA. Determining optimum design parameters of foldable product using response surface methodology and genetic algorithm. Engineering. 2020;12(11):839–50.
21. Khajeh M. Optimization of microwave-assisted extraction procedure for zinc and copper determination in food samples by Box-Behnken design. J Food Compos Anal. 2009;22(4):343–6.
22. Kurtulba? E, Pekel AG, Bilgin M, Makris DP, ?ahin S. Citric acid-based deep eutectic solvent for the anthocyanin recovery from Hibiscus sabdariffa through microwave-assisted extraction. Biomass Convers Biorefinery. 2022;12(2):351–60.
23. Riswanto FDO, Rohman A, Pramono S, Martono S. Application of response surface methodology as mathematical and statistical tools in natural product research. J Appl Pharm Sci. 2019;9(10):125–33.
24. Bobo-García G, Davidov-Pardo G, Arroqui C, Vírseda P, Marín-Arroyo MR, Navarro M. Intra-laboratory validation of microplate methods for total phenolic content and antioxidant activity on polyphenolic extracts, and comparison with conventional spectrophotometric methods. J Sci Food Agric. 2014;95(1):204–9.
25. Shang X, Tan JN, Du Y, Liu X, Zhang Z. Environmentally-friendly extraction of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves with deep eutectic solvents and evaluation of their antioxidant activities. Molecules. 2018;23(9):2110.
26. Shang X, Chu D, Zhang J, Zheng Y, Li Y. Microwave-assisted extraction, partial purification and biological activity in vitro of polysaccharides from bladder-wrack (Fucus vesiculosus) by using deep eutectic solvents. Sep Purif. 2021;259:118169.
27. Kurtulba? E, Pekel AG, Bilgin M, ?ahin S. Microwave-aided extraction of bioactive materials from Mandarin leaves: deep eutectic liquids versus conventional solvents. Biomed J Sci Tech Res. 2019;23(2):17269–73.
28. Oktaviani ND, Kartini K, Hadiyat MA, Rachmawati E, Wijaya AC, Hayun H, et al. A green extraction design for enhancing flavonoid compounds from Ixora javanica flowers using a deep eutectic solvent. R Soc Open Sci. 2020;7(10):201116.
29. Sun Y, Liu D, Chen J, Ye X, Yu D. Effects of different factors of ultrasound treatment on the extraction yield of the all-trans-β-carotene from citrus peels. Ultrason Sonochem. 2011;18(1):243–9.
30. Lorenzetti AS, Vidal E, Silva MF, Domini C. Native fluorescent natural deep eutectic solvents for green sensing applications: curcuminoids in curcuma longa powder. ACS Sustainable Chem Eng. 2021;9:15.
31. Rodríguez-Martínez B, Ferreira-Santos P, Alfonso IM, Martínez S, Genisheva Z, Gullón B. Deep eutectic solvents as a green tool for the extraction of bioactive phenolic compounds from avocado peels. Molecules. 2022;27(19):6646.
32. Mulia K, Krisanti E, Terahadi F, Putri S. Selected natural deep eutectic solvents for the extraction of α-mangostin from mangosteen (Garcinia mangostana L.) pericarp. Int J Technol. 2015;7:1211–20.
33. Kurtulbas E. Microwave-assisted extraction of Prunus cerasus L. Peels: citric acid- based deep eutectic solvents. J Turkish Chem Soc. 2022;9(2):433–42.
34. Franklin DS, Guhanathan S. Investigation of citric acid-glycerol based pH-sensitive biopolymeric hydrogels for dye removal applications: a green approach. Ecotoxicol Environ Saf. 2015;121:80–6.
35. Özmatara Bat M. Environmentally friendly extraction of antioxidants from elettaria cardamomum seeds with glucose-citric acid-based natural deep eutectic solvent. Rec Agric Food Chem. 2021;1(1-2):12–8.
36. Rao KV, Murthy PBGSN. Modeling and optimization of tool vibration and surface roughness in boring of steel using RSM, ANN and SVM. J Intell Manuf. 2018;29(7):1533–43.
37. Swamy GJ, Sangamithra A, Chandrasekar V. Response surface modeling and process optimization of aqueous extraction of natural pigments from Beta vulgaris using Box-Behnken design of experiments. Dye Pigment. 2014;111:64–74.
38. Raissi S, Farsani RE. Statistical process optimization through multi-response surface methodology. World Acad Sci Eng Technol. 2009;39(3):280–4.
39. Munaeni W, Widanarni W, Yuhana M, Setiawati M, Wahyudi A. Phytochemical analysis and antibacterial activities of Eleutherine bulbosa (Mill.) Urb. extract against Vibrio parahaemolyticus. Asian Pac J Trop Biomed. 2019;9(9):397–404.