INTRODUCTION
The COVID-19 infection is one of the fatal pandemics faced by mankind that began in late 2019. The pandemic was etiologically linked to a naive coronavirus known as severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2). While the patients became serologically negative in 3–4 weeks, a large number of patients continued to experience symptoms for months. Moreover, while the viral load typically accumulates in the respiratory system, with constant replication, the virus reaches other vital organs causing multiorgan damage with persistent symptoms, termed “post-COVID-19 syndrome” (PCS) or “long COVID” [1]. Long COVID is the term used when symptoms last longer than 4 weeks from the start of the infection, whereas PCS is the term used when symptoms last more than 12 weeks [2]. Long COVID is highly unpredictable due to its variety in terms of symptom intensity, time period, frequency, severity of initial illness, and patient characteristics. Extreme weariness, exhaustion, shortness of breath, cognitive fog, long-term loss of taste and smell, and musculoskeletal problems, particularly arthritic pain are some of the most typical PCS symptoms and are cumulatively termed “myalgic encephalomyelitis.” Shortness of breath and weariness associated with COVID-19 may be present for more than 6 months with a decreased quality of life [3]. Numerous investigations have demonstrated that some moderately unwell COVID-19 patients still had aberrant lung functions and structural abnormalities up to 6 months [4–6]. A German follow-up study on COVID-19 patients (n = 100) revealed myocardial inflammation and cardiac disturbances in 60% and 78% of subjects irrespective of the severity of their underlying condition [7]. According to data from the COVID-19 symptom research, age has an impact on the likelihood of developing protracted COVID, with rates ranging from 1% to 2% for individuals in their twenties to roughly 5% for those in their sixties [8,9]. Although COVID-19 has a low overall mortality rate (1.4%–2.3%), individuals with comorbidities appear to have an enhanced likelihood of severe and prolonged illness with higher death incidence [10,11]. The majority of the research that is now available has demonstrated an association between diabetic mellitus (DM) and enhanced disease severity, acute respiratory distress syndrome, and death [10,12,13]. This review will explore the diabetes manifestations and associated challenges in COVID-19 and PCS.
PCS AND DIABETES MELLITUS
Type 2 diabetes mellitus (T2DM) and COVID-19 have an antagonistic relationship [14,15]. Uncontrolled diabetes worsens COVID-19 with a predisposition for increased morbidity. Inadequate diabetes management, progression from prediabetes to manifest diabetes, an enhanced ratio of new diabetics, and a rise in corticosteroid-induced diabetes have all been related to the COVID-19 pandemic [14,16]. Theoretically, chronic diabetic patients may have an increased likelihood of predisposition to PCS. Microvascular injury, which is specifically caused by long-term uncontrolled diabetes, can be worse in people who are hospitalized and have SARS-CoV-2 infection. Diabetes also enhances the risk of hospitalization, having a severe and critical COVID-19 condition, and the need for mechanical ventilation support, all of which might increase the risk that PCS will manifest. The available data refute the hypothesis that COVID-19 risk is elevated in DM patients [17]. However, it was found in COVID-19 that diabetes mellitus was an independent predictor of mortality, invasive ventilation, or admission to an intensive care unit (hazard ratio 1.59, 95% CI: 1.03–2.45) [18]. From a group of 52 people, 20 survivors were reported. The most prevalent underlying comorbidity among patients in the critical care unit (ICU) was diabetes mellitus (22%) [12]. Guan et al. [10] observed that among the 1,099 confirmed COVID-19 patients from China with severe disease, 173 patients were related to a higher prevalence of diabetes mellitus (16.2%) than mild disease (5.7%). In addition, in COVID-19 cases reported by the Chinese Centre for Disease Control and Prevention involving the data of 72,314 patients, higher mortality was recorded in diabetics (7.3%) when compared with overall COVID-19 patients (2.3%) [11].
With somewhat divergent results, two significant studies have analyzed the problem of chronic diabetes that develops when a diabetes diagnosis coexists with SARS-CoV-2 infection. Newly diagnosed diabetes (NDD) was prevalent in younger patients compared to those with pre-existing DM, and a relationship exists between lower glycemic parameters and insulin needs, a longer hospital stay, greater inflammatory parameters, and enhanced vulnerability for admission to the intensive care unit. Cromer et al. [19] reported that among 1,902 COVID-19 patients, 64 NDD patients exist among which 36 (56.3%) still had diabetes, and 26 (40.6%) experienced a relapse to pre-diabetes. These inter-relationships suggested the potential role of stress hyperglycemia as a crucial physiological mechanism behind COVID-19 and DM regression. Another significant study reporting for a cohort of 181,280 COVID-19-positive individuals who survived for the initial 1 month of COVID-19 infection demonstrated a higher risk of incident diabetes (hazard ratio (HR) 1:40, 95% CI: 1:36–1:44), enhanced burden (13:46, 95% CI: 12:11–14:84), and the risk of antihyperglycaemic medications use (HR 1:85, 1:78–1:92) with excess burden (12:35, 11:36–13:38) in people with COVID-19 [20]. These patients were at an increased risk and burden of post-acute care, regardless of whether patients were nonhospitalized, hospitalized, or admitted to critical care.
Another meta-analysis carried out by Ssentongo et al. [21] to determine the prevalence of newly discovered diabetes among COVID-19 survivors demonstrated a higher risk of diabetes incidence by 66% when COVID-19 was present. Age, sex, or research quality did not affect the risk in any way. In this comprehensive investigation and meta-analysis, the COVID-19 survivors were at increased predisposition for the incidence of NDD.
Zhang et al. [22] conducted a comprehensive review and meta-analysis to assess the incidence of diabetes in 10 post-COVID-19 groups. The authors reported a relative risk of 1.62-fold for diabetes incidence among post-COVID-19 patients compared to noninfected individuals. Despite the lack of a statistically significant relative risk for undifferentiated diabetes, subgroup analysis showed significant vulnerability for the development of DM regardless of age, gender, follow-up duration, and the COVID-19 severity level. These findings remain constant with the inclusion of confounder variables. Patients suffering from previous upper respiratory tract infections had a 1.2-fold higher vulnerability of diabetes incidence following COVID-19 in contrast to the general population who had a 1.82-fold higher risk, per the subgroup analysis. This emphasizes how crucial it is for clinicians to keep an eye on their patients’ glucose metabolism throughout the PCS. Patients with COVID-19 infection had a 1.7-fold higher chance of developing diabetes mellitus as per the subgroup analysis.
POTENTIAL ASSOCIATION BETWEEN LONG COVID, DIABETES, AND INFLAMMATION
The connection between DM and COVID-19 severity was supported by a variety of pathophysiological explanations (Fig. 1). The first pathophysiology is the compromised innate immune system among patients with uncontrolled diabetes which undisputedly serves as the first line of defense against SARS-CoV-2 infection [23]. In addition, DM is a disorder that promotes inflammation and is characterized by an excessive and heightened cytokine response. This was shown in COVID-19-infected individuals, where inflammatory markers viz. interleukin-6 (IL-6), C-reactive protein, and ferritin were markedly greater in the serum of DM patients when compared with controls. This inter-relationship demonstrates the enhanced vulnerability of diabetes patients to encounter an inflammatory cytokine storm resulting in acute respiratory distress syndrome (ARDS), shock, and PCS. Moreover, this study further revealed that D-dimer levels were higher in COVID-19 individuals with DM than in those without DM [24], presumably pointing to an overactive hemostatic system. The pre-existence of DM relatively enhances the occurrence of pro-thrombotic hypercoagulable conditions while COVID-19 infection triggers the coagulation cascade. Therefore, the amalgamation of DM and COVID-19 infection can cause severe thromboembolic consequences and eventually mortality [25,26]. Furthermore, COVID-19 can exacerbate insulin resistance among diabetic patients, specifically obese people with pre-existing insulin resistance or a complete lack of insulin. High quantities of IL-6, IL-1β, tumor necrosis factor-α (TNF-α), monocyte chemoattractant protein-1 (MCP-1), and inducible protein-10 (IP-10) in obese diabetic patients may amplify the cytokine response and worsen insulin resistance and can be triggered by even a mild COVID-19 infection [27]. A2-Hermans-Schmid glycoprotein fetuin A, which has been reported to be associated with impaired insulin sensitivity [28], is also elevated in the blood by SARS-CoV; it is unknown if SARS-CoV-2 can do the same. Not to mention, COVID-19 frequently results in hypokalemia, which has been connected to decreased angiotension-II degradation and enhanced pulmonary angiotensin-converting enzyme 2 (ACE2) [29]. Hypokalemia may cause patients with T1DM and T2DM to have worsening glucose control [30].
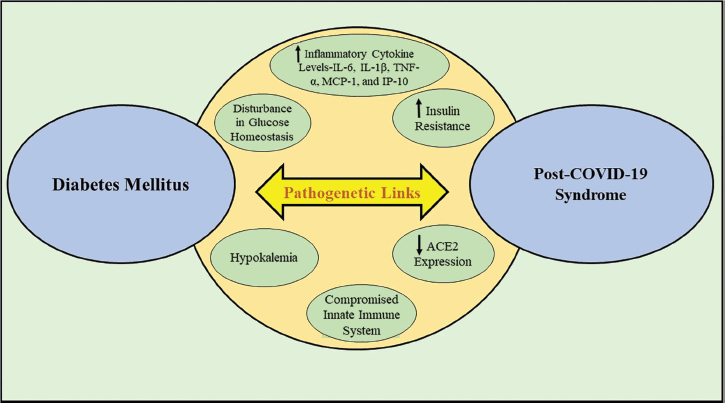 | Figure 1. Pathophysiological connections between diabetes mellitus and COVID-19. [Click here to view] |
INTERPLAY OF ACE2 BETWEEN LONG COVID AND DIABETES
ACE2 has been considered one of the key players in the association of DM and COVID-19 by the researchers as shown in Figure 2. The lungs, kidneys, stomach, and blood vessel epithelial cells constitutively express ACE2, a type 1 integral membrane protein. The original function of ACE2 is to convert angiotensin-II and partially angiotensin-I into smaller peptides and angiotensin (1–9), respectively. The ACE2/Ang (1–7) system has also been recorded to exhibit protective action against the lethal H5N1 strain of avian influenza [31], and it also plays a critical role in ARDS lung protection by acting as an important anti-inflammatory and antioxidant. Glycosylation may be responsible for the lower expression of ACE2 in DM patients, which could explain why COVID-19 carries a higher risk of severe lung injury and ARDS [29,32]. Despite how absurd it may sound, COVID-19 would suffer from overexpressing ACE2. ACE2 functions as a receptor for SARS-CoV-2 as it enters host pneumocytes [33]. This is where DM drugs such as ACE inhibitors (ACEis) and angiotensin-receptor blockers (ARBs) become tricky. In patients with DM (and hypertension) using ACEi or ARBs, the expression of ACE2 is noticeably enhanced as an outcome of the increased levels of Ang-II and Ang-I. As a result, using drugs that stimulate ACE2 would make it simpler for SARS-CoV-2 to enter pneumocytes, thus increasing the severity and lethality of the sickness [34]. In addition, pioglitazone and liraglutide are recently connected with ACE2 overexpression in animal studies [34,35]. However, these studies have considerable limitations in not accounting for the baseline therapy. In addition, a recent study found that seriously and critically ill COVID-19 individuals had a higher incidence of hypokalemia caused by renal potassium wasting. It is hypothesized that the viral infiltration downregulates ACE2, which causes an increase in aldosterone secretion, an increase in potassium loss from the urine, and a decrease in angiotensin-II degradation. In fact, it has been proposed that early serum potassium normalization is a predictor of a successful outcome in COVID-19 [36]. Because the virus breaks down the enzyme, ACE2 overexpression encourages SARS-CoV-2 entry but does not shield against lung injury.
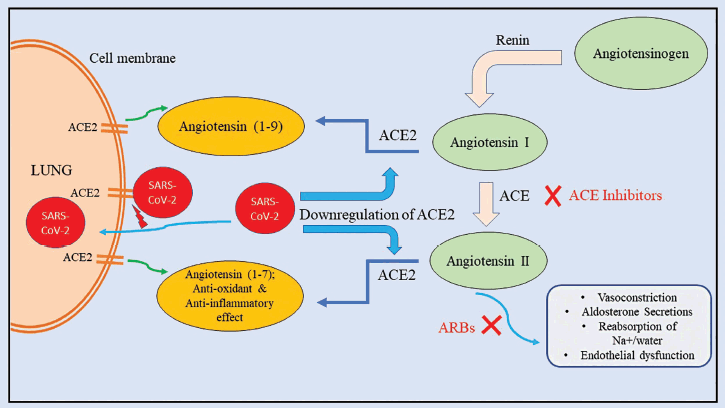 | Figure 2. Interplay of ACE2 between long COVID and diabetes. [Click here to view] |
IMPACT ON PANCREATIC β CELLS AND ROLE OF ANTIHYPERGLYCAEMIC DRUGS
The direct influence of COVID-19 on pancreatic β cells is still unclear. Conflicting reports are available related to the presence of SARS-CoV-2 receptors in pancreatic β cells. While single-cell Ribonucleic acid (RNA) surveys and immunohistochemistry of pancreatic islets deny the presence of SARS-CoV-2 co-receptor ACE2 in pancreatic β cells, other studies have discovered these receptors along with other associated co-receptors such as transmembrane serine protease (TMPRSS) and neuropilin 1 (NRP1) in cells [37–40]. Diabetes ketoacidosis and inflammatory indicators such as ferritin, C-reactive protein, leukocyte and platelet counts, and D-dimer are not definitively linked. However, the level of viral invasion must be substantial for the virus to produce a considerable loss of β-cell function and affect insulin secretion on its own. Furthermore, SARS-CoV-2 infection has been shown to reduce pancreatic insulin levels and production as well as to cause cell apoptosis [41,42]. Therefore, it is conceivable that SARS-CoV-2 could change glucose metabolism in a variety of ways, which could result in diabetes incidentally or speed up the progression of prediabetes into full-blown diabetes.
The alleged anti-inflammatory effects of diabetic drugs including metformin, pioglitazone, sodium-glucose co-transporter-2 inhibitors (SGLT2-Is), and incretin-based medications should also be considered in the pathophysiologic processes of PCS-related hyperglycemia [43]. According to a number of investigations, ACE2 receptors and the enzyme receptor dipeptidyl peptidase 4 (DPP4) may work together as binding targets [44]. The preliminary connections, however, appear to be tenuous as SARS-CoV-2 and DPP4 inhibitors had no appreciable beneficial effect on infection incidence. Owing to their particular side effect profile, SGLT2-I may cause harm in COVID-19-infected individuals. Furthermore, due to the potency, flexibility, anti-inflammatory advantages, absence of pharmaceutical interactions, and effectiveness against diabetic ketoacidosis, insulin therapy ultimately proves to be the most effective therapeutic option for COVID-19 patients with hyperglycemia [43,45].
MANAGEMENT OF DIABETES MELLITUS IN PCS
To avoid the extreme level of death associated with COVID-19, it is highly important to avoid the occurrence of type 2 diabetes, put it into control when it manifests, and adequately treat it with medication. Research studies have provided ample evidence that overweight, obese, and type 2 diabetes have contributed to the excess deaths in COVID-19 [46,47]. This makes it more important than ever for professionals to advocate for improvements in the population’s nutrition and lifestyle. Individually tailored, evidence-based treatments for weight management, behavior change, psychotherapy, and health promotion must also receive significant support from those on the front lines. Instead of only addressing complex patients in hospitals and clinics, diabetes experts should work with primary care colleagues to oversee prevention/remission and ensure appropriate medication for less challenging cases.
Evidence already indicated that type 2 diabetes can be reversed in primary care during its early years in up to half of the cases through a low-calorie diet, although practiced in a few locations [48]. The relatively enhanced mortality of diabetic people during the COVID-19 pandemic argues for the rigorous implementation of nationwide diabetes remission programs with consequential positive influence over the health and economy of society.
The recent 5 years have been momentous for type 2 diabetes drug discovery. Two novel pharmacological drugs—SGLT2 inhibitors and glucagon-like peptide 1 (GLP-1) receptor agonists—reduce weight and reduce the incidence of heart disease and stroke for the first time reliably. These modern pharmacological interventions, particularly injection-only GLP-1 receptor agonists, are not, however, widely used for the condition. There is a dearth of experience in the administration of these recently approved medicines in secondary care due to the simple reason that the majority of diabetic people are treated in primary care. Many diabetic patients approach secondary care only after the development of diabetic complications like neuropathy or if they have chronic uncontrolled conditions, despite the fact that there are some examples of good practices, such as locally extended services that are focused on diabetes. Management of diabetes in the post-COVID era requires a more dynamic two-way relationship between primary and secondary care, so that a larger diabetic population can take advantage of an array of available treatment options with evidence-based learning of new treatment options as well [49]. The development and flexible implementation of a comprehensive “care plan” with the assistance of persons with diabetes could improve and enhance each person’s capacity for self-management.
The implementation of this approach has been hampered by the notion that each sector is already functioning at capacity and that any modification could overwhelm one or the other. The need to enhance control to avoid complications should now be a main and urgent impetus for cooperative engagement specifically taking into account the level of diabetes morbidity and death across COVID-19. In addition, increased comfort with virtual conferencing may serve as a catalyst for better inter-professional collaboration between primary and secondary care. Increased use of telemedicine further outreaches people who might not have otherwise attended while also making it easier to conduct suitable “distance” consultations.
While the present manuscript has used the latest updates about the diabetes manifestations in long COVID, the limitations of being a narrative review like the inability to produce new data and provide conclusive answers for the contradictory findings reported in the literature cannot be out-ruled. The other minor limitations may include unintentional bias due to the nonavailability of studies in subscribed platforms or most recently published studies during the publication process of the present review. Nevertheless, the present study provided the most comprehensive insights about the major work done in the field of diabetes and long COVID.
CONCLUSION
Research evidence clearly implies the complex interaction between post-COVID-19 and diabetes mellitus with consequent high risk of developing a serious illness, acute respiratory distress syndrome, and death. Post-COVID diabetes falls under the umbrella of illnesses that are now classified as long COVID. In addition, people with diabetes mellitus may find it difficult to maintain blood sugar management due to the concurrent COVID-19. Furthermore, it is claimed that during PCS, higher risk and burden of diabetes as well as the use of antihyperglycaemic drugs exist. Among the nonhospitalized, hospitalized, and ICU-admitted individuals COVID-19 infected individuals, the risks of NDD and management of pre-existing diabetes enhance proportionately with the severity of the acute illness. While the enhanced risk and predisposition have been demonstrated between diabetes and PCS, it is yet to be established whether chronic infection can directly cause diabetes. This depends on the progression of viral infection and consequent cellular damage to the pancreatic β cells. In addition, the existence or absence of molecular mimicry, a concept that involves cross-reactive immunity against epitopes that SARS-CoV-2 virus and pancreatic β cells share, as well as the patient’s immunological profile may have an impact. The existing research shows that COVID-19 patients should use post-acute care measures to identify and treat their diabetes because diabetes is one component of the complex extended COVID condition. The epidemiologic impact of “COVID-diabetes pandemic” is tragically disproportionately greater in developing countries because the majority of the vulnerable population resides there. To provide the greatest care, the problem needs a multimodal strategy that addresses both personalized treatment and difficulties with public health. A considerable percentage of COVID-19-infected individuals are also at risk for developing chronic glucometabolic sequelae as a part of PCS, which is characterized by persistent illness and ambiguous quality of life.
AUTHOR CONTRIBUTIONS
All authors have made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Dennis A, Wamil M, Alberts J, Oben J, Cuthbertson DJ, Wootton D, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. 2021;11(3):e048391. CrossRef
2. Ambrosino P, Lanzillo A, Maniscalco M. COVID-19 and post-acute COVID-19 syndrome: from pathophysiology to novel translational applications. Biomedicines. 2022;10(1):47. CrossRef
3. Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: a systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. 2022;11(2):269. CrossRef
4. Heiss R, Grodzki DM, Horger W, Uder M, Nagel AM, Bickelhaupt S. High-performance low field MRI enables visualization of persistent pulmonary damage after COVID-19. Magn Reson Imaging. 2021;76:49–51. CrossRef
5. Moreno-Pérez O, Merino E, Leon-Ramirez JM, Andres M, Ramos JM, Arenas-Jiménez J, et al. Post-acute COVID-19 syndrome. Incidence and risk factors: a mediterranean cohort study. J Infect. 2021;82(3):378–83. CrossRef
6. Shah AS, Wong AW, Hague CJ, Murphy DT, Johnston JC, Ryerson CJ, et al. A prospective study of 12-week respiratory outcomes in COVID-19-related hospitalisations. Thorax. 2021;76(4):402–4. CrossRef
7. Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J, et al. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19). JAMA Cardiol. 2020;5(11):1265–73. CrossRef
8. Baraniuk C. How long does covid-19 immunity last? BMJ. 2021;373:n1605. CrossRef
9. O’Kelly B, Vidal L, Avramovic G, Broughan J, Connolly SP, Cotter AG, et al. Assessing the impact of COVID-19 at 1-year using the SF-12 questionnaire: data from the anticipate longitudinal cohort study. Int J Infect Dis. 2022;118:236–43. CrossRef
10. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical characteristics of coronavirus disease 2019 in China. N J Emerg Med. 2020;58(4):711–2. CrossRef
11. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA. 2020;323(13):1239–42. CrossRef
12. Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. CrossRef
13. Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180(7):934–43. CrossRef
14. Ghosh A, Anjana RM, Shanthi Rani CS, Jeba Rani S, Gupta R, Jha A, et al. Glycemic parameters in patients with new-onset diabetes during COVID-19 pandemic are more severe than in patients with new-onset diabetes before the pandemic: NOD COVID India Study. Diabetes Metab Syndr. 2021;15(1):215–20. CrossRef
15. Unnikrishnan R, Misra A. Diabetes and COVID19: a bidirectional relationship. Nutr Diabetes. 2021;11(1):21. CrossRef
16. Misra A, Ghosh A, Gupta R. Heterogeneity in presentation of hyperglycaemia during COVID-19 pandemic: a proposed classification. Diabetes Metab Syndr. 2021;15(1):403–6. CrossRef
17. Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43(6):867–9. CrossRef
18. Guan W, Liang W, Zhao Y, Liang H, Chen Z, Li Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. CrossRef
19. Cromer SJ, Colling C, Schatoff D, Leary M, Stamou MI, Selen DJ, et al. Newly diagnosed diabetes vs. pre-existing diabetes upon admission for COVID-19: associated factors, short-term outcomes, and long-term glycemic phenotypes. J Diabetes Complicat. 2022;36(4):108145. CrossRef
20. Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10(5):311–21. doi: 10.1016/s2213-8587(22)00044-4 CrossRef
21. Ssentongo P, Zhang Y, Witmer L, Chinchilli VM, Ba DM. Association of COVID-19 with diabetes: a systematic review and meta-analysis. Sci Rep. 2022;12(1):20191. CrossRef
22. Zhang T, Mei Q, Zhang Z, Walline JH, Liu Y, Zhu H, et al. Risk for newly diagnosed diabetes after COVID-19: a systematic review and meta-analysis. BMC Med. 2022;20(1):444. CrossRef
23. Jafar N, Edriss H, Nugent K. The effect of short-term hyperglycemia on the innate immune system. Am J Med Sci. 2016;351(2):201–11. CrossRef
24. Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;36(7):e3319. CrossRef
25. Dunn E, Grant P. Type 2 diabetes: an atherothrombotic syndrome. Curr Mol Med. 2005;5(3):323–32. CrossRef
26. Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: knowledge in progress. Diabetes Res Clin Pract. 2020;162:108142. CrossRef
27. Kassir R. Risk of COVID-19 for patients with obesity. Obes Rev. 2020;21(6);e13034. CrossRef
28. Wan J, Sun W, Li X, Ying W, Dai J, Kuai X, et al. Inflammation inhibitors were remarkably up-regulated in plasma of severe acute respiratory syndrome patients at progressive phase. Proteomics. 2006;6(9):2886–94. CrossRef
29. Pal R, Bhansali A. COVID-19, diabetes mellitus and ACE2: the conundrum. Diabetes Res Clin Pract. 2020;162:108132. CrossRef
30. Liamis G, Liberopoulos E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. World J Clin Cases. 2014;2(10):488–96. CrossRef
31. Zou Z, Yan Y, Shu Y, Gao R, Sun Y, Li X, et al. Angiotensin-converting enzyme 2 protects from lethal avian influenza A H5N1 infections. Nat Commun. 2014;5:3594. CrossRef
32. Tikellis C, Thomas MC. Angiotensin-converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept. 2012;2012:256294. CrossRef
33. Wrapp D, Wang N, Corbett KS, Goldsmith JA, Hsieh CL, Abiona O, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–3. CrossRef
34. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4):e21. CrossRef
35. Romaní-Pérez M, Outeiriño-Iglesias V, Moya CM, Santisteban P, González-Matías LC, Vigo E, et al. Activation of the GLP-1 receptor by liraglutide increases ACE2 expression, reversing right ventricle hypertrophy, and improving the production of SP-A and SP-B in the lungs of type 1 diabetes rats. Endocrinology. 2015;156(10):3559–69. CrossRef
36. Chen D, Li X, Song Q, Hu C, Su F, Dai J, et al. Assessment of hypokalemia and clinical characteristics in patients with coronavirus disease 2019 in Wenzhou, China. JAMA Netw Open. 2020;3(6):e2011122. CrossRef
37. Veres A, Faust AL, Bushnell HL, Engquist EN, Kenty JHR, Harb G, et al. Charting cellular identity during human in vitro β-cell differentiation. Nature. 2019;569(7756):368–73. CrossRef
38. Coate KC, Cha J, Shrestha S, Wang W, Gonçalves LM, Almaça J, et al. SARS-CoV-2 cell entry factors ACE2 and TMPRSS2 are expressed in the microvasculature and ducts of human pancreas but are not enriched in β cells. Cell Metab. 2020;32(6):1028–40.e4. CrossRef
39. Kusmartseva I, Wu W, Syed F, Van Der Heide V, Jorgensen M, Joseph P, et al. Expression of SARS-CoV-2 entry factors in the pancreas of normal organ donors and individuals with COVID-19. Cell Metab. 2020;32(6):1041–51.e6. CrossRef
40. Fignani D, Licata G, Brusco N, Nigi L, Grieco GE, Marselli L, et al. SARS-CoV-2 receptor angiotensin I-converting enzyme type 2 (ACE2) is expressed in human pancreatic β-cells and in the human pancreas microvasculature. Front Endocrinol (Lausanne). 2020;11:596898. doi: 10.3389/fendo.2020.596898. CrossRef
41. Müller JA, Groß R, Conzelmann C, Krüger J, Merle U, Steinhart J, et al. SARS-CoV-2 infects and replicates in cells of the human endocrine and exocrine pancreas. Nat Metab. 2021;3(2):149–65. CrossRef
42. Wu CT, Lidsky PV, Xiao Y, Lee IT, Cheng R, Nakayama T, et al. SARS-CoV-2 infects human pancreatic β cells and elicits β cell impairment. Cell Metab. 2021;33(8):1565–76.e5. CrossRef
43. Al-kuraishy HM, Al-Gareeb AI, Alblihed M, Guerreiro SG, Cruz-Martins N, Batiha GES. COVID-19 in relation to hyperglycemia and diabetes mellitus. Front Cardiovasc Med. 2021;8:644095. CrossRef
44. Valencia I, Peiró C, Lorenzo Ó, Sánchez-Ferrer CF, Eckel J, Romacho T. DPP4 and ACE2 in diabetes and COVID-19: therapeutic targets for cardiovascular complications? Front Pharmacol. 2020;11:1161. CrossRef
45. Lim S, Bae JH, Kwon HS, Nauck MA. COVID-19 and diabetes mellitus: from pathophysiology to clinical management. Nat Rev Endocrinol. 2021;17(1):11–30. CrossRef
46. McGurnaghan SJ, Weir A, Bishop J, Kennedy S, Blackbourn LAK, McAllister DA, et al. Risks of and risk factors for COVID-19 disease in people with diabetes: a cohort study of the total population of Scotland. Lancet Diabetes Endocrinol. 2021;9(2):82–93. CrossRef
47. Niedzwiedz CL, O’Donnell CA, Jani BD, Demou E, Ho FK, Celis-Morales C, et al. Ethnic and socioeconomic differences in SARS-CoV-2 infection: prospective cohort study using UK Biobank. BMC Med. 2020;18(1):160. CrossRef
48. Lean MEJ, Leslie WS, Barnes AC, Brosnahan N, Thom G, McCombie L, et al. Durability of a primary care-led weight-management intervention for remission of type 2 diabetes: 2-year results of the DiRECT open-label, cluster-randomised trial. Lancet Diabetes Endocrinol. 2019;7(5):344–55. CrossRef
49. Xin Y, Davies A, McCombie L, Briggs A, Messow CM, Grieve E, et al. Type 2 diabetes remission: economic evaluation of the DiRECT/counterweight-plus weight management programme within a primary care randomized controlled trial. Diabet Med. 2019;36(8):1003–12. CrossRef