INTRODUCTION
Morus of the family Moraceae is a small plant genus having 19 species worldwide [1]. Economically important species are Morus alba (white mulberry), Morus indica (MI, Indian mulberry), Morus nigra (MN, black mulberry), and Morus rubra (red mulberry). China has 11 Morus species of which 5 are endemic and 1 is introduced [2].
Morus alba L., comprising M. alba var. alba and M. alba var. multicaulis, is a fast-growing monoecious and deciduous shrub or medium-sized tree without buttress [2−5]. Young plants produce multiple stems via coppicing. The bark is brownish–gray with vertical fissures, lenticels, and white or cream-colored latex. Leaves of M. alba (MA) are glossy green, alternately arranged, cordate at the base, and acuminate at the apex. Leaf margins are serrated, leaf petioles are long and slender, and leaf blades vary from unlobed to almost palmate. Fruits are drupes or sorosis that are white when young, turning reddish when mature, and black when ripe [2−5]. Leaves, twigs, and maturing fruits of MA are shown in Figure 1.
The whole plant, leaf, fruit, twig, and root of MA have medicinal values. Chemical constituents comprise steroids, tannins, phytosterols, glycosides, alkaloids, carbohydrates, proteins, and amino acids, as well as saponins, triterpenes, phenols, flavonoids, benzofurans, anthocyanins, polysaccharides, anthraquinones, and glycosides [5−7]. Pharmacological properties include antioxidant, antimicrobial, anti-inflammatory, anti-diabetic, hypolipidemic, anti-obesity, anti-atherosclerotic, neuroprotective, hepatoprotective, tyrosinase inhibitory, and cardioprotective [5−7]. The beneficial effects of MA leave against cardiometabolic risks have been reviewed [8]. The chemical constituents, medicinal properties, clinical trials, and patents of twigs of MA (Ramulus Mori) have recently been reviewed [9].
In this review, the clinical studies of Morus species (mostly MA) to date are briefly described. These studies are categorized as anti-diabetic properties (2007−2022) and other pharmacological properties (2001−2021) in chronological order. Relevant to the findings of these studies are mention of compounds, their classes, and bioactivities.
CLINICAL STUDIES
Anti-diabetic properties
There are 23 clinical studies on anti-diabetic properties of Morus species (Table 1). Five studies were undertaken in Japan; three studies each in the USA, Korea, China, and India; and two studies each in Iran and the UK. Single studies were conducted in Thailand and Poland. All studies were on MA except one study on MN. Plant parts of MA clinically tested were mostly leaves with fruits and twigs lesser studied.
OTHER PHARMACOLOGICAL PROPERTIESS
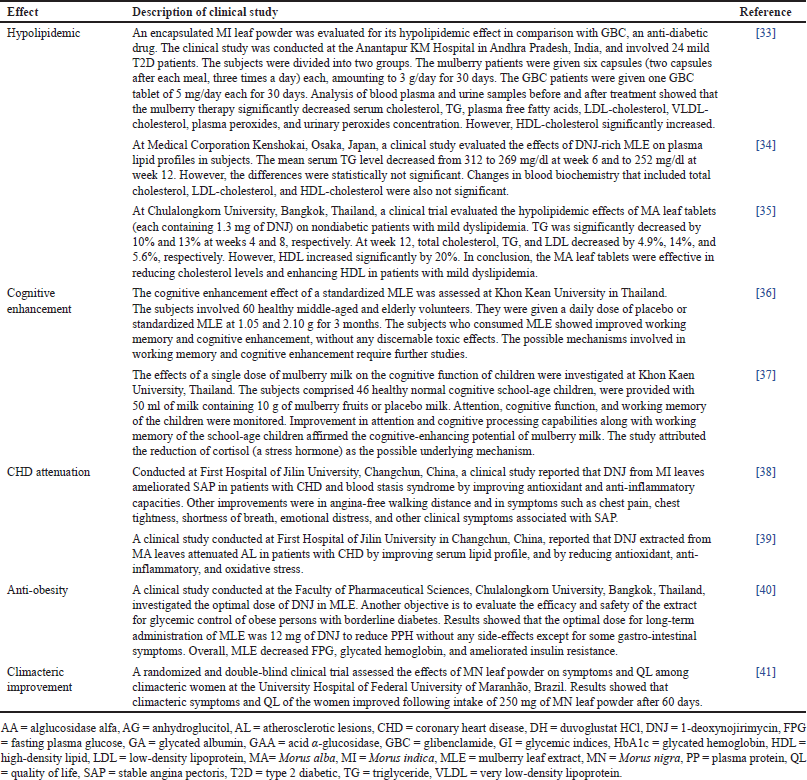
There are nine clinical studies on other pharmacological properties of Morus species (Table 2). Four studies were undertaken in Thailand, two studies in China, and single studies were conducted in Japan, India, and Brazil. One study was tested on MN and MI each. Clinical studies on other pharmacological properties of Morus species include hypolipidemic (3), cognitive enhancement (2), coronary heart disease (CHD) attenuation (2), anti-obesity (1), and climacteric improvement (1).
 | Figure 1. Leaves (left), twigs (middle), and maturing fruits (right) of Morus alba. [Click here to view] |
BIOACTIVE COMPOUNDS
Anti-diabetic
Compounds in MA leaves, fruits, and twigs with anti-diabetic activities include 1-deoxynojirimycin (DNJ), quercetin, dihydroquercetin kaempferol, rutin, chlorogenic acid chalcomoracin, morachalcone, and isobavachalcone [6,42,43]. Steppogenin-4’-O-β-D-glucoside and mulberroside A from the root bark of MA significantly reduced the fasting blood glucose level in alloxan-induced diabetic mice [44]. Rutin and quercetin-3-O-β-D-glucoside, two anti-diabetic flavonoids from the fruit of MA, improved glucose uptake in 3T3-L1 adipocytes [45]. The mechanism involved Akt-mediated insulin signaling pathway or AMP-activated protein kinase activation. A high-purity polysaccharide from mulberry leaf extract (MLE) (99.8% purity) exhibited anti-diabetic effects in streptozotocin-induced diabetic rats with effects equivalent to glibenclamide (GBC), an anti-diabetic drug [46].
 | Table 1. Clinical studies on anti-diabetic properties of Morus species. [Click here to view] |
DNJ, an alkaloid from MA leaves and twigs, is a potent α-glucosidase inhibitor and is effective in suppressing high blood glucose levels in human subjects, thus preventing T2D [4,9]. In diabetic mice, DNJ significantly decreased serum glucose and insulin levels, improved serum lipid contents, and reversed insulin resistance [47]. DNJ prevents the secretion of insulin and thus lowers fasting and post-prandial blood glucose levels associated with T2D [48]. Out of 21 clinical studies on anti-diabetic activities, 12 studies have been attributed to DNJ (Table 1).
 | Table 2. Clinical studies on other pharmacological properties of Morus species. [Click here to view] |
Hypolipidemic
Mulberroside A was a stilbenoid isolated and purified from the ethanol root extract of MA while oxyresveratrol was produced from enzymatic conversion. Both compounds exhibited hypolipidemic effects in rats on a high-cholesterol diet. Rats orally treated with mulberroside A and oxyresveratrol significantly decreased serum lipids, coronary artery risk index, and atherogenic index [49]. From the leaf of MA, a benzofuran derivative, a flavonoid, and an alkaloid displayed potent lipolytic activity in 3T3-L1 cells with values from 15.4% to 21.2% [43]. Rutin and quercetin-3-O-β-D-glucoside, two anti-diabetic flavonoids from the fruit of MA, reduced lipid accumulation in adipocytes [45].
Anti-obesity
The ability of MLE to suppress obesity and reduce visceral adipose tissues has been attributed to polyphenols such as quercetin, kaempferol, rutin, caffeic acid, and chlorogenic acid [6]. From MLE, 2’,7-dihydroxy-4’-methoxy-8-prenylflavan (flavan), isobavachalcone, and morachalcone B (chalcones) inhibited 3T3-L1 preadipocytes with IC50 values of 37, 43, and 48 μM, respectively [50]. A pectic polysaccharide from MFE, named JS-MP-1, displayed an anti-obesity effect by inhibiting pre-adipocytes via reducing fat cells and adipose tissue [51].
Cognitive enhancement
Mulberrofuran G (2.13 and 9.72 μM) and albanol B (2.47 and 1.39 μM) from the root bark of MA possessed strong acetylcholinesterase and butyrylcholinesterase inhibitory activities, respectively [52]. These activities showed their ability to treat cognitive dysfunction associated with Alzheimer’s disease (AD). Among four moracins isolated from the root of MA, the inhibition of BACE1, a beta-secretase enzyme in AD, moracin S was the strongest [53].
CHD attenuation
CHD is a common cardiovascular disease that causes human disability and death [54]. Among the various symptoms of CHD are angina pectoris, blood stasis syndrome, and atherosclerosis [38,39]. The underlying mechanisms of CHD are associated with inflammatory stress responses [55]. Compounds in Morus species that possess anti-inflammatory properties include DNJ and oxyresveratrol. DNJ, the main component in MA alkaloid tablets, possessed anti-inflammatory properties [56]. The alkaloid checked inflammation via regulation of mitogen-activated kinase signaling. DNJ markedly down-regulated interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) cytokine levels in lipopolysaccharide (LPS)-induced RAW 264.7 and bone marrow-derived macrophage cells. Oxyresveratrol, a stilbenoid, is another ingredient of MA that exerts anti-inflammatory activity via inhibition of leukocyte migration, and involvement of mitogen-activated ERK (MEK)/extracellular signal-regulated kinase (ERK) signaling [57]. Oxyresveratrol from MA also inhibited LPS-induced translocation of nuclear kappa B and cyclooxygenase-2 activity in RAW 264.7 cells [58,59]. The anti-inflammatory activity of oxyresveratrol has also been reported in RAW 264.7 cells, Jurkat leukemic T cells, and C28/I2 chondrocytes [60]. Quercetin 3-(6-malonylglucoside), a flavonol from MLE, attenuated atherosclerosis in low-density lipoprotein (LDL) receptor-deficient mice [61]. Among 36 compounds from the twig of MA, albanin D and 3-methyl-1-phenyl-1,3-butadiene exhibited the strongest anti-inflammatory activity of 4.1 and 2.2 μM by inhibiting NO production in RAW 264.7 cells [62]. The potent anti-inflammatory activity of prenylated flavonoids from the root of MA and MN has been reported [63]. Noteworthy is kuwanon C with an IC50 value of 1.7 μM. Albanol, an arylbenzofuran derivative from the root bark of MA, had the strongest anti-inflammatory effects towards RAW 264.7 cells, followed by sanggenon B and sanggenon D [64].
CONCLUSION
Objectives of clinical studies on MA include the effective dosage, duration, timing, and administration. Materials used include mulberry tea, MLE powder, and confections containing enriched compounds such as DNJ, and standardized extract, e.g., reducose. Subjects are children, middle-aged adults, and elderly people, including people with CHD, impaired glucose tolerance (IGT), Pompe disease (PD), dyslipidemia, and climacteric symptoms. Some clinical studies on MA are designed to compare diabetic patients, people with dyslipidemia, and healthy or nondiabetic volunteers. Diabetic patients include post-prandial pre-diabetic, and borderline subjects. Placebo groups serve as controls. Further research is needed on the anti-diabetic mechanisms of mulberry leaves at the molecular level, which may involve multiple pathways. While most clinical trials have shown that mulberry leaves regulate blood glucose and lipid metabolism, research focusing on the safety of mulberry leaves is lacking. Studies are therefore needed to understand the distribution, absorption, metabolism, and excretion of mulberry compounds.
AUTHOR CONTRIBUTIONS
The author made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. The author is eligible to be an author as per the International Committee of Medical Journal Editors (ICMJEs) requirements/guidelines.
FINANCIAL SUPPORT
Assoc. Prof. Eric W. C. Chan, the Lead and Sole Author, acknowledges that the funds for the publication of this review in the Journal of Applied Pharmaceutical Science (JAPS) as article processing charges (APCs) are provided by UCSI University. The author is grateful for the World’s Top 2% Scientist Research Grant, awarded by CERVIE (Grant Code: T2S-2023/004).
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Ozturk M, Kamili AN, Altay V, Rohela GK. Mulberry: from botany to phytochemistry. New York, NY: Springer International Publication AG; 2023. 191 pp.
2. Wu Z, Zhou ZK, Gilbert MG. Moraceae. Flora China. 2003;5:22−6.
3. Lim TK. Morus alba. In: edible, medicinal and non-medicinal plants: volume 3, Fruits. New York, NY: Science + Business Media B.V.; 2011. pp 399−429. CrossRef
4. Chan EWC, Lye PY, Wong SK. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin J Nat Med. 2016;14(1):17−30. CrossRef
5. Batiha GE, Al-Snafi AE, Thuwaini MM, Teibo JO, Shaheen HM, Akomolafe AP, et al. Morus alba: a comprehensive phytochemical and pharmacological review. Naunyn-Schmiedeberg Arch Pharmacol. 2023;396:1399−413. CrossRef
6. Chen C, Mohamad Razali UH, Saikim FH, Mahyudin A, Mohd Noor NQ. Morus alba L. plant: Bioactive compounds and potential as a functional food ingredient. Foods. 2021;10(3):689−717. CrossRef
7. Tang C, Bao T, Zhang Q, Qi H, Huang Y, Zhang B, et al. Clinical potential and mechanistic insights of mulberry (Morus alba L.) leaves in managing type 2 diabetes mellitus: focusing on gut microbiota, inflammation, and metabolism. J Ethnopharmacol. 2023:116143. CrossRef
8. Thaipitakwong T, Numhom S, Aramwit P. Mulberry leaves and their potential effects against cardiometabolic risks: a review of chemical compositions, biological properties and clinical efficacy. Pharm Biol. 2018;56(1):109−18. CrossRef
9. Chan EWC, Chan HT, Wong SK. An overview of chemical constituents, medicinal properties, clinical trials, and patents of twigs of Morus alba (Ramulus Mori). World J Chin Tradit Med (In Press). CrossRef
10. Kimura T, Nakagawa K, Kubota H, Kojima Y, Goto Y, Yamagishi K, et al. Food-grade mulberry powder enriched with 1-deoxynojirimycin suppresses the elevation of post-prandial blood glucose in humans. J Agric Food Chem. 2007;55(14):5869−74. CrossRef
11. Mudra M, Ercan-Fang N, Zhong L, Furne J, Levitt M. Influence of mulberry leaf extract on the blood glucose and breath hydrogen response to ingestion of 75 g sucrose by type 2 diabetic and control subjects. Diabetes Care. 2007;30(5):1272−4. CrossRef
12. Nakamura M, Nakamura S, Oku T. Suppressive response of confections containing the extractive from leaves of Morus alba on postprandial blood glucose and insulin in healthy human subjects. Nutr Metabol. 2009;6(1):1−10. CrossRef
13. Asai A, Nakagawa K, Higuchi O, Kimura T, Kojima Y, Kariya J, et al. Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on post-prandial glycemic control in subjects with impaired glucose metabolism. J Diabetes Investig. 2011;2(4):318−23. CrossRef
14. Nakamura S, Hashiguchi M, Yamaguchi Y. Hypoglycemic effect of Morus alba leaf extract on post-prandial blood glucose and insulin levels in patients with type 2 diabetes treated with sulfonylurea hypoglycemic agents. J Diabetes Metab. 2011;2(9):1−6. CrossRef
15. Chung HI, Kim J, Kim JY, Kwon O. Acute intake of mulberry leaf aqueous extract affects post-prandial glucose response after maltose loading: randomized double-blind placebo-controlled pilot study. J Funct Food. 2013;5(3):1502−6. CrossRef
16. Banu S, Jabir NR, Manjunath NC, Khan MS, Ashraf GM, Kamal MA, et al. Reduction of post-prandial hyperglycemia by mulberry tea in type-2 diabetes patients. Saudi J Biol Sci. 2015;22(1):32−6. CrossRef
17. Kim JY, Ok HM, Kim J, Park SW, Kwon SW, Kwon O. Mulberry leaf extract improves postprandial glucose response in prediabetic subjects: a randomized, double-blind placebo-controlled trial. J Med Food. 2015;18(3):306−13. CrossRef
18. Hwang SH, Li HM, Lim SS, Wang Z, Hong JS, Huang B. Evaluation of a standardized extract from Morus alba against α-glucosidase inhibitory effect and post-prandial anti-hyperglycemic in patients with impaired glucose tolerance: a randomized double-blind clinical trial. Evid-Based Complement Altern Med. 2016;2016:8983232. CrossRef
19. Li M, Huang X, Ye H, Chen Y, Yu J, Yang J, et al. Randomized, double-blinded, double-dummy, active-controlled, and multiple-dose clinical study comparing the efficacy and safety of mulberry twig (Ramulus Mori, Sangzhi) alkaloid tablet and acarbose in individuals with type 2 diabetes mellitus. Evid-Based Complement Altern Med. 2016;2016:7121356. CrossRef
20. Józefczuk J, Malikowska K, Glapa A, Stawinska-Witoszynska B, Nowak JK, Bajerska J, et al. Mulberry leaf extract decreases digestion and absorption of starch in healthy subjects—a randomized, placebo-controlled, cross-over study. Adv Med Sci. 2017;62(2):302−6. CrossRef
21. Kishnani P, Tarnopolsky M, Roberts M, Sivakumar K, Dasouki M, Dimachkie MM, et al. Duvoglustat HCl increases systemic and tissue exposure of active acid α-glucosidase in Pompe patients co-administered with alglucosidase α. Mol Ther. 2017;25(5):1199−208. CrossRef
22. Lown M, Fuller R, Lightowler H, FraserA, Gallagher A, Stuart B, et al. Mulberry-extract improves glucose tolerance and decreases insulin concentrations in normo-glycaemic adults: results of a randomised double-blind placebo-controlled study. PLoS One. 2017;12(2):e0172239. CrossRef
23. Riche DM, Riche KD, East HE, Barrett EK, May WL. Impact of mulberry leaf extract on type 2 diabetes (Mul-DM): a randomized, placebo-controlled pilot study. Complement Ther Med. 2017;32:105−8. CrossRef
24. Wang R, Li Y, Mu W, Li Z, Sun J, Wang B, et al. Mulberry leaf extract reduces the glycemic indexes of four common dietary carbohydrates. Medicine. 2018;97(34):e11996. CrossRef
25. Mela DJ, Cao XZ, Dobriyal R, Fowler MI, Lin L, Joshi M, et al. The effect of 8 plant extracts and combinations on post-prandial blood glucose and insulin responses in healthy adults: a randomized controlled trial. Nutr Metabol. 2020;17(51):1−12. CrossRef
26. Momeni H, Salehi A, Absalan A, Akbari M. Hydro-alcoholic extract of Morus nigra reduces fasting blood glucose and HbA1c% in diabetic patients, probably via competitive and allosteric interaction with alpha-glucosidase enzyme; a clinical trial and in silico analysis. J Complement Integr Med. 2021;19(3):763−9. CrossRef
27. Thondre PS, Lightowler H, Ahlstrom L, Gallagher A. Mulberry leaf extract improves glycaemic response and insulinaemic response to sucrose in healthy subjects: results of a randomized, double blind, placebo-controlled study. Nutr Metab. 2021;18(1):1−9. CrossRef
28. Fongsodsri K, Thaipitakwong T, Rujimongkon K, Kanjanapruthipong T, Ampawong S, Reamtong O, et al. Mulberry-derived 1-deoxynojirimycin prevents type 2 diabetes mellitus progression via modulation of retinol-binding protein 4 and haptoglobin. Nutrients. 2022;14(21):4538. CrossRef
29. Mela DJ, Cao XZ, Govindaiah S, Hiemstra H, Kalathil R, Lin L, et al. Dose-response efficacy of mulberry fruit extract for reducing post-prandial blood glucose and insulin responses: randomized trial evidence in healthy adults. Br J Nutr. 2022;11:1−24. CrossRef
30. Qu L, Liang XC, Tian GQ, Zhang GL, Wu QL, Huang XM, et al. Efficacy and safety of mulberry twig alkaloids tablet for treatment of type 2 diabetes: a randomized, double-blind, placebo-controlled multi-centre clinical study. Chin J Integr Med. 2022;28(4):304−11. CrossRef
31. Taghizadeh M, Zadeh AM, Asemi Z, Farrokhnezhad AH, Memarzadeh MR, Banikazemi Z, et al. Morus alba leaf extract affects metabolic profiles, biomarkers inflammation and oxidative stress in patients with type 2 diabetes mellitus: a double-blind clinical trial. Clin Nutr. 2022;49:68−73. CrossRef
32. Takahashi M, Mineshita Y, Yamagami J, Wang C, Fujihira K, Tahara Y, et al. Effects of the timing of acute mulberry leaf extract intake on postprandial glucose metabolism in healthy adults: a randomised, placebo-controlled, double-blind study. Eur J Clin Nutr. 2023:1−6. CrossRef
33. Andallu B, Suryakantham V, Srikanthi BL, Reddy GK. Effect of mulberry (Morus indica L.) therapy on plasma and erythrocyte membrane lipids in patients with type 2 diabetes. Clin Chim Acta. 2001;314:47−53. CrossRef
34. Kojima Y, Kimura T, Nakagawa K, Asai A, Hasumi K, Oikawa S, et al. Effects of mulberry leaf extract rich in 1-deoxynojirimycin on blood lipid profiles in humans. J Clin Biochem Nutr. 2010;47(2):155−61. CrossRef
35. Aramwit P, Petcharat K, Supasyndh O. Efficacy of mulberry leaf tablets in patients with mild dyslipidemia. Phytother Res. 2011;25:365−9. CrossRef
36. Wattanathorn J, Tong-un T, Muchimapura S, Wannanon P, Thukhammee W, Anulukanapakorn K, et al. Evaluation of safety and cognitive enhancing effect of Morus alba leaves extract in healthy older adults. PharmaNutrition. 2014;2(3):102. CrossRef
37. Thukham-Mee W, Wattanathorn J, Kirisattayakul W, Wannanon P. Effect of single administration of mulberry milk on the cognitive function of 6–12-year-old children: results from a randomized, placebo-controlled, crossover study. Oxid Med Cell Longev. 2020;2020:6123759. CrossRef
38. Ma Y, Lv W, Gu Y, Yu S. 1-Deoxynojirimycin in mulberry (Morus indica L.) leaves ameliorates stable angina pectoris in patients with coronary heart disease by improving antioxidant and anti-inflammatory capacities. Front Pharmacol. 2019;10:569−78. CrossRef
39. Wang Y, Yu Z, Jiang J, Li Y, Yu S. Mulberry leaf attenuates atherosclerotic lesions in patients with coronary heart disease possibly via 1-deoxynojirimycin: a placebo- controlled, double-blind clinical trial. J Food Biochem. 2021;45(1):e13573. CrossRef
40. Thaipitakwong T, Supasyndh O, Rasmi Y, Aramwit P. A randomized controlled study of dose-finding, efficacy, and safety of mulberry leaves on glycemic profiles in obese persons with borderline diabetes. Complement Ther Med. 2020;49:102292. CrossRef
41. Costa JP, Brito HO, Galvão-Moreira LV, Brito LG, Costa-Paiva L, Brito LM. Randomized double-blind placebo-controlled trial of the effect of Morus nigra L. (black mulberry) leaf powder on symptoms and quality of life among climacteric women. Int J Gynecol Obstet. 2020;148(2):243−52. CrossRef
42. Hunyadi A, Martins A, Hsieh TJ, Seres A, Zupkó I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS One. 2012;7(11):e50619. CrossRef
43. Li HX, Jo E, Myung CS, Kim YH, Yang SY. Lipolytic effect of compounds isolated from leaves of mulberry (Morus alba L.) in 3T3-L1 adipocytes. Nat Prod Res. 2018;32(16):1963−6. CrossRef
44. Zhang M, Chen M, Zhang HQ, Sun S, Xia B, Wu FH. In vivo hypoglycemic effects of phenolics from the root bark of Morus alba. Fitoterapia. 2009;80(8):475−7. CrossRef
45. Lim SH, Yu JS, Lee HS, Choi CI, Kim KH. Antidiabetic flavonoids from fruits of Morus alba promoting insulin-stimulated glucose uptake via Akt and AMP-activated protein kinase activation in 3T3-L1 adipocytes. Pharmaceutics. 2021;13(4):526. CrossRef
46. Zhang Y, Ren C, Lu G, Cui W, Mu Z, Gao H, et al. Purification, characterization and anti-diabetic activity of a polysaccharide from mulberry leaf. Regul Toxicol Pharmacol. 2014;70(3):687−95. CrossRef
47. Hu TG, Wen P, Shen WZ, Liu F, Li Q, Li EN, et al. Effect of 1-deoxynojirimycin isolated from mulberry leaves on glucose metabolism and gut microbiota in a streptozotocin-induced diabetic mouse model. J Nat Prod. 2019;82(8):2189−200. CrossRef
48. Ramappa VK, Srivastava D, Singh P, Kumar U, Singh V. Mulberry 1-deoxynojirimycin (DNJ): an exemplary compound for therapeutics. J Hortic Sci Biotechnol. 2020;95(6):679−86. CrossRef
49. Jo SP, Kim JK, Lim YH. Antihyperlipidemic effects of stilbenoids isolated from Morus alba in rats fed a high-cholesterol diet. Food Chem Toxicol. 2014;65:213−8. CrossRef
50. Yang Y, Yang X, Xu B, Zeng G, Tan J, He X, et al. Chemical constituents of Morus alba L. and their inhibitory effect on 3T3-L1 preadipocyte proliferation and differentiation. Fitoterapia. 2014;98:222−7. CrossRef
51. Choi JW, Synytsya A, Capek P, Bleha R, Pohl R, Park YI. Structural analysis and anti-obesity effect of a pectic polysaccharide isolated from Korean mulberry fruit Oddi (Morus alba L.). Carbohydr Polym. 2016;146:187−96. CrossRef
52. Kuk EB, Jo AR, Oh SI, Sohn HS, Seong SH, Roy A, et al. Anti-Alzheimer’s disease activity of compounds from the root bark of Morus alba L. Arch Pharm Res. 2017;40:338−49. CrossRef
53. Seong SH, Ha MT, Min BS, Jung HA, Choi JS. Moracin derivatives from morus radix as dual BACE1 and cholinesterase inhibitors with antioxidant and anti-glycation capacities. Life Sci. 2018;210:20−8. CrossRef
54. Awerbach JD, Krasuski RA, Camitta MG. Coronary disease and modifying cardiovascular risk in adult congenital heart disease patients: should general guidelines apply? Prog Cardiovasc Dis. 2018;61:300−7. CrossRef
55. Wirtz PH, von Känel R. Psychological stress, inflammation, and coronary heart disease. Curr Cardiol Rep. 2017;19:111−21. CrossRef
56. Cao H, Ji W, Liu Q, Li C, Huan Y, Lei L, et al. Morus alba L. (Sangzhi) alkaloids (SZ-A) exert anti-inflammatory effects via regulation of MAPK signaling in macrophages. J Ethnopharmacol. 2021;280:114483. CrossRef
57. Chen YC, Tien YJ, Chen CH, Beltran FN, Amor EC, Wang RJ, et al. Morus alba and active compound oxyresveratrol exert anti-inflammatory activity via inhibition of leukocyte migration involving MEK/ERK signaling. BMC Complement Altern Med. 2013;13(45):1−10. CrossRef
58. Chung KO, Kim BY, Lee MH, Kim YR, Chung HY, Park JH, et al. In vitro and in vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J Pharm Pharmacol. 2003;55(12):1695−700. CrossRef
59. Wei J, Chen JR, Pais EM, Wang TY, Miao L, Li L, et al. Oxyresveratrol is a phytoestrogen exerting anti-inflammatory effects through NF-κB and estrogen receptor signaling. Inflammation. 2017;40:1285−96. CrossRef
60. Likhitwitayawuid K. Oxyresveratrol: sources, productions, biological activities, pharmacokinetics, and delivery systems. Molecules. 2021;26(14):4212. CrossRef
61. Enkhmaa B, Shiwaku K, Katsube T, Kitajima K, Anuurad E, Yamasaki M, et al. Mulberry (Morus alba L.) leaves and their major flavonol quercetin 3-(6-malonylglucoside) attenuate atherosclerotic lesion development in LDL receptor-deficient mice. J Nutr. 2005;135(4):729−34. CrossRef
62. Tran HNK, Kim JA, Rho SS, Woo MH, Choi JS, Lee JH, et al. Anti-inflammatory activities of compounds from twigs of Morus alba. Fitoterapia. 2017;120:17−24. CrossRef
63. Zelovaa H, Hanakovaa Z, Cermakova Z, Smejkal K, Dall Acqua S, Babula P, et al. Evaluation of anti-inflammatory activity of prenylated substances isolated from Morus alba and Morus nigra. J Nat Prod. 2014;77(6):1297−303. CrossRef
64. Wu YX, Kim YJ, Kwon TH, Tan CP, Son KH, Kim T. Anti-inflammatory effects of mulberry (Morus alba L.) root bark and its active compounds. Nat Prod Res. 2020;34(12):1786−90. CrossRef