INTRODUCTION
Comprising many layers of epithelial cells, the human skin is the largest organ in the human body [1,2]. The epidermis, dermis, and hypodermis are its three primary layers [1]. The skin’s outermost layer, or epidermis, is a complicated and multilayered structure system that is divided into several layers, or strata [1,3]. Starting from the most superficial layer, these include the stratum corneum, stratum lucidum, stratum granulosum, stratum spinosum, and the stratum basale (the deepest portion of the epidermis) [1]. In addition to these layers, the epidermis comprises specialized cells which include keratinocytes, melanocytes, Langerhans’ cells, and Merkel’s cells [1]. The epidermal portion is followed by the dermis and the hypodermis (deepest layer of the skin) [1,3]. Owing to the skin’s structure it functions as the body’s first line of defense, serving as a barrier to ward off infections, damaging UVA and UVB rays, trauma, and damage, and regulating sweating and water loss [1–4]. The skin microbiome, which is home to a range of microbial communities that preserve the balance within the skin and produce healthy, glowing skin, is another component of the skin’s defensive system [5]. An appropriately functioning skin microbiota is one of the factors that affect optimal skin functioning [6]. It is involved in triggering the immune response and acts as an inflammatory mediator against viruses and infections [4,5]. The skin microbiome is composed of bacteria like Staphylococcus aureus and Staphylococcus epidermidis. Out of the 90% total microbial flora, Actinobacteria (52%), Firmicutes (24%), Proteobacteria (16%), and Bacteroidetes (6%) are the four classes of microbiota predominantly found in human skin [6,7]. The other species found in the skin microbiome are Acinetobacter, Micrococcus, Streptococcus, Corynebacterium, and Propionibacterium acne [6,7]. The human skin serves as the body’s first line of defense, yet it is greatly impacted by multiple factors that contribute to its senescence across all age groups. These elements can be broadly classified into two categories: intrinsic, or chronological factors and extrinsic, or environmental, factors. Phenotypical age-related data collection by preferred reporting item for systematic review and meta-analyses meta-analytical guidelines by Wong and Chew [8] revealed that increasing exposure to extrinsic factors like smoking [8], blue light from the screens [9], air pollution [10], and ultraviolet (UV) light are major contributors towards natural and premature skin aging [3,11]. In addition to these, age, gender, and ethnicity are fundamental intrinsic aging elements that cause pigmentation and skin aging [12]. In the recent and upcoming years, the worldwide population, including both developed and developing nations, will be experiencing increased premature skin aging because of their lifestyles and increased exposure to extrinsic aging factors [13]. Japan and South Korea have the oldest populations of all of them [14]. Particularly among the public, 90% of women are starting to learn more about anti-aging ingredients and skin-care routines and there is a projected market surge for anti-aging products in the next 10 years as shown in Figure 1 [13,14]. However, modern-day issues demand futuristic answers; merely adding active ingredients to your regular skincare regimen is not going to fix it. Instead, the latest developments in the skin-care sector; active substances like retinoids formulated within nano-formulations have shown to be the solution [15,16].
The term “retinoid” is broad and encompasses both synthetic and natural forms of vitamin A analogs, such as retinol [17]. In the skincare industry, a variety of retinoids and their derivatives, such as tretinoin, isotretinoin, adapalene, retinaldehyde, retinyl palmitate (RP), retinol esters, and retinoic acid esters, are used topically to treat acne and aging skin [18]. RP, which is found in eggs, chicken, and beef liver, as well as dairy products such as whole milk, yogurt, cheese, and butter, is a dietary source of vitamin A [19]. Vitamin A is produced by the body from absorbed beta-carotenoids found in squash, cantaloupe, sweet potatoes, pumpkins, carrots, and mangoes for people who are vegan or lactose intolerant [20]. As a naturally occurring endogenous photoprotector, β-carotene prevents the damaging effects of UV radiation on the skin [19]. Numerous research and meta-analyses have also provided evidence for it [21,22]. The polar terminal end, conjugated side chain, and cyclohexenyl ring are the three components that make up the general structure of retinoid molecules [17]. These three fundamental components make up the majority of naturally occurring retinoids, including retinol, retinaldehyde, and retinoic acid as shown in Table 1 [17]. Retinol—a renowned derivative of vitamin A1 [23], is used as an over-the-counter (OTC) medication in the cosmeceutical industry as well as in the form of nutritional supplements [24]. It is a lipophilic molecule that exists as yellow or orange crystals [24]. In addition to its anti-aging properties, retinol supports brain development, growth, vision, hemopoiesis, wound healing, and epithelial cell differentiation [24,25]. Tretinoin—a prescription medicine has shown efficacious responses to improve wrinkling, hyperpigmentation, shallowness, and lentigines caused dire photoaging within a period of a month and effects that lasted for 2 years [26]. Although vitamin A is a stable molecule, exposure to UV radiation renders it inactive [24,26]. The vulnerability of the skin to UV radiation without effective sun protection is a leading cause of early signs of skin senescence that can be controlled by appropriate usage of retinoids [27]. Retinoids are wonder drug molecules that find use in anti-aging therapy for the skin primarily in photoaging and sequential aging [28,29]. Retinoids are fat-soluble vitamins primarily used for acne vulgaris treatment and skin rejuvenation therapy, and their effect can be enhanced by the appropriate employment of nanotechnology and the use of nanoparticles in their formulations [30]. Retinoids or other vitamin A derivatives such as retinoic acid work against the aging process by reducing the presence of skin creasing, and crow’s feet and by increasing the epidermal thickness coupled with increasing the cell turnover rate [31,32]. Retinoids are formulated in a variety of topical preparations like serums, creams, and moisturizers, leave-on or rinse-off cosmetics as described in Table 2. They cause a tingling and a stinging feeling or irritancy after their application on the skin [29,33]. Very often, they also lead to the development of scales on the facial skin and lead to purging and desquamation [29]. These adverse effects of retinoids decrease patient compliance with them [33]. To overcome these problems, nanocarriers are employed for topical retinoid drug delivery in the form of liposomes, solid lipid nanoparticles (SLNs), nanostructured lipid carriers (NLCs), polymeric micelles, and microemulsions [34]. Nanoparticles exhibit controlled drug release, targeted drug delivery, and strong drug adhesion to the skin and are highly specific in their drug delivery [34].
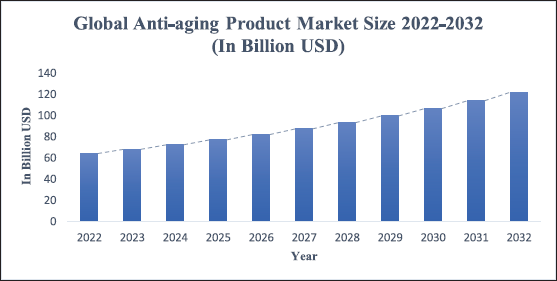 | Figure 1. Graphical representation of projected surge in global anti-aging product market size from the year 2022 to 2023 in billion US dollars. [Click here to view] |
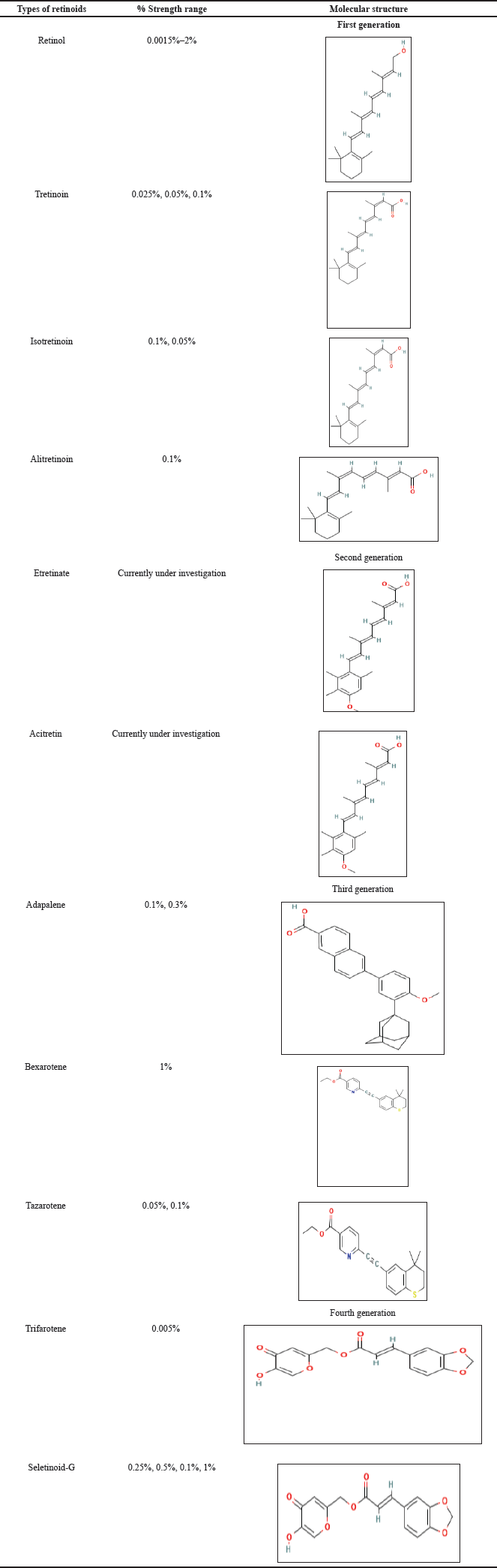 | Table 1. Classification and concentration of retinoids. [Click here to view] |
This review focuses on the increasing demand for skin-care formulations that include retinoids as the primary active ingredient which is formulated into nanotechnological vesicles to maximize patient compliance and minimize side effects in the everyday skincare routine of individuals. For the generation exposed to a greater degree of extrinsic and intrinsic aging triggers, these nano-drug retinoid-loaded vesicles will help to prevent early indications of aging and pigmentation, rejuvenate the skin, and attain a healthy, glowing complexion. The general audience is yet unaware of nano-drug delivery that has promising long-term advantages above easily available marketed cosmetics. To raise public awareness about the advantages of nanotechnology and retinoids, and how their combination rejuvenates the skin without requiring invasive and expensive dermatological procedures, considering the growing market trends for anti-aging products is the central aim of this review.
METHODS AND LITERATURE SEARCH
To prepare this manuscript, literature review, data integration, and collecting processes were initiated in September 2022 and continued until December 2023. Finding novel cosmeceuticals that delay skin aging and promote skin rejuvenation by understanding their mechanism of action, pharmacokinetics, and formulation strategies was the key objective of the data-gathering process. The primary goal in preparing this manuscript was to emphasize the significance of skincare regimens that incorporate cutting-edge novel cosmeceuticals providing nano-drug delivery, resulting in a healthier and more balanced skin barrier that can adapt to changing environmental conditions rather than spending a lot of money on invasive dermatological treatments. ScienceDirect (https://www.sciencedirect.com), PubMed (https://pubmed.ncbi.nlm. nih.gov), and Google Scholar (https://scholar.google.com/) search engines were used to conduct the literature search. Keywords like “nano-drug delivery,” “nano-particles” and “Retinol” were used to obtain maximum data. PubChem (https://pubchem.ncbi.nlm.nih.gov/) was used to describe the structures of Retinoids and their derivatives in more detail and contributed to 13 references. A comprehensive search was done to include the marketed preparations from the global as well as the Indian market and pharmacies. For this review paper, 120 publications were chosen after carefully weighing the data from the literature survey.
SKIN REJUVENATION
The art of skin rejuvenation involves using surgical and non-surgical methods to restore the skin’s most natural, healthy texture and tone by promoting collagen synthesis and reducing fine lines and wrinkles through a variety of mechanisms [15,35–38]. Chemical peeling, ablative [39] and non-ablative laser photo-rejuvenation [39,40], and injectable skin biostimulation and rejuvenation are examples of surgically invasive procedures [36,38,41]. Despite their promising outcomes, they come at a considerable cost [36,38,41]. Botox (Botulinum toxin) fillers [42], microdermabrasion [43], and laser hair removal [44] are the most favored non-invasive dermatological procedures [35,45]. In the last 10 years, most people have turned to at-home skin care to enhance their skin instead of intrusive and invasive procedures like aesthetic plastic surgeries that break the bank [35]. The cosmeceutical industry has witnessed a significant surge of 82% in the use of anti-aging active ingredients such as retinoids due to their ability to improve fine lines and wrinkles and reduce hyperpigmentation when formulated within nano-drug vesicles, in contrast to other active species that may only provide transient benefits [35].
Retinoid classification
An assortment of retinoids find usage for a wide range of skin concerns according to their varying concentrations. Based on the molecular structure retinoids can be classified into four generations [24,46–58]. The molecular structures and concentration strength of retinoids commonly employed in formulations are shown in Table 1.
The aging process
Aging is a natural phenomenon. However, the causes of aging can be natural or man-made. Aging is seen more predominantly on facial skin than in the other parts of the body [1]. The following Figure 2 depicts a young, healthy skin section depicting the presence of hyaluronic acid (HA) and collagen that supports and provides elasticity to the skin.
Aging is characterized by decreased collagen synthesis, fine lines, facial sagging, and depletion of antioxidants such as Vit A, Vit C, and Vit E. The two processes by which aging takes place are intrinsic or chronological aging and extrinsic or photoaging processes [59,61].
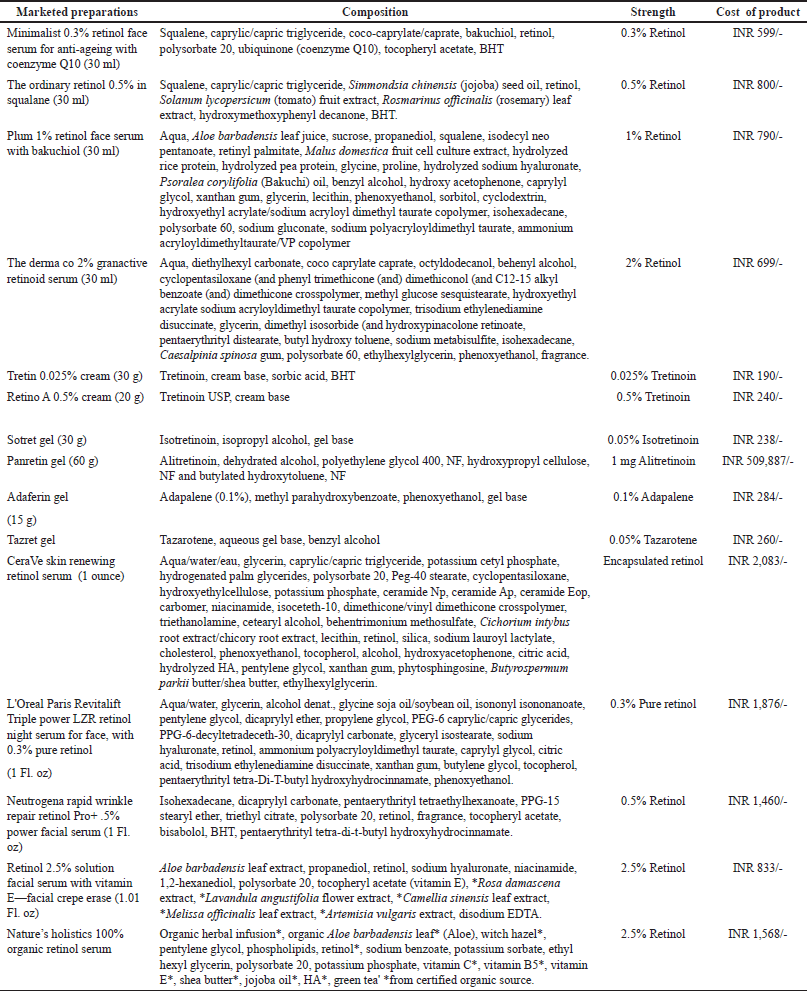 | Table 2. Marketed preparations of retinoids available on a global market scale. [Click here to view] |
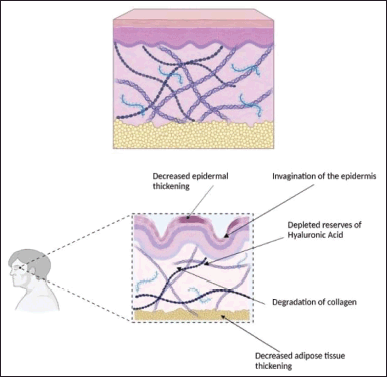 | Figure 2. Healthy skin section with optimum collagen and HA deposition (above) and a skin snippet depicting decreased collagen and HA deposition because of intrinsic aging and photoaging (below). [Click here to view] |
Intrinsic aging
Chronological aging is referred to as intrinsic aging [2,62,63]. It accounts for 10% of skin damage leading to aging [6,62]. Constant generation of reactive oxygen species (ROS) is the cause of skin damage [64]. ROS is responsible for damage to vital cellular components like DNA, enzymes, cell, and plasma membranes [6,62,63,65]. The release of activator protein-1 (AP-1) is stimulated by the activation of the mitogen-activated protein kinases (MAPK) pathway, which is triggered by the generation of ROS [64,66]. The release of AP-1 causes matrix metalloproteinases (MMP) to activate [62], which in turn decreases the synthesis of collagen and elastin fibers and accelerates their breakdown in the skin [64]. Wrinkles and sagging are common indicators of aging skin caused due to decreased levels of collagen and elastin [62] as shown in Figure 3. Despite the presence of predisposing factors genetics contain a pivotal importance in the aging process accompanied by the depletion in the sex hormone levels [62]. The eukaryotic chromosome is made of distinct parts, the telomere being the last portion [62,67]. As we age, the cell divisions that take place also increase; the human telomere (TTAGGG) shortens with each increasing cell division [62,67]. Although a greater number of cells possess the potential of about 60–70 postnatal doublings, over the period, they do not proliferate but remain viable and become more susceptible to apoptosis [62,67]. This process is called the biological clock [8,12,62,63,68]. Along with this process, some signaling molecules increase while some decrease in number [62,63]. A decrease or increase in the number of signaling molecules like chemokines and cytokines leads to the damaging of several skin functions and results in fibroblast senescence [62,68]. All these factors decrease the viability of the cells and hence aging occurs. Some of the prominent clinical features of aging are the appearance of benign cherry angiomas, fine lines, and wrinkles along with sloppiness of the skin [12,62]. A decreased volume of dermis and melanocytes is observed [62]. The number of blood vessels also decreases leading to a reduction in blood supply and nerve innervations to the epidermis and dermis [62]. A sparse number of melanocytes leads to greying of hair as well [62]. The age pigment “lipofuscin” marks cell damage and is responsible for the appearance of visible small brown spots on the skin [62,69]. The following Figure 2, depicts a young, healthy skin section depicting the presence of HA and collagen that supports and provides elasticity to the skin.
Extrinsic/photo aging
Environmental factors such as prolonged subjection of the skin to the sun, air pollution like dust and aerosols, blue light [32], tobacco inhalation, alcoholism, smoking, inadequate hydration, stress, poor sleep schedules, and having a diet devoid of essential nutrients contribute to the extrinsic aging process [9,62,70,71]. It accounts for 90% of skin damage leading to aging [62]. About 80% of extrinsic aging occurs from the vulnerability of the skin to UV radiation and is a common cause of early signs of skin senescence that leads to the generation of ROS [62,64,71,72]. The UV radiations can be categorized into UVA and UVB radiations [62]. Both UV radiations exert different effects on the skin. The UVA radiations can percolate into the subcutaneous layers of the skin involving the epidermis and dermis [62]. UVA radiations are held accountable for photoaging and premature aging. The UVA rays can also pass through the glasses of the windows. These radiations cause damage to the dermal layer leading to alterations along with a rise in the collagen-degrading enzymes and xeroderma pigmentosum factor (XPF) primarily found in the epidermis [62]. The beginning of the presence of fine creases on the skin is a result of XPF since it causes invagination of the epidermal layer [62]. On the contrary, UVB radiations do not perforate into the subcutaneous skin layers and get absorbed into the superficial layer of the skin—the epidermis [62,72]. The epidermis consists of specialized cells called keratinocytes and melanocytes [62]. The keratinocytes are responsible for keratin production which provides firmness and elasticity to the skin [62]. The melanocytes are concerned with the production of a pigment called melanin that imparts color to the skin [62]. The UVB radiations attack the DNA of keratinocytes and melanocytes present in the epidermis which results in the production of specific proteolytic enzymes upon being exposed to the UV rays [62,72]. Such affected cells appear sunburnt after 8–12 hours of sun exposure due to the occurrence of UV fingerprints which are characterized by the formation of thymidine dimers [62]. With increasing age, the lysis of thymidine bonds ceases leading to irreversible mutations [62]. Extrinsic aging owes to the lowered expression of type VII collagen in keratinocytes which leads to skin creasing and decreased levels of collagen type I [73]. In contrast, to the healthy skin section, the aged skin tends to show drastically different properties like invagination of the epidermis coupled with decreased collaged and HA deposition as depicted in Figure 2.
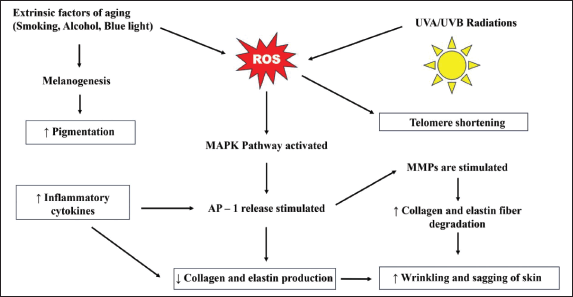 | Figure 3. Mechanism of generation of ROS and its effect on the skin aging process. [Click here to view] |
Mechanism of action of topical retinoids in the anti-aging process
Post-absorption retinoids bind to different nucleic acid and cytosolic receptors at the molecular level to exert their action that initiates immune variations, inflammatory responses, and different cell programs such as cell differentiation and proliferation for epidermal thickening [27,47,74]. Cytosolic retinol binding proteins (CRBP I and II) and cytosolic retinoic acid binding proteins (CRABP I and II) are protein transporters essential to activate the receptors so that subsequent binding to nuclear receptors occurs [47]. Retinoids bind to nuclear receptors like retinoic acid receptors (RAR) and retinoid X receptors (RXR) present within the nucleus of skin cells [27,47,74]. These receptors are ligand-dependent transcription factors, wherein retinoic acid is the natural ligand for the RAR superfamily while 9-cis-retinoic acid is the natural ligand for the RXR superfamily [47]. The nuclear RAR family has three types of isotypes namely RARα, RARβ, and RARγ [47,75]. Human skin predominantly contains heterodimers of RXRα and RARγ [27,75]. Post binding, RAR, and RXR form dimers [47]. Dimers formed can be homodimers (RAR/RAR) or heterodimers (RAR/RXR) [74]. These dimers bind to the suitable retinoid responsive elements (RAREs and RXREs) that induce successive gene expression which leads to cell differentiation [27,47,74]. The mechanism of action of retinoids to repair photodamaged skin can be sought from both the RARs and RXRs as shown in Figure 4 [27]. RARE elements are located near the gene promoter sequences to which retinoid binding occurs [47]. The absence of retinoid-responsive elements leads to competitive inhibition and inhibited gene expression [47,74]. Other transcription factors like AP-1 that regulate gene expression and cytokine proliferation are desensitized thus impacting cell differentiation [47,74]. Topical retinoids act by inducing different cell programs like cell differentiation and proliferation that pave the way for epidermal thickening which helps in skin rejuvenation [27,74,76]. Retinoid derivatives like retinol, or all-trans retinol are extremely lipophilic due to the polarity of the terminal group [45]. A chemical with excellent lipophilicity readily passes through the phospholipid bilayer membrane of the cell and has good absorbability [45]. Retinoic acid has a highly polar group at the terminal position hence greater absorption in contrast to retinaldehyde which has a weak polar group at the terminal position and hence has poor absorption rates [45]. These structural benefits confer upon retinoids several avenues for skin rejuvenation at a much lower cost and pain than invasive dermal procedures for skin rejuvenation [45].
Studies conducted on tretinoin
Retinoids are widely known for their anti-aging benefits for the skin which help in skin firming, inducing elasticity, decreasing shallowness of skin increasing the epidermal thickness, and minimizing the fine creasing and wrinkling of the skin. Some of the retinoids that exert effective benefits are tretinoin, isotretinoin, adapalene, accutane, and retinols [77]. Tretinoin is mostly used for treatment against chronological or intrinsic aging [27,78]. Weiss et al. [79] demonstrated the efficacy of tretinoin by conducting randomized, double-blind studies on an assembly of 30 subjects for a short period of 4 months by using 0.1% tretinoin versus a vehicle [79]. The results exhibited a noticeable increase in glycosamine glycans, a depletion in shallowness, fine lines and wrinkles, compaction of stratum corneum, and an enhancement in perceptible roughness [79,80]. Leyden et al. [81] have proved the efficacy of tretinoin by conducting randomized, double-blind studies on a group of 30 subjects for an extended period of 6 months by using 0.05% tretinoin versus a vehicle. The results showed a reduction in shallowness, fine lines, and wrinkles, and an enhancement in hyperpigmentation [81]. Tretinoin proved to be an effective treatment for clinical signs of photoaging [27]. High strength tretinoin has disadvantages like irritation and dermatitis therefore low strength tretinoin comes to the rescue for patients who are allergic to typically higher concentrations of tretinoin (0.05%) [27]. The US Food and Drug Administration has recognized 0.02% tretinoin as the standard treatment for all skin types [27]. In conclusion, tretinoin primarily acts by amplifying the epidermal thickness, improving fine lines and wrinkles, compaction of stratum corneum, and reducing the laxity of photoaged skin [27]. Along with the anti-aging benefits, tretinoin exerts severe side effects such as irritation, scaling, redness, dermatitis, and dryness. A first-generation retinoid—retinol provides an equal anti-aging benefit but with fewer side effects and reactions. Retinol is chemically known as all-trans-retinol or vitamin A alcohol [27,81]. It comes from the family of endogenous natural retinoids and is a precursor required to produce retinoic acid and retinal [27,81].
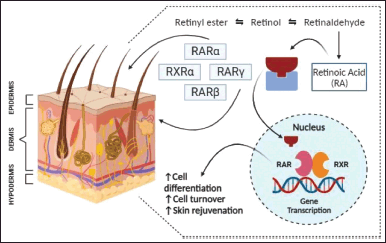 | Figure 4. Mechanism of action and gene transcription of natural and synthetic retinoids in skin rejuvenation. [Click here to view] |
In comparison with tretinoin; retinol exhibited lesser signs of erythema and irritation [34,82]. But Fisher et al. [83], demonstrated that tretinoin is 20 times more effective than retinol. Nonetheless, retinol has effective anti-aging benefits such as epidermal thickening, keratinocyte proliferation, and a fundamental improvement in fine lines and wrinkles but with fewer side effects and aggravation [34].
Studies conducted on retinol
A cream tested by Oddos et al. [84], containing retinol with HA and dihydroxymethylchromone (DMC) demonstrates the retained anti-aging and skin rejuvenating benefits of retinol when paired with key ingredients like HA and DMC on the facial skin. The study was conducted for a period of 8 weeks with a dermal application of the cream [84]. A low concentration of retinol—0.1% when formulated into a cream using HA and DMC in the formulation exhibits a long-term anti-aging benefit on the facial skin [84]. HA is a mucopolysaccharide that helps to hold water molecules in the skin and supports collagen development [73]. Likewise, HA increases keratinocyte expansion through the stimulation of the CD44 receptor in the skin [84]. UV light exposure decreases the CD44 expression which is regressed by the presence of retinol [84]. Furthermore, the addition of DMC in the formulation provides a synergistic action to that of retinol [84]. Kaufman et al. [85], conducted a study by using an eye cream formulated with retinoids and alpha hydroxy acid (AHA, lactic acid) [85]. AHAs are alcoholic carboxylic acids procured from natural substances like fruits and fermented milk products which help in increasing skin hydration and enhancing the rate of keratinocyte proliferation and increased thickening of the dermis [86]. An effective combination of retinoid and AHA-based eye cream was studied with a regular nighttime application for 12 weeks which revealed remarkable advancement in the reduction of darkness and puffiness of the eyes along with reduced lines and wrinkles in the under-eye region [85]. Farris et al. [87], tested a topical cream containing 0.5% retinol along with 4.4% niacinamide, 1% resveratrol, and 1.1% hexylresorcinol all put together in a nourishing moisturizer base [88]. This study was carried out over a period of 10 weeks with 25 subjects who had a moderate number of fine lines, wrinkles, and cutaneous hyperpigmentation [87]. The usage of antioxidants in retinol-based creams provides a synergistic effect in anti-aging therapy. Topically used 5% niacinamide (vitamin B3) is an antioxidant that exhibits anti-aging and skin brightening benefits as elucidated in results of studies conducted by Bissett et al. [89], with 50 female Caucasians for 4-week intervals. Reduced levels of hyperpigmentation were observed in the [89]. Resveratrol is a potent antioxidant that has effective anti-aging benefits and is extensively used in skin care formulations [90]. Furthermore, it is very helpful in reducing signs of photoaging [90] as it possesses the unique capacity to perforate into the subcutaneous layers of the skin and stimulate the increase of collagen III concentrations [91]. Schlessinger et al. [92], stated that hexylresorcinol is an efficient ingredient in anti-aging therapy for the skin. In conclusion, a combination topical cream evaluated by Farris et al. [87], demonstrates efficient anti-aging activity and helps in reducing hyperpigmentation and cohesively increasing the evenness of the skin. Piérard-Franchimont et al. [93], carried out controlled clinical trials with retinol formulations which demonstrated a significant depletion in fine lines after 12 weeks of application with reduced levels of MMP. In conclusion, retinol is an efficient treatment for aging skin, but its efficacy entirely depends on the vehicle being used for its delivery as it is highly unstable and gets degraded easily upon exposure to UV radiation [27].
Adverse effects of retinoids
Antithetical to the good benefits that retinoids have to offer they come with their own set of side effects commonly called the “retinoid reaction.” Retinoids tend to cause a tingling and a stinging feeling after their application on the skin [29]. Very often, they also lead to the development of redness, dryness, and scales on the facial skin [29]. Retinoids are a potent form of vitamin A which is primarily used for targeting skin concerns like acne, hyperpigmentation, fine lines, and wrinkles. Studies suggest that continual usage of retinoids by oral or topical dosage forms leads to a rise in serum vitamin A levels. Increased Vit A levels are harmful to the growing fetus. Therefore, oral, and topical usage of retinoids is not recommended for pregnant women and lactating mothers [94]. Studies have shown that retinoids like isotretinoin and accutane should be avoided during pregnancy as it leads to fetal retinoid syndrome and the risk of developing this syndrome stands high at 35% if pregnant females use retinoids [72,95]. Fetal retinoid syndrome is a rare case of birth defect that involves congenital malformations and teratogenic effects which results in cardiovascular and central nervous system abnormalities [95].
Marketed preparations of retinoids
Retinoids are formulated in a variety of topical preparations like serums, creams, and moisturizers, leave-on or rinse-off cosmetics. The various preparations commonly used are described in Table 2 along with the ingredients used in the formulation.
Nanotechnological approach in retinoid-based cosmeceuticals
Nanotechnology refers to the improved absorption or penetration of the active ingredient species into the body because of the highly reduced size of the drugs [34]. It focuses on nano-drug delivery at specific targets in the body and is a very useful method of topical and dermatological nano-drug delivery [34,96]. Nanotechnology in cosmeceuticals is implied through “nanocarriers” that target specific skin concerns and provide sustained release of drugs [97]. The skin has several factors that influence the rate of absorption and penetration of active ingredients like intermolecular drug interactions and the physicochemical properties of the nanoparticles like particle size and partition coefficient [34]. Moreover, the skin is in continual contact with the environment making it more vulnerable to wear and tear. At the cellular level, epidermal cell cohesion and the presence of lipids in the stratum corneum impose a barrier to the effective absorption of the drugs [98]. Considering these barriers, nanotechnology is an efficient method for better absorption and penetration of active ingredients [34]. The nanoparticles or nanocarriers used in skincare formulations help in adhering the active ingredient to the skin for a prolonged time without much degradation or spoilage and do not disturb the normal functions of the skin [97]. Furthermore, better absorption and penetration of drugs in the skin are observed owing to the small size of the active pharmaceutical ingredient (API) [34]. Nanocarriers are colloidal particles with a size range of 10–1,000 nm. The skin penetration mechanism is influenced by the rigidity, hydrophobic nature, particle size, and particle charge of the nanoparticles [34,99]. These small nanocarriers are available as liposomes, SLNs, NLCs, polymeric micelles, and hydrogels [34]. Nanoparticles exhibit controlled drug release, targeted drug delivery, and strong drug adhesion to the skin [34,99,100]. They are highly specific in their drug delivery thus leading to a reduced percentage of active ingredients used in the formulations and fewer side effects are observed [34,99]. A sunscreen formulation tested against its non-nanosized formulation not having TiO2 and ZnO nanoparticles had lower penetration than their nanosized counterparts [34,97].
Nanotechnological delivery for retinoids
Retinoids are linked with an extensive number of side effects post-application while some adverse reactions take place due to the route of administration. To overcome these side effects, Nano formulations have been developed that help in effective drug delivery, improved penetration mechanisms, and a significant reduction of retinoid reactions post-dermal application [49,101]. Retinoids especially retinol a highly popular OTC anti-aging treatment are formulated either within serums, creams, or nano gels for their increased therapeutic action. Nano formulations are favorable as they have low toxicity and lower biodegradation levels [34]. It consists of various delivery systems for efficient drug penetration and increased skin bioavailability, the formulations widely used are lipid-based, polymer-based, and metal-based [34,97]. Retinoids are typically formulated using lipid-based nanosystems which include liposomes, niosomes, SLNs, NLCs, and nanoemulsions. These nanoparticles help in the effective drug delivery of poorly soluble substances across the skin barrier along with the addition of excipients that aid the absorption of drugs through the skin. Among these nano-drug delivery systems, liposomes hold immense importance and are by far the highest used materials for Retinoid formulations.
Liposomes
Liposomes are small, spherical vesicles that are prepared from phospholipids and cholesterol and they deliver both lipophilic and hydrophilic drugs [102]. Due to their small size, they have a higher penetration rate through the superstratum [74]. Based on their size liposomes are classified into three types:
a. Multilamellar vesicles (300–5,000 nm) [74].
b. Large unilamellar vesicles (100–300 nm) [74].
c. Small unilamellar vesicles (20–100 nm) [74].
Due to their size and penetration powers, liposomes hold immense importance and are by far the highest-used material for retinoid formulations. Studies conducted by the Korean Society [30,103] have shown that retinol when incorporated into lamellar liposomes exhibits a slower degradation and an increased shelf life as compared to its non-liposomal counterparts. Studies conducted by [104] use multi-layered liposomal delivery technology which incorporates 0.5% retinol coupled with niacinamide and Terminalia chebula [104]. Retinol is an OTC drug that is spontaneously converted to retinoic acid for exerting its therapeutic action. A 12-week in-vivo study involved topical application of this formulation which had positive outcomes such as patient compliance mitigation of irritation and reduced fine lines [104]. Transferosomes, flexible liposomal counterparts, were loaded with RP to assess its penetration in the skin [105]. The development of RP-loaded transferosome cream was assessed in three distinct systems [105]. In the first investigation, only 7.64% of the RP was able to pass the membrane throughout 30 hours in vitro over a synthetic membrane [105]. The RP-containing transferosomes were effective in breaking through the membrane and releasing the RP [105]. Franz-cells were used for full-thickness pig skin penetration testing, wherein 63% RP was effectively released in the second research than with its plain RP counterparts [105]. In-vivo topical application of transferosome RP cream was performed on six healthy volunteers but the results showed no difference between the transferosome RP cream and its non-transferosome counterpart in terms of compatibility [105]. Overall, this study developed a transferosome RP-loaded cream that is a good candidate for achieving targeted drug delivery [105].
Solid–lipid nanoparticles
SLNs are comprised of a solid lipid core enclosed within a layer of surfactants [74,106]. SLNs deliver both hydrophilic and lipophilic drugs and their size ranges between 40 and 1,000 nm [107]. It has been introduced to induce enhanced dermal absorption [108] and to overcome the drawbacks of liposome-based delivery systems [108,109] SLNs exhibit a sustained drug release and extended fortification of active compounds and a reduction in chemical degradation [97,103,108–110] when the core is filled with the drug and burst release when the shell is loaded with API [74]. A comparable in-vitro study conducted by [111] reported prolonged release of vitamin A palmitate for up to 24 hours, which was used as the primary API embedded in the core of SLN [111,112]. The developed gel had twice higher drug retention in the superstratum with enhanced hydration and no skin irritancy in contrast to the traditional gel prepared [111]. Jun et al. [113], developed a cream containing, 0.1% retinol-loaded solid lipid nanocarriers employing the vacuum emulsification method (VLN-ROL) to maintain the stability of retinol in the formulation. This prepared cream was evaluated on porcine skin and a 3D human skin model [113]. Application of this VLN-ROL-containing cream to the porcine skin demonstrated better retinol retention as compared to other commercial products procured from BASF [113]. Similarly, the 3D human skin model exhibited increased thickness of the superstrata, a boost in collagen levels, and lessened irritation in contrast to other formulations tested [113]. NLCs are the next generation of SLNs developed to counterfeit the drawbacks of SLNs in terms of their core loading capacity [74] NLCs contain both solid and liquid lipids in their cores in contrast to SLNs [112].
Microemulsion
Microemulsions are colloidal nanosystems that exist as transparent, monophasic systems which are thermodynamically stable. They contain an organic phase and aqueous phase dispersed in a solution which is stabilized by the addition of a mixture of surface-active agents coupled with co-surfactants [113,114]. Microemulsions are of potential use in targeted delivery for dermal applications, lessened skin irritancy, and improved bioavailability of drugs [115] Tretinoin lacks water solubility and is a chemically unstable, photo-unstable ingredient that is it undergoes degradation upon exposure to light [116]. Topical application of tretinoin leads to redness and skin irritation [27]. The incorporation of efficient carriers for the drug helps to transport the drug through an aqueous vehicle and has also shown increased rates of drug stability and drug retention [116]. As per the studies conducted by Špaglová et al. [117], the potential of a new microemulsion was studied and prepared using carbopol as a wetting agent incorporating tretinoin (TRE) as the active ingredient which was later dispersed in a hydrogel for effortless application purposes [116,117]. This study was conducted in comparison with a hydrogel formulated with xanthan gum as a wetting agent with a similar procedure [116,117]. A comparison of permeation enhancers used in the microemulsion proved that the tretinoin penetration rates were 2.5 times higher with increased drug retention and improved skin bioavailability of tretinoin-after-dermal-application-of carbopol-based hydrogel [116,117]. Algahtani et al. [118], prepared a nano-emulgel system containing RP as the API for increasing the drug transport across the skin and overcoming the side effects caused by the application of retinoid derivative—RP. This study assessed the stability of RP and its hydrophobicity [118]. Stability evaluation studies of the nano-emulgel suggest increased UVA stability upon exposure to direct UVA rays as compared to that of pure RP exposed [118]. This increased UV stability is obtained by the virtue of encapsulation of RA within the nano-emulgel [118].
Niosomes
Niosomes are second-generation nanotechnological vesicles enclosed by an admixture of non-ionic surface-active agents like cetyl alcohol, polysorbates, and cholesterol instead of phospholipids [74]. It overcomes the drawbacks posed by liposomes and provides better penetration mechanisms through the superstrata, decreased skin irritancy, and enhanced stability [74]. Niosomes can deliver both hydrophilic and lipophilic drugs. Studies conducted by Manconi et al. [119], compare the in vitro delivery of trans retinoic acid (TRA) or tretinoin-loaded niosomes and liposomes. After evaluating the incorporation of vesicles in-vitro by Franz cells on newborn pig skin and the high-performance liquid chromatography method it was concluded that formulations containing niosomes had a greater skin bioavailability as compared to other formulations evaluated in this study [119]. Surfactants also play a crucial role in deciding the absorption and retention of the TRA niosomes and liposomes. TRA niosomes were formulated using two non-ionic surfactants, ORAMIX CG110 and ORAMIX NS 10. The former showed more targeted (trans) dermal delivery than the latter, which showed improved TRA retention within the skin [119].
CONCLUSION AND OUTLOOK
Skin aging is an intricate and complex process that is being comprehended from its mechanism of action and is a field that is very much sought for treatment via topical and ingestible modes of action. Primarily skin ages due to intrinsic and extrinsic predisposing factors, which may differ according to different parts of the world. Signs of skin aging are shown in people above the age of 35. Premature aging has become a common concern due to several predisposing factors, such as those that accelerate the aging process of the skin. However, society at large, especially women over the age of 35, has begun to become more conscious of their appearance. Fluctuating work schedules, and disturbed sleep patterns make it even more difficult to maintain healthy, youthful-looking skin without the use of cutting-edge novel cosmeceuticals. The ability to rejuvenate and restore the natural glow of skin has been made possible by the development of innovative anti-aging cosmeceutical solutions that incorporate nanotechnological vesicle-loaded drug delivery techniques. Although intrinsic factors are beyond one’s control, the rate of extrinsic or photo-induced aging can be restrained with the appropriate usage of anti-aging molecules like retinoids in the form of tretinoin, isotretinoin, retinol, and adapalene formulated within the topical route of administration and seldom via an oral route. The key function of retinoids is to mitigate the signs of aging and help achieve healthy and well-maintained skin even in older adults. However, retinoids are accompanied by a lot of adverse reactions that lead to a decline in patient compliance. To overcome these adverse effects, retinoids are formulated within nanotechnological vesicles and other potential delivery systems to reap the benefit without the adverse effects. Polymeric micelles, liposomes, SLNs, and microemulsion-based hydrogels have opened a new era of targeted drug delivery systems with little to no side effects. SLNs are potential nanotechnological delivery systems, their presence in sunscreens as TiO2 (titanium dioxide) and ZnO (zinc dioxide) loaded SLNs helps prevent sun damage due to UV radiations and can benefit in photodamage of the skin preventing fine lines and wrinkles. Before SLNs; Liposomes were used extensively in cosmeceuticals but owing to their side effects now SLNs are used further. Considerable research is being carried out to minimize the chemical, particle-particle, and particle-skin interactions that contribute to the instability of nanoparticles. Evaluation of patient health and safety post-exposure to nanoparticles is still under evaluation. Emerging studies for skin rejuvenating cosmeceuticals focus on developing newer derivatives of retinol possessing fewer side effects, but due to the developmental cost involved the process is slowed down. Research is now being carried out to find retinol-mimetic ancient medicines like PADMA-28 that stimulate procollagen synthesis and help fight the signs of aging. Overall evolving research will focus on the development of innovative retinoid derivatives like N-formyl aspartate, drugs having receptor selectivity like seletinoid-G, combination therapy of API having synergistic effects, retinyl ascorbate co-drug for better acceptability and ancient medicines having therapeutic benefits. In conclusion, continued help and support of dermatologists is required for the endorsement of newer drug delivery technologies and their potential benefits.
ACKNOWLEDGMENTS
The authors are very grateful to the Advisory management and Dean, School of Health Science and Technology, Department of Pharmaceutical Sciences, Dr. Vishwanath Karad, MIT-World Peace University, Pune for providing an opportunity to carry out the work and extending constant support and encouragement throughout the work.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
REFERENCES
1. Yousef H, Alhajj M, Sharma S. Anatomy, skin (integument), epidermis. [Updated 2022 Nov 14]. [Internet]. Treasure Island, FL: StatPearls Publishing; 2023. Available from: https://www.ncbi.nlm.nih.gov/books/NBK470464/
2. Chambers ES, Vukmanovic-Stejic M. Skin barrier immunity and ageing. Immunology. 2020 Jun;160(2):116–25. CrossRef
3. Kim JC, Park TJ, Kang HY. Skin-aging pigmentation: who is the real enemy? Cells. 2022 Aug 16;11(16):2541. CrossRef
4. Bonifant H, Holloway S. A review of the effects of ageing on skin integrity and wound healing. Br J Community Nurs. 2019 Mar 1;24(Sup3):S28–33. CrossRef.
5. Luna PC. Skin microbiome as years go by. Am J Clin Dermatol. 2020;21(Suppl 1):12–7. CrossRef
6. Skowron K, Bauza-Kaszewska J, Kraszewska Z, Wiktorczyk-Kapischke N, Grudlewska-Buda K, Kwieci?ska-Piróg J, et al. Human skin microbiome: impact of intrinsic and extrinsic factors on skin microbiota. Microorganisms. 2021;9(3):543. CrossRef
7. Grice EA, Kong HH, Conlan S, Deming CB, Davis J, Young AC, et al. Topographical and temporal diversity of the human skin microbiome. Science. 2009;324:1190–2.
8. Wong QYA, Chew FT. Defining skin aging and its risk factors: a systematic review and meta-analysis. Sci Rep. 2021 Nov 11;11(1):22075. CrossRef
9. Campiche R, Curpen SJ, Lutchmanen-Kolanthan V, Gougeon S, Cherel M, Laurent G, et al. Pigmentation effects of blue light irradiation on skin and how to protect against them. Int J Cosmet Sci. 2020 Aug;42(4):399–406. CrossRef.
10. Schikowski T, Huls A. Air pollution and skin aging. Curr Environ Health Rep. 2020;7:58–64. CrossRef
11. Gilchrest BA. Skin aging and photoaging: an overview. J Am Acad Dermatol. 1989;21:610–3. CrossRef
12. Gilchrest BA, Szabo G, Flynn E, Goldwyn RM. Chronologic and actinically induced aging in human facial skin. J Investig Dermatol. 1983;80:81s–5s. CrossRef
13. He X, Wan F, Su W, Xie W. Research progress on skin aging and active ingredients. Molecules. 2023 Jul 20;28(14):5556. CrossRef
14. Peng D. The Status quo of population aging and the development of the social security system in china. Chin Soc Secur Rev. 2023;7:31–47. Chinese.
15. Farris P, Draelos ZD, Felipe de Oliveira Stehling L. Novel facial treatment regimen improves aging skin appearance. J Drugs Dermatol. 2021 Mar 1;20(3):274–8. CrossRef.
16. Dayan SH, Bacos JT, Ho TT, Gandhi ND, Gutierrez-Borst S, Kalbag A. Topical skin therapies in subjects undergoing full facial rejuvenation. J Cosmet Dermatol. 2019 Jun;18(3):798–805. CrossRef.
17. Sani BP, Hill DL. Structural characteristics of synthetic retinoids. Methods in enzymology. Academic Press; 1990. Vol. 189, pp 43–50, ISSN 0076-6879, ISBN 9780121820909. doi: https://doi.org/10.1016/0076-6879(90)89274-L. Available from: https://www.sciencedirect.com/science/article/pii/007668799089274L
18. Stratigos AJ, Katsambas AD. The role of topical retinoids in the treatment of photoaging. Drugs. 2005;65(8):1061–72. CrossRef.
19. Alaluf S, Heinrich U, Stahl W, Tronnier H, Wiseman S. Dietary carotenoids contribute to normal human skin color and UV photosensitivity. J Nutr. 2002;132:399–403.
20. Britton G, Liaaen-Jensen S, Pfander H. Carotenoids. Basel, Swtizerland: Birkhäuser; 2008.
21. Köpcke W, Krutmann J. Protection from sunburn with beta-carotene—a meta-analysis. Photochem Photobiol. 2008;84:284–8. CrossRef
22. Sies H, Stahl W. Nutritional protection against skin damage from sunlight. Annu Rev Nutr. 2004;24:173–200. CrossRef
23. Bondi A, Sklan D. Vitamin A and carotene in animal nutrition. Prog Food Nutr Sci. 1984;8(1–2):165–91.
24. PubChem [Internet]. PubChem compound summary for CID 445354, retinol. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2023 Aug 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Retinol
25. Sadick N, Edison BL, John G, Bohnert KL, Green B. An advanced, physician-strength retinol peel improves signs of aging and acne across a range of skin types including melasma and skin of color. J Drugs Dermatol. 2019 Sep 1;18(9):918–23.
26. Sitohang IBS, Makes WI, Sandora N, Suryanegara J. Topical tretinoin for treating photoaging: a systematic review of randomized controlled trials. Int J Womens Dermatol. 2022 Mar 25;8(1):e003. CrossRef.
27. Mukherjee S, Date A, Patravale V, Korting HC, Roeder A, Weindl G. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Interv Aging. 2006;1(4):327–48. CrossRef.
28. Herndon JH Jr, Jiang LI, Kononov T, Fox T. An open label clinical trial to evaluate the efficacy and tolerance of a retinol and vitamin C facial regimen in women with mild-to-moderate hyperpigmentation and photodamaged facial skin. J Drugs Dermatol. 2016 Apr;15(4):476–82.
29. Dhaliwal S, Rybak I, Ellis SR, Notay M, Trivedi M, Burney W, et al. Prospective, randomized, double-blind assessment of topical bakuchiol and retinol for facial photoageing. Br J Dermatol. 2019 Feb;180(2):289–96. CrossRef.
30. Oliveira MB, do Prado AH, Bernegossi J, Sato CS, Lourenço Brunetti I, Scarpa MV, et al. Topical application of retinyl palmitate-loaded nanotechnology-based drug delivery systems for the treatment of skin aging. Biomed Res Int. 2014;2014:632570. CrossRef
31. Kong R, Cui Y, Fisher GJ, Wang X, Chen Y, Schneider LM, et al. A comparative study of the effects of retinol and retinoic acid on histological, molecular, and clinical properties of human skin. J Cosmet Dermatol. 2016 Mar;15(1):49–57. CrossRef.
32. Riahi RR, Bush AE, Cohen PR. Topical retinoids: therapeutic mechanisms in the treatment of photodamaged skin. Am J Clin Dermatol. 2016 Jun;17(3):265–76. CrossRef.
33. Fu JJ, Hillebrand GG, Raleigh P, Li J, Marmor MJ, Bertucci V, et al. A randomized, controlled comparative study of the wrinkle reduction benefits of a cosmetic niacinamide/peptide/retinyl propionate product regimen vs. a prescription 0.02% tretinoin product regimen. Br J Dermatol. 2010 Mar;162(3):647–54. CrossRef.
34. Gupta S, Bansal R, Gupta S, Jindal N, Jindal A. Nanocarriers and nanoparticles for skin care and dermatological treatments. Indian Dermatol Online J. 2013 Oct;4(4):267–72. CrossRef.
35. Bowler PJ. Impact on facial rejuvenation with dermatological preparations. Clin Interv Aging. 2009;4:81–9. CrossRef
36. Nelson BR, Majmudar G, Griffiths CE, Gillard MO, Dixon AE, Tavakkol A, et al. Clinical improvement following dermabrasion of photoaged skin correlates with synthesis of collagen I. Arch Dermatol. 1994;130:1136–42. CrossRef
37. Jager C, Brenner C, Habicht J, Wallic R. Bioactive reagents used in mesotherapy for skin rejuvenation in vivo induce diverse physiological processes in human skin fibroblasts in vitro—a pilot study. Exp Dermatol. 2011;21:70–80.
38. Baumann L. Skin ageing and its treatment. J Pathol. 2007;211:241–51. CrossRef
39. Sadick NS. Update on non-ablative light therapy for rejuvenation: a review. Lasers Surg Med. 2003;32:120–8. CrossRef
40. Hardaway CA, Ross EV. Nonablative laser skin remodeling. Dermatol Clin. 2002;20:97–111, ix. CrossRef
41. Ganceviciene R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. Skin anti-aging strategies. Dermatoendocrinol. 2012 Jul 1;4(3):308–19. CrossRef.
42. Camargo CP, Xia J, Costa CS, Gemperli R, Tatini MD, Bulsara MK, et al. Botulinum toxin type A for facial wrinkles. Cochrane Database Syst Rev. 2021 Jul 5;7(7):CD011301. CrossRef.
43. Spencer JM. Microdermabrasion. Am J Clin Dermatol. 2005;6:89–92. CrossRef
44. Ross EV, Domankevitz Y. Hair removal with blended 755/1064 nm laser energy. Lasers Surg Med. 2021 Oct;53(8):1020–5. CrossRef.
45. Redfern CPF. Chapter one—vitamin A and its natural derivatives. In: Pohl E, editor. Methods in enzymology. Academic Press; 2020. Vol. 637, pp 1–25. ISSN 0076-6879, ISBN 9780128201442. CrossRef
46. Kolli SS, Pecone D, Pona A, Cline A, Feldman SR. Topical retinoids in acne vulgaris: a systematic review. Am J Clin Dermatol. 2019 Jun;20(3):345–65. CrossRef.
47. Zasada M, Budzisz E. Retinoids: active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol Alergol. 2019 Aug;36(4):392–7. CrossRef.
48. PubChem [Internet]. PubChem compound summary for CID 444795, tretinoin. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2023 Aug 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Tretinoin
49. Milosheska D, Roškar R. Use of retinoids in topical antiaging treatments: a focused review of clinical evidence for conventional and nanoformulations. Adv Ther. 2022 Dec;39(12):5351–75. CrossRef.
50. PubChem [Internet]. PubChem compound summary for CID 5282379, isotretinoin. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2023 Aug 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Isotretinoin
51. PubChem [Internet]. PubChem compound summary for CID 449171, alitretinoin. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2023 Aug 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Alitretinoin
52. PubChem [Internet]. PubChem compound summary for CID 5282375, etretinate. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2023 Aug 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Etretinate
53. PubChem [Internet]. PubChem compound summary for CID 5284513, acitretin. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2023 Aug 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Acitretin
54. PubChem [Internet]. PubChem compound summary for CID 60164, adapalene. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2023 Aug 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Adapalene
55. PubChem [Internet]. PubChem compound summary for CID 82146, bexarotene. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2023 Aug 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Bexarotene
56. PubChem [Internet]. PubChem compound summary for CID 5381, tazarotene. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2023 Aug 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Tazarotene
57. PubChem [Internet]. PubChem compound summary for CID 11518241, trifarotene. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2023 Aug 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/Trifarotene
58. PubChem [Internet]. PubChem compound summary for CID 70691610, seletinoid G. Bethesda, MD: National Library of Medicine (US), National Center for Biotechnology Information; 2004 [cited 2023 Aug 26]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/seletinoid-G
59. Kafi R, Kwak HS, Schumacher WE, Cho S, Hanft VN, Hamilton TA, et al. Improvement of naturally aged skin with vitamin A (retinol). Arch Dermatol. 2007 May;143(5):606–12. CrossRef
60. Dobos G, Lichterfeld A, Blume-Peytavi U, Kottner J. Evaluation of skin ageing: a systematic review of clinical scales. Br J Dermatol. 2015;172:1249–61. CrossRef
61. Krutmann J, Bouloc A, Sore G, Bernard BA, Passeron T. The skin aging exposome. J Dermatol Sci. 2017;85:152–61. CrossRef
62. Puizina-Ivic N. Skin aging. Acta Dermatovenerol Alp Pannonica Adriat. 2008 Jun;17(2):47–54.
63. Makrantonaki E, Zouboulis CC. William J. Cunliffe scientific awards. Characteristics and pathomechanisms of endogenously aged skin. Dermatology. 2007;214:352–60. CrossRef
64. Masaki H. Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci. 2010;58(2):85–90. ISSN 0923-1811. CrossRef
65. Zasada M, Budzisz E, Erkiert-Polguj A. A clinical anti-ageing comparative study of 0.3 and 0.5% retinol serums: a clinically controlled trial. Skin Pharmacol Physiol. 2020;33(2):102–16. CrossRef.
66. Cargnello M, Roux PP. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol Mol Biol Rev. 2012 Jun;76(2):496. CrossRef.
67. Ostler EL, Wallis CV, Aboalchamat B, Faragher RG. Telomerase and the cellular lifespan: implications of the aging process. J Pediatr Endocrinol Metab. 2000;13(Suppl 6):1467–76.
68. Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann N Y Acad Sci. 2007;1119:40–50. CrossRef
69. Dayan D, Abrahami I, Buchner A, Gorsky M, Chimovitz N. Lipid pigment (lipofuscin) in human perioral muscles with aging. Exp Gerontol. 1988;23(2):97–102. CrossRef.
70. Lever L, Kumar P, Marks R. Topical retinoic acid for treatment of solar damage. Br J Dermatol. 1990 Jan;122(1):91–8. CrossRef.
71. Morgado-Carrasco D, Gil-Lianes J, Jourdain E, Piquero-Casals J. Oral supplementation and systemic drugs for skin aging: a narrative review. Actas Dermosifiliogr. 2023 Feb;114(2):114–24. English, Spanish. CrossRef.
72. Honeybrook A, Bernstein E. Oral isotretinoin and photoaging: a review. J Cosmet Dermatol. 2020 Jul;19(7):1548–54. CrossRef.
73. Gold MH, Biron JA, Wilson A, Nelson DB. Efficacy and tolerability of a hyaluronic acid-based serum and a peptide-rich cream for the face and neck in subjects with photodamaged skin. J Cosmet Dermatol. 2022 Aug;21(8):3458–63. CrossRef.
74. Lalrengpuii J, Raza K, Mishra A, Shukla R. Retinoid nanoparticulates: approachable gateway for acne treatment. Health Sci Rev. 2022;2022:100042. ISSN 2772-6320. CrossRef
75. Shin JW, Kwon SH, Choi JY, Na JI, Huh CH, Choi HR, et al. Molecular mechanisms of dermal aging and antiaging approaches. Int J Mol Sci. 2019 Apr 29;20(9):2126. CrossRef.
76. Katsambas AD, Katoulis AC. Topical retinoids in the treatment of aging of the skin. Adv Exp Med Biol. 1999;455:477–82. CrossRef.
77. Zasada M, Budzisz E. Randomized parallel control trial checking the efficacy and impact of two concentrations of retinol in the original formula on the aging skin condition: pilot study. J Cosmet Dermatol. 2020 Feb;19(2):437–43. CrossRef.
78. Hernandez-Perez E, Khawaja HA, Alvarez TY. Oral isotretinoin as part of the treatment of cutaneous aging. Dermatol Surg. 2000 Jul;26(7):649–52. CrossRef.
79. Weiss JS, Ellis CN, Headington JT, Tincoff T, Hamilton TA, Voorhees JJ. Topical tretinoin improves photoaged skin. A double-blind vehicle-controlled study. JAMA. 1988 Jan 22–29;259(4):527–32. Erratum in: JAMA 1988 Aug 19;260(7):926. Erratum in: JAMA 1988 Jun 10;259(22):3274.
80. Essendoubi M, Gobinet C, Reynaud R, Angiboust JF, Manfait M, Piot O. Human skin penetration of hyaluronic acid of different molecular weights as probed by Raman spectroscopy. Skin Res Technol. 2016 Feb;22(1):55–62. CrossRef.
81. Leyden JJ, Grove GL, Grove MJ, Thorne EG, Lufrano L. Treatment of photodamaged facial skin with topical tretinoin. J Am Acad Dermatol. 1989 Sep;21(3 Pt 2):638–44. CrossRef.
82. Draelos ZD, Peterson INR A double-blind, comparative clinical study of newly formulated retinol serums vs tretinoin cream in escalating doses: a method for rapid retinization with minimized irritation. J Drugs Dermatol. 2020 Jun 1;19(6):625–31. CrossRef.
83. Fisher GJ, Datta SC, Talwar HS, Wang ZQ, Varani J, Kang S, et al. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature. 1996 Jan 25;379(6563):335–9. CrossRef.
84. Oddos T, Roure R, Leyden J, Bruère V, Bertin C. A placebo-controlled study demonstrates the long-lasting anti-aging benefits of a cream containing retinol, dihydroxymethylchromone (DMC) and hyaluronic acid. J Cosmet Dermatol Sci Appl. 2012;02(02):051–9. CrossRef
85. Kaufman J, Callender V, Young C, Jones P, Wortzman M, Nelson D. Efficacy and tolerability of a retinoid eye cream for fine to moderate wrinkles of the periorbital region. J Drugs Dermatol. 2022 Sep 1;21(9):932–7. CrossRef.
86. Babilas P, Knie U, Abels C. Cosmetic and dermatologic use of alpha hydroxy acids. J Dtsch Dermatol Ges. 2012 Jul;10(7):488–91. English, German. CrossRef.
87. Farris P, Zeichner J, Berson D. Efficacy and tolerability of a skin brightening/anti-aging cosmeceutical containing retinol 0.5%, niacinamide, hexylresorcinol, and resveratrol. J Drugs Dermatol. 2016 Jul 1;15(7):863–8.
88. Shariff R, Du Y, Dutta M, Kumar S 5th, Thimmaiah S, Doraiswamy C, et al. Superior even skin tone and anti-ageing benefit of a combination of 4-hexylresorcinol and niacinamide. Int J Cosmet Sci. 2022 Feb;44(1):103–17. CrossRef.
89. Bissett DL, Oblong JE, Berge CA. Niacinamide: a B vitamin that improves aging facial skin appearance. Dermatol Surg. 2005 Jul;31(7 Pt 2):860–5; discussion 865. CrossRef.
90. Baxter RA. Anti-aging properties of resveratrol: review and report of a potent new antioxidant skin care formulation. J Cosmet Dermatol. 2008 Mar;7(1):2–7. CrossRef.
91. Ratz-lyko A, Arct J. Resveratrol as an active ingredient for cosmetic and dermatological applications: a review. J Cosmet Laser Ther. 2019;21(2):84–90. CrossRef.
92. Schlessinger J, Saxena S, Mohr S. Assessment of a novel anti-aging hand cream. J Drugs Dermatol. 2016 Apr;15(4):496–503.
93. Piérard-Franchimont C, Castelli D, Cromphaut IV, Bertin C, Ries G, Cauwenbergh G, et al. Tensile properties and contours of aging facial skin. A controlled double-blind comparative study of the effects of retinol, melibiose-lactose and their association. Skin Res Technol. 1998 Nov;4(4):237–43. CrossRef.
94. Stern RS, Rosa F, Baum C. Isotretinoin and pregnancy. J Am Acad Dermatol. 1984 May;10(5 Pt 1):851–4. CrossRef.
95. Mondal D, Shenoy SR, Mishra S. Retinoic acid embryopathy. Int J Appl Basic Med Res. 2017 Oct–Dec;7(4):264–5. CrossRef.
96. Chaudhary M, Khan A, Gupta M. Skin ageing: pathophysiology and current market treatment approaches. Curr Aging Sci. 2020;13(1):22–30. CrossRef.
97. Raszewska-Famielec M, Flieger J. Nanoparticles for topical application in the treatment of skin dysfunctions—an overview of dermo-cosmetic and dermatological products. Int J Mol Sci. 2022 Dec 15;23(24):15980. CrossRef.
98. Gupta V, Mohapatra S, Mishra H, Farooq U, Kumar K, Ansari MJ, et al. Nanotechnology in cosmetics and cosmeceuticals—a review of latest advancements. Gels. 2022;8:173. CrossRef
99. Shah KA, Date AA, Joshi MD, Patravale VB. Solid lipid nanoparticles (SLN) of tretinoin: potential in topical delivery. Int J Pharm. 2007 Dec 10;345(1–2):163–71. CrossRef.
100. Patzelt A, Mak WC, Jung S, Knorr F, Meinke MC, Richter H, et al. Do nanoparticles have a future in dermal drug delivery? J Control Release. 2017;246:174–82. CrossRef
101. Fex G, Johannesson G. Retinol transfer across and between phospholipid bilayer membranes. Biochim Biophys Acta. 1988 Oct 6;944(2):249–55. CrossRef.
102. Chmykh YG, Nadeau JL. Characterization of retinol stabilized in phosphatidylcholine vesicles with and without antioxidants. ACS Omega. 2020;5:18367–75. CrossRef
103. Pisetpackdeekul P, Supmuang P, Pan-In P, Banlunara W, Limcharoen B, Kokpol C, et al. Proretinal nanoparticles: stability, release, efficacy, and irritation. Int J Nanomedicine. 2016 Jul 21;11:3277–86. CrossRef.
104. Handler M, Adams-Woodford A, Ayres P, Giancola G, Diaz I. Facial aging improvement case study using a novel combination of retinol, niacinamide, and Terminalia chebula. J Drugs Dermatol. 2022 Jul 1;21(7):784–8. CrossRef.
105. Pena-Rodríguez E, Moreno MC, Blanco-Fernandez B, González J, Fernández-Campos F. Epidermal delivery of retinyl palmitate loaded transfersomes: penetration and biodistribution studies. Pharmaceutics. 2020;12:112. CrossRef
106. Blasi P, Giovagnoli S, Schoubben A, Ricci M, Rossi C. Solid lipid nanoparticles for targeted brain drug delivery. Adv Drug Deliv Rev. 2007 Jul 10;59(6):454–77. CrossRef.
107. Pardeike J, Hommoss A, Müller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–84. CrossRef
108. Müller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002 Nov 1;54(Suppl 1):S131–55. CrossRef.
109. Morales JO, Valdés K, Morales J, Oyarzun-Ampuero F. Lipid nanoparticles for the topical delivery of retinoids and derivatives. Nanomedicine (Lond). 2015 Jan;10(2):253–69. CrossRef.
110. Souto EB, Müller RH. Lipid nanoparticles: effect on bioavailability and pharmacokinetic changes. Handb Exp Pharmacol. 2010;(197):115–41. CrossRef.
111. Pople PV, Singh KK. Development and evaluation of topical formulation containing solid lipid nanoparticles of vitamin A. AAPS PharmSciTech. 2006;7(4):91. CrossRef.
112. Puglia C, Bonina F. Lipid nanoparticles as novel delivery systems for cosmetics and dermal pharmaceuticals. Exp Opin Drug Deliv. 2012;9(4):429–41. CrossRef
113. Jun SH, Kim H, Lee H, Song JE, Park SG, Kang NG. Synthesis of retinol-loaded lipid nanocarrier via vacuum emulsification to improve topical skin delivery. Polymers (Basel). 2021 Mar 8;13(5):826. CrossRef.
114. Chen L, Tan F, Wang J, Liu F. Microemulsion: a novel transdermal delivery system to facilitate skin penetration of indomethacin. Pharmazie. 2012 Apr;67(4):319–23.
115. Park H, Mun S, Kim YR. UV and storage stability of retinol contained in oil-in-water nanoemulsions. Food Chem. 2019 Jan 30;272:404–10. CrossRef.
116. Moghimipour E, Salimi A, Leis F. Preparation and evaluation of tretinoin microemulsion based on pseudo-ternary phase diagram. Adv Pharm Bull. 2012;2(2):141–7. CrossRef.
117. Špaglová M, Papadakos M, Cuchorová M, Matušová D. Release of tretinoin solubilized in microemulsion from carbopol and xanthan gel: in vitro versus ex vivo permeation study. Polymers (Basel). 2023 Jan 9;15(2):329. CrossRef.
118. Algahtani MS, Ahmad MZ, Ahmad J. Nanoemulgel for improved topical delivery of retinyl palmitate: formulation design and stability evaluation. Nanomaterials (Basel). 2020 Apr 28;10(5):848. CrossRef.
119. Manconi M, Sinico C, Valenti D, Lai F, Fadda AM. Niosomes as carriers for tretinoin: III. A study into the in vitro cutaneous delivery of vesicle-incorporated tretinoin. Int J Pharm. 2006;311(1–2):11–9. CrossRef