INTRODUCTION
Alzheimer’s disease (AD) is the predominant form of dementia, making up at least 2/3rd of dementia cases among individuals aged 65 and older [1]. It is predicted that 40 million individuals worldwide currently experience dementia and that by the year 2050, that figure will have doubled every 20 years [2]. Neurofibrillary tangles (NFTs) and plaques containing tau are obligatory for the diagnosis of AD [3]. By impairing mitochondrial activity, these aggregation complexes cause a pathological cascade that results in the degeneration of synapses and neurons [4]. Memory and cognitive impairment are caused by the death of the hippocampus and cortical neurons in this condition [5]. Different hypotheses were intended for AD. Khachaturian was the first to put forth the calcium hypothesis, arguing that persistent intracellular calcium abnormalities are the root causes of neurodegenerative diseases such as AD [6]. A recent Swedish study revealed that older women who regularly take calcium supplements may actually raise their risk of contracting dementias such as Alzheimer’s [7]. There is more and more evidence that the disruption of neuronal calcium ion (Ca2+) homeostasis that comes with ageing may be part of what causes AD [6]. Disrupted Ca2+ could result in synaptic impairments and encourage the buildup of amyloid beta (Aβ) plaques and NFT [8].
The amyloid precursor protein (APP) significantly contributes to the pathophysiology of AD in great part due to the sequential proteolytic cleavages that result in the production of β-amyloid peptides [9]. APP is cleaved by three categories of proteases: α-, β-, and γ-secretases. β- and γ-secretase cleave at the N- and C-terminal ends of the Aβ region, respectively, releasing Aβ, whereas α-secretase cleaves within the Aβ sequence. γ-Secretase performs cleavage at multiple adjacent sites, resulting in Aβ species comprising 39–43 amino acid residues [10]. The Aβ peptide, generated from β- and γ-secretase processing of APP, has garnered significant interest as a key factor in AD [11]. Under typical physiological conditions, Aβ serves a regular function and maintains a low concentration in vivo. Nevertheless, elements such as aging, oxidative stress, and genetic mutation lead to the disturbance of Aβ homeostasis. This disruption leads to the buildup and aggregation of Aβ, giving rise to the formation of oligomers and fibers, and ultimately leading to the formation of plaque deposits in brains [12]. Aβ is produced in normal individuals, yet under specific conditions, it can aggregate, initiating the onset of the disease. Extensive evidence underscores that Aβ oligomers are primarily responsible for neuronal dysfunction and the progression of AD [13]. When APP levels are aberrant, A builds up, causing tau to get phosphorylated and aggregate, eventually resulting in NFTs. These NFTs are formed of hyperphosphorylated tau protein fibres that are entangled, insoluble, and concentrated in AD neurons [14].
Tau is widely recognized as a protein associated with microtubules in neurons [15]. Tau is essential for neurons, as it binds and maintains the stability of microtubules and also regulates axonal transport. These functions are controlled by phosphorylation events at specific sites [16]. Post-translational modifications such as phosphorylation, acetylation, and ubiquitination influence the role of tau. In disease conditions, regulation of the equilibrium between phosphorylation and de-phosphorylation is disrupted, resulting in increased abnormal (“hyper”) phosphorylation [17]. Tauopathies refer to neurodegenerative diseases characterized by hyperphosphorylated accumulations of the microtubule-associated protein tau [18]. Atypical phosphorylation (“hyperphosphorylation”) and aggregation of tau proteins are distinguishing features of AD [19].
According to another theory, normal brain function depends on metal homeostasis in the central nervous system (CNS) because metals work as enzyme cofactors and are important parts of both intra- and inter-neuronal communication [20]. In addition, heavy metals encourage oxidative damage that results in neuronal death in several regions of the brain with deficiencies in behaviour, memory, and cognition [21]. Ionic substances, such as aluminum, copper, zinc, and iron, have been associated with the development of extracellular beta-amyloid plaques and intracellular hyperphosphorylation of neurofibrillary tau tangles [22]. Al-induced memory and learning problems appear to be the result of a complex pathology [23]. In addition, these heavy metals cause an overactive inflammatory response, which leads to an imbalance between anti-inflammatory and pro-inflammatory cytokines. This is a key factor in the destruction of brain tissue and the development of neurodegenerative diseases [24].
It is generally known that activation of the microglia is a significant inducer of neuroinflammation including, in particular, AD and Parkinson’s diseases [25]. Therefore, inhibiting microglial activation is a primary objective in the quest to improve the survival of neuronal cells [26]. These aggregation complexes interfere with the operation of the mitochondria, which sets off a pathological chain reaction that ultimately results in the death of synapses and neurons [27].
Oxidative stress in the brain is increasingly recognized as a possible role in the aging process and in age-related neurodegenerative diseases [28]. There is a significant lack of cholinergic activity in both the cortical and hippocampus regions of patients with AD, as indicated by numerous pieces of data [29]. AD is characterized by a fundamental process that is responsible for the clinical manifestations of the disease [30]. This process involves the degeneration and death of neurons [31].
There are a significant number of studies that describe the preventive benefits of different polyphenols against AD [32]. In addition, certain polyphenolic substances have been noted to exhibit a capacity to impede amyloid fibrillation [33]. This review article provides a condensed overview of the recent research that has been conducted on the link between Chrysin and AD, as well as its possible role as a revolutionary molecule in many clinical applications [34].
Chrysin is an inherently occurring hydroxylated flavonoid that can be found in bee propolis, honey, and a variety of plants, including mushrooms, passion flowers, carrots, Passiflora caerulea, Pelargonium crispum, carrots, Passiflora incarnata, and Oroxylum indicum [35]. The chemical formula of chrysin is C15H10O4, and its other name is 5,7-dihydroxy-2-phenyl-4H-1-benzopyran-4-one [36]. The significance of oxygenation at the C-3 position is a trait that is unique to flavones and can be seen to be present in chrysin. This feature distinguishes flavones from other types of molecules [37]. In contrast to the majority of flavonoids, which have either one (often at C-4′) or two hydroxy (C3′, C4′-di, ortho hydroxyl) functional groups in ring-B, these flavonoids only have one hydroxy group (Fig. 1) [38]. The number of hydroxyl groups and where they are located, in addition to the configuration of the polyphenol rings, may all have an influence on the inhibitory actions of the compound [39]. Chrysin, originally discovered in 1949 by Gösta Linstedt at the KTH Royal Institute of Technology in Stockholm, was initially derived from the wood of pine trees [40].
In its chemical composition, chrysin has two benzene rings (A and B) and a pyran-like heterocyclic ring that contains oxygen (Fig. 1). Because of the conjugation, the double bond between C2 and C3 and ring B in the chemical structure of chrysin is going to be coplanar with the rings A and C. Each of these three rings has undergone structural alterations that primarily influence unique biological outcomes [41].
Chrysin possesses a diversity of pharmacological effects, including anticancer, antiapoptotic, antioxidant, neuroprotective, anti-inflammatory, antihemolytic, and antihypertensive [42]. Figure 1 presents a visual representation of the numerous pharmacological activities that have been described [40]. Due to the fact that it can treat a wide variety of conditions through a variety of different pathways, chrysin has garnered a lot of interest recently for the benefits it provides [43]. Chrysin provides prominent neuroprotective properties and decreases neuroinflammation [44]. Chrysin exhibits the ability to overwhelm cognitive impairment and possesses efficacious encephalo-protective properties [45].
CHRYSIN IN AD
The pathophysiology of AD primarily involves two key elements: the accumulation of A and the hyperphosphorylation of tau protein [46]. There is a connection between oxidative stress, the activation of microglia, neuroinflammation, and AD [47].
Chrysin inhibit the aggregation of amyloid
The amyloid hypothesis is one of the established hypotheses for AD [48]. It proposes that the buildup of Aβ peptide is the key factor that leads to the incidence of AD [49]. The formation of Aβ requires the proteolysis of the APP, which results in the evolution of 39–43 amino acids in the Aβ [50]. The β-secretase and γ-secretase enzymes work in tandem to carry out this particular proteolysis in a step-by-step fashion [51]. The protein is cleaved by each secretase at a distinct cleavage site, which results in the creation of a variety of different APP fragments [52]. One of these fragments is the solvable A isoform, which is the more neurotoxic form [53]. In contrast, the insoluble form encourages the generation of reactive oxygen species (ROS) [54]. Therefore, mutations in γ-secretase and β-secretase that hoist up their APP expression and increase their enzymatic activity might be plausible reasons for the buildup of Aβ [55]. The search for anti-amyloid medicines has emerged as a prominent method in AD-related research [56]. This is due to the fact that amylin is believed to be the originator of events that promote neurotoxicity and the clinical signs of AD [57]. Various flavonoids are strongly associated with this hypothesis [58]. Quercetin is one of the flavonoids that has previously been recognized as preventing the aggregation of amylin and disaggregating its fibers [59].
According to the findings of an in-silico and in-vitro study, chrysin has the ability to avert the aggregation of human amylin [60]. Chrysin is able to inhibit the emergence of amyloid aggregates, as demonstrated by the findings of the thioflavin T binding turbidimetry assay [61]. Compatible with thio-flavin T binding, chrysin was reported to prevent the production of amyloid aggregates [62]. In addition, the evaluation of Chrysin’s molecular interactions with amylin indicated that the protein had a substantial binding affinity for amylin [63]. Chrysin’s molecular docking and in vitro results suggest that it may have potential therapeutic applications, one of which is the prevention of amylin aggregation [59]. Chrysin-loaded chitosan nanoparticles were shown to have properties that prevented the aggregation of ROS and Aβ1-42 proteins in a different body of study [64]. In addition, the activity of the enzymes butyryl cholinesterase (BChE) and acetyl cholinesterase (AChE) is inhibited to a significant degree by compounds having heterocyclic motifs, such as tacrine, donepezil, rivastigmine, and galantamine, which serve in the capacity of potent inhibitors of both enzymes [65]. In addition to this, the results of investigations on the kinetics and docking of chrysin generated conclusions that were consistent with these findings [66]. According to research that was achieved in 2019 by Taslimi et al. [67] chrysin has the ability to inhibit acetylcholinesterase and BChE, which results in anti-cholinergic action. This activity was tested on rats that had numerous organs damaged as a result of cyclophosphamide [68]. Chrysin, carvacrol, zingerone, hesperidin, and naringin are natural phenols known for their remarkable suppression effects against human carbonic anhydrase catalysts I and II, as well as α-glucosidase, AChE, and BChE enzymes [69]. These phenols also block the activity of naringin, which has been demonstrated to have anti-cancer properties [70]. The combination of chrysin and luteolin was found to have a protective effect against the development of advanced glycation end products and the aggregation of albumin protein that was generated by glyoxal in in-vitro and molecular docking investigations. These results were obtained by combining the two compounds [71].
Chrysin inhibits the calcium influx-induced β amyloid aggregation and apoptosis
Multiple studies have identified calcium influx and the development of ROS as potential mechanisms responsible for Aβ-induced neurotoxicity [72]. Studies propose that the main occurrence subsequent to Aβ treatment of cultured neurons and neuroblastoma involves calcium influx, most likely facilitated by the L voltage-sensitive calcium channel [73]. This is due to the fact that blocking this channel and/or calcium chelation averts all other consequences [74].
The accumulation of especially (Ca2+), metal ions can accelerate the production of amyloid plaques and NFT [75]. However, there has been no comprehensive investigation into the processes by which Ca2+ affects the evolution of AD [76]. In light of this fact, the current article provides a synopsis of the methods by which Ca2+ is transported into and out of cells and organelles, such as the cell, mitochondrial, endoplasmic reticulum, and lysosomal membranes, to influence the equilibrium of intracellular Ca2+ levels [77]. In addition, Ca2+ dyshomeostasis plays a significant part in the regulation of the pathogenesis of AD by having an effect on the formation and aggregation of peptides as well as the phosphorylation of tau protein [78]. This is in addition to the fact that a disruption in the metabolic balance of Ca2+ can have an effect on the cognitive abilities and memories of people who have AD [79]. Chrysin was able to reduce both the passive cutaneal anaphylaxis and the systemic pseudo-allergy that was observed in an in vivo animal model. The chrysin inhibited Laboratory of Allergic Diseases 2 cell degranulation, Ca2+ inflow, and adenosine 5′-triphosphate content in a dose-dependent manner, which led to substantial suppression of these processes depicted in Figure 2. Chrysin was able to decrease pseudo-allergic reactions by inhibiting the PLC/IP3/Ca2+ route as well as the ERK/STAT3 serine 727 pathway, all of which are downstream of MrgX2 [80]. Furthermore, these substances inhibited apoptosis by reducing mitochondrial dysfunction, including mitigating the mislaying of membrane potential, suppressing Ca2+ accumulation, and regulating the ratio of Bax/Bcl-2 [81]. Moreover, chrysin hindered the expression of cyclooxygenase 2 (COX-2) and inducible nitric oxide synthase (iNOS), leading to the suppression of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), nitric oxide (NO), and prostaglandin E2 (PGE2) [82]. Significantly, all the compounds demonstrated anti-inflammatory effects by inhibiting the nuclear factor-kappa beta (NF-Κb)/mitogen-activated protein kinase (MAPK) pathway [83]. Excessive activation of calcium-calmodulin-dependent protein kinase kinase 2 or AMP-activated protein kinase alone is adequate to trigger the loss of dendritic spines. Conversely, chrysin hinders the activity of these factors and safeguards hippocampal neurons from the synaptotoxic impact of Aβ42 oligomers [84].
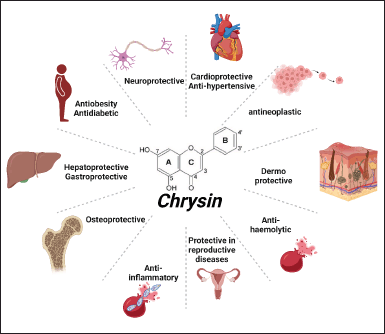 | Figure 1. Chemical structure and pharmacological activities of chrysin. [Click here to view] |
Chrysin suppress microglial activation
Under typical circumstances in the brain, microglia are responsible for regulating homeostasis and providing a line of shielding against damage [85]. Activated microglia exhibit a diversity of individual traits, including pro-inflammatory (M1-like) and anti-inflammatory (M2-like) phenotypes [86]. Depending on the individual traits activated, microglia can produce cytotoxic or neuroprotective effects [87]. However, overactive microglia can cause the production of proinflammatory and cytotoxic substances, which can lead to the evolution of progressive neurological disorders such as AD, ischemia, and Parkinson’s diseases (PD) [88]. Hence, inhibiting the animating of microglia may play a significant role in preserving the well-being of neuronal cells [89]. Reactive glial cells may lead to variations in the typical functioning of the CNS [90]. In spite of the fact that glial activation is initially advantageous, glial reactivity that is excessive and persists for an extended time might result in an inflammatory response that has detrimental consequences on neuronal cells (Fig. 3) [91]. Reactive glial cells are responsible for the production of a number of neurotrophic compounds, but they are also responsible for the production of agents that have the potential to be neurotoxic [92]. It has been hypothesized that the neurotoxic effect of reactive glia is mediated by NO, pro-inflammatory cytokines, and ROS [93]. Chrysin ability to inhibit the expression of cytosine-cytosine-adenosine-adenosine-thymidine/magnifying the binding protein δ in microglial cells leads to beneficial outcomes, including anti-inflammatory and neuroprotective effects [41]. In addition, chrysin increased the level of interleukin-4 while inhibiting the secretion of interferon, tumor necrosis factor, interleukin-1, interleukin-2, interleukin-6, and interleukin-12 by splenic mononuclear cells [94]. These substances have the capability to decrease the expression of pro-inflammatory cytokines (IL-6, TNF-α, and IL-1β) and neurotoxic mediators (NO, PGE2, iNOS, and COX-2), thereby reducing inflammatory markers and protecting against neural damage [95]. Experimental data demonstrates that these substances exert anti-neuroinflammatory effects by regulating pertinent signaling pathways NF-κB, Janus kinase/Signal transducers and activators of transcripition, MAPKs, phosphatidylinostiol 3-kinase/Akt (protein kinase B), and nuclear factor erythroid 2-related factor 2/heme oxygenase 1) [96].
Chrysin inhibits heavy metal-induced AD
Metals, such as lead, zinc, aluminum, copper, and others, are believed to be associated with various neurological conditions, with many of these conditions linked with an elevation in ROS production [97]. Including this, there is mounting product to suggest that the metal might amplify oxidative and inflammatory reactions, which can result in harm to the tissue [98]. Chrysin helps to remediate learning and memory deficiencies caused by aluminum, and also helps to untangle some of the relevant underlying mechanisms [99]. In addition, lead is a toxic heavy metal and its exposure causes cognitive decline, imbalance of pro- and anti-inflammatory cytokines, and suppression of the hippocampal long-term potentiation (LTP) induction [100]. In addition, after exposure to lead, its concentration increases in both vital fluid and brain cells, leading to the apoptosis of neurons [101]. In an investigation involving lead-exposed rats, the administration of chrysin improved cognitive function, mitigated hippocampal LTP impairment, regulated inflammatory responses, lowered lead levels, and prevented neuronal cell death [102]. According to the findings, chrysin ameliorates the cognitive deficit, perhaps by reducing the malfunctioning of hippocampal synapses, modulating the inflammatory response, lowering lead concentrations, and preventing neuronal death (Fig. 4) [103].
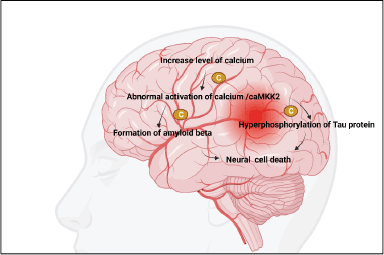 | Figure 2. Role of calcium in AD. [Click here to view] |
Chrysin inhibits oxidative stress in AD
A contrast in the redox state can cause oxidative stress in the brain [104]. This can be caused by the production of an excessive amount of ROS or by a failure in the antioxidant system [105]. Oxidative stress is a process that becomes more prevalent in an aging brain [106]. Patients with AD have brains with a significant amount of oxidative damage, which is related to an abnormal accumulation of Aβ and the deposition of NFT [107]. After administering Aβ25–35, considerable oxidative stress was observed, evident by a significant rise in the levels of thiobarbituric acid reactive substance, acetylcholinesterase, and a decrease in the activities of glutathione peroxidase, glutathione reductase, reduced glutathione, superoxide dismutase, catalase, and Vitamin C [108]. Chrysin administration at doses of 25 and 50 mg/kg body weight reversed the memory deficits observed in rats induced with Aβ25–35 [109]. Memory loss caused by exposure to Aβ25–35 was reversed in rats by giving them chrysin at doses of 25 and 50 mg/kg of body weight [110]. Chrysin treatment has the potential to reduce oxidative damage, as shown in attenuating levels of thiobarbituric acid reactive substance and acetylcholinesterase, as well as a repair in the activity of antioxidant enzymes [41].
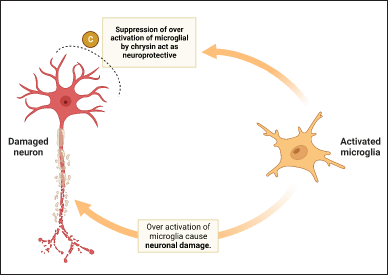 | Figure 3. Role of chrysin on microglia cell. [Click here to view] |
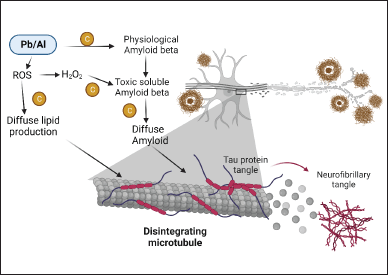 | Figure 4. Role of chrysin in heavy metal-induced AD. [Click here to view] |
Chrysin inhibits inflammation in AD
It has been demonstrated that Aβ itself can operate as a pro-inflammatory agent, which leads to the activation of the inflammatory machinery [111]. Inflammation is a physiological response that has two main objectives. One is to shield the body from potentially deleterious stimuli, and the second is to initiate the healing process to bring the tissue back to its normal state of homeostasis [112]. The expression of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α can be elicited by activated microglia, which can then have an effect on the surrounding neurons and have the potential to play a potentially negative function [113]. Recent investigation has shown that activation of pro-inflammatory cytokines has many roles in both neurodegeneration and neuroprotection processes [114]. In addition, research on the origins of AD has shown that microglia are the initiator of the Aβ protein [115]. This protein is pro-inflammatory and is responsible for the activation of a number of other inflammatory components [116]. The application of chrysin resulted in a decrease in the expression levels of TNF-α, TBARS, caspase-3, and caspase-8. Chrysin considerably reduced inflammation by bringing down the expression of NF-kB/p65/IKK-β and the level of TNF-α [117]. In addition, chrysin had a strong inhibitory effect on apoptosis, by stimulation of Bcl-2 expression and the downregulation of caspase-3 expressions and Bax [118]. In inclusion, chrysin was able to reduce nitro-oxidative stress by restoring normal levels of 8-OHdG, NO, TBARS, CAT, and GSH, as well as manganese superoxide dismutase, NADPH oxidase 4, endothelial nitric oxidase synthase), and nucleotides expression [119]. Chrysin also had a powerful inhibitory effect on the expressions of inducible COX-2 and NO synthase (iNOS) [120]. In addition, chrysin effectively hindered the activation of two essential signaling molecules associated with neuroinflammation: (c-Jun N-terminal kinase) JNK and NF-kB [121].
Formulations of chrysin in the management of AD
Natural substances have been shown to be effective against neurodegenerative disease through different molecular pathways, such as the prevention of the evolution of ROS, the elimination of the deteriorated biomolecules before their buildup has an effect on cell metabolism, and the improvement of disease circumstances [122,123]. However, the distribution of natural compounds into the CNS is limited by the presence of the blood–brain barrier as well as the antagonistic pharmacokinetic features of natural compounds [124]. To reduce the severity of this issue and improve the transport of a drug into the brain at a therapeutically appropriate dose, it is necessary to come up with an innovative and applicable method [125]. According to the findings of numerous research, nanoformulations and microneedles incorporating natural ingredients, such as ferulic acid, quercetin, chrysin, piperine, curcumin, resveratrol, huperzine, berberine, baicalein, and hesperetin, have demonstrated significant potential in enhancing neurodegenerative conditions [126].
A comparison was conducted between the effect of treatment using lipid-core nanocapsules loaded with chrysin and without chrysin. Chrysin was observed to increase the levels of Glutathione reductase (GR), Glutathione peroxidase (GPx), Glutathione S-transferase (GST), and Catalase (CAT), while simultaneously reducing net positive suction head and ROS [127]. Both the hippocampus and the prefrontal cortex were shown to have increased levels of IL-10, which decreases the levels of proinflammatory mediators such as TNF-α and IL-1β [128]. The binding of pharmaceuticals is made possible by magnetic nanoparticles through entrapping the medications on the particles, covalent attachment, or adsorption [129]. In this investigation, we used chrysin-loaded magnetic PEGylated silica nanospheres (MChRPNPs) that were broadly defined and had the potential for enhanced protective features against the oxidative stress generated by amyloid [130]. In rat hippocampus cell cultures, the interactions of MChRPNPs with Aβ were observed [131]. An anti-Alzheimer’s effect was observed in the rat hippocampus region when chrysin-loaded solid lipid nanoparticles were tested against Aβ25–35 caused oxidative stress [110]. It was discovered that the chrysin formulation was entirely effective in treating AD [110]. The formulation of chrysin (0.5 mg/kg) using nose-to-brain administration of transfersomal and composite vesicles showed a protective effect against doxorubicin-induced cognitive impairment in rats. It achieved this by reducing oxidative stress and inhibiting the TLR4/NF-kB/NLRP3 pathways [132]. The similar research was conducted by another researchers too [133,134].
Examples of formulation of chrysin
In comparison to chrysin suspension, it was discovered that chrysin-loaded nano-emulsion formulation significantly improved drug delivery to the hippocampus of rats [135]. The diabetic rats treated with chrysin-loaded nano-vesicles showed the greatest therapeutic benefit. To combat diabetes, putting chrysin onto nanovesicles has the potential to be investigated [136]. In MCF-7 human breast cancer cells, selenium-containing chrysin and quercetin successfully inhibited clonal development and hampered TrxR activity, which resulted in apoptotic cell death [137].
CONCLUSION AND FUTURE PERSPECTIVES
Chrysin has established itself as a useful polyphenol and is currently the subject of a significant amount of research. Chrysin is capable of a diverse range of biological activities, some of which include the protection against oxidative stress, inflammation, and neurodegeneration. Chrysin also possesses a wide spectrum of biological activity. The current review showed that chrysin has neuroprotective advantages in AD by lowering the aggregation of amyloid, activation of calcium, the association of heavy metals, and neuroinflammation. In spite of the numerous pre-clinical studies that have highlighted the conceivable function of chrysin in different neurological disorders, there is still a paucity of clinical data. This is mostly due to the fact that chrysin has a low bioavailability and metabolically unstable. Because of the importance of the blood–brain barrier in the progression of AD, we also placed an emphasis on the development of novel methods of administration and nanotechnology-based drug delivery systems.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Odeku OA, Ajala TO. Nanodelivery systems for Alzheimer’s disease: prospects of natural therapeutic agents. J Appl Pharm Sci. 2021;11(11):001–10.
2. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s Dement. 2013 Jan 1;9(1):63–75.
3. Sharma VK, Mehta V, Singh TG. Alzheimer’s disorder: epigenetic connection and associated risk factors. Curr Neuropharmacol. 2020 Aug 1;18(8):740–53.
4. Jellinger KA. Basic mechanisms of neurodegeneration: a critical update. J Cell Mol Med. 2010 Mar;14(3):457–87. CrossRef
5. Hambright WS, Fonseca RS, Chen L, Na R, Ran Q. Ablation of ferroptosis regulator glutathione peroxidase 4 in forebrain neurons promotes cognitive impairment and neurodegeneration. Redox Biol. 2017 Aug 1;12:8–17. CrossRef
6. Alzheimer’s Association Calcium Hypothesis Workgroup. Calcium hypothesis of Alzheimer’s disease and brain aging: a framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement. 2017 Feb;13(2):178–82.e17. CrossRef
7. Goldberg TH, Chavin SI. Preventive medicine and screening in older adults. J Am Geriatr Soc. 1997 Mar;45(3):344–54. CrossRef
8. Torres AK, Jara C, Park-Kang HS, Polanco CM, Tapia D, Alarcón F, et al. Synaptic mitochondria: an early target of amyloid-β and tau in Alzheimer’s disease. J Alzheimers Dis. 2021 Jan 1;84(4):1391–414. CrossRef
9. Zheng H, Koo EH. Biology and pathophysiology of the amyloid precursor protein. Mol Neurodegener. 2011 Dec;6(1):27. CrossRef
10. Nunan J, Small DH. Regulation of APP cleavage by α-, β- and γ-secretases. FEBS Lett. 2000 Oct 13;483(1):6–10. CrossRef
11. Turner PR, O’Connor K, Tate WP, Abraham WC. Roles of amyloid precursor protein and its fragments in regulating neural activity, plasticity and memory. Prog Neurobiol. 2003 May 1;70(1):1–32. CrossRef
12. Huang YR, Liu RT. The toxicity and polymorphism of β-amyloid oligomers. Int J Mol Sci. 2020 Jun 24;21(12):4477. CrossRef
13. Sadigh-Eteghad S, Sabermarouf B, Majdi A, Talebi M, Farhoudi M, Mahmoudi J. Amyloid-beta: a crucial factor in Alzheimer’s disease. Med Princ Pract. 2015 Nov 27;24(1):1–10. CrossRef
14. Chen SY, Gao Y, Sun JY, Meng XL, Yang D, Fan LH, et al. Traditional Chinese medicine: role in reducing β-amyloid, apoptosis, autophagy, neuroinflammation, oxidative stress, and mitochondrial dysfunction of Alzheimer’s disease. Front Pharmacol. 2020 Apr 22;11:497. CrossRef
15. Guo T, Noble W, Hanger DP. Roles of tau protein in health and disease. Acta Neuropathol. 2017 May;133(5):665–704. CrossRef
16. Mi K, Johnson GV. The role of tau phosphorylation in the pathogenesis of Alzheimer’s disease. Curr Alzheimer Res. 2006 Dec 1;3(5):449–63. CrossRef
17. Hill E, Wall MJ, Moffat KG, Karikari TK. Understanding the pathophysiological actions of tau oligomers: a critical review of current electrophysiological approaches. Front Mol Neurosci. 2020 Aug 20;13:155. CrossRef
18. Badiola N, Suárez-Calvet M, Lleó A. Tau phosphorylation and aggregation as a therapeutic target in tauopathies. CNS Neurol Disord Drug Targets. 2010 Dec 1;9(6):727–40. CrossRef
19. Tepper K, Biernat J, Kumar S, Wegmann S, Timm T, Hübschmann S, et al. Oligomer formation of tau protein hyperphosphorylated in cells. J Biol Chem. 2014 Dec 5;289(49):34389–407. CrossRef
20. Das N, Raymick J, Sarkar S. Role of metals in Alzheimer’s disease. Metab Brain Dis. 2021 Oct;36(7):1627–39. CrossRef
21. Aragão WAB, Teixeira FB, Fagundes NCF, Fernandes RM, Fernandes LMP, da Silva MCF, et al. Hippocampal dysfunction provoked by mercury chloride exposure: evaluation of cognitive impairment, oxidative stress, tissue injury and nature of cell death. Oxid Med Cell Longev. 2018 Oct;2018:7878050. CrossRef
22. Zatta P, Drago D, Bolognin S, Sensi SL. Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharmacol Sci. 2009 Jul 1;30(7):346–55. CrossRef
23. Zhao HH, Di J, Liu WS, Liu HL, Lai H, Lü YL. Involvement of GSK3 and PP2A in ginsenoside Rb1’s attenuation of aluminum-induced tau hyperphosphorylation. Behav Brain Res. 2013 Mar 15;241:228–34. CrossRef
24. Mezzaroba L, Alfieri DF, Colado Simão AN, Vissoci Reiche EM. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology. 2019 Sep 1;74:230–41. CrossRef
25. Ma MW, Wang J, Zhang Q, Wang R, Dhandapani KM, Vadlamudi RK, et al. NADPH oxidase in brain injury and neurodegenerative disorders. Mol Neurodegener. 2017 Dec;12(1):7. CrossRef
26. Kriska J, Hermanova Z, Knotek T, Tureckova J, Anderova M. On the common journey of neural cells through ischemic brain injury and Alzheimer’s disease. Int J Mol Sci. 2021 Sep 7;22(18):9689. CrossRef
27. Bhatia V, Sharma S. Role of mitochondrial dysfunction, oxidative stress and autophagy in progression of Alzheimer’s disease. J Neurol Sci. 2021 Feb 15;421:117253. CrossRef
28. Elfawy HA, Das B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: etiologies and therapeutic strategies. Life Sci. 2019 Feb 1;218:165–84. CrossRef
29. Hampel H, Mesulam MM, Cuello AC, Farlow MR, Giacobini E, Grossberg GT, et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain. 2018 Jul 1;141(7):1917–33. CrossRef
30. Orad RI, Shiner T. Differentiating dementia with Lewy bodies from Alzheimer’s disease and Parkinson’s disease dementia: an update on imaging modalities. J Neurol. 2022 Feb;269(2):639–53. CrossRef
31. Bucchia M, Merwin SJ, Re DB, Kariya S. Limitations and challenges in modeling diseases involving spinal motor neuron degeneration in vitro. Front Cell Neurosci. 2018 Mar 6;12:61. CrossRef
32. Caruana M, Cauchi R, Vassallo N. Putative role of red wine polyphenols against brain pathology in Alzheimer’s and Parkinson’s disease. Front Nutr. 2016 Aug 12;3:31. CrossRef
33. Jahic Mujkic A, Tušek Žnidaric M, Berbic S, Žerovnik E. Synergy of the inhibitory action of polyphenols plus vitamin C on amyloid fibril formation: case study of human stefin B. Antioxidants (Basel). 2021 Sep 15;10(9):1471. CrossRef
34. Wang Y, Rao Y, Lin Z, Sa R, Yin Y, Zhang X, et al. Current status and trends of research on anthracycline-induced cardiotoxicity from 2002 to 2021: a twenty-year bibliometric and visualization analysis. Oxid Med Cell Longev. 2022 Aug 11;2022:6260243. CrossRef
35. Sabry HA, Zahra MM, Haredy SA, Amer AS. Neuroprotective impacts of chrysin against clonazepam induced cognitive deficits in male rats. J Appl Pharm Sci. 2023 Jul 4;13(7):174–85.
36. Gao S, Siddiqui N, Etim I, Du T, Zhang Y, Liang D. Developing nutritional component chrysin as a therapeutic agent: bioavailability and pharmacokinetics consideration, and ADME mechanisms. Biomed Pharmacother. 2021 Oct 1;142:112080. CrossRef
37. Krysa M, Szymanska-Chargot M, Zdunek A. FT-IR and FT-Raman fingerprints of flavonoids—a review. Food Chem. 2022 Nov 1;393:133430. CrossRef
38. Alcaraz M, Olivares A, Achel DG, García-Gamuz JA, Castillo J, Alcaraz-Saura M. Genoprotective effect of some flavonoids against genotoxic damage induced by X-rays in vivo: relationship between structure and activity. Antioxidants (Basel). 2021 Dec 30;11(1):94. CrossRef
39. Heim KE, Tagliaferro AR, Bobilya DJ. Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. J Nutr Biochem. 2002 Oct 1;13(10):572–84. CrossRef
40. Lin CM, Wang BW, Pan CM, Fang WJ, Chua SK, Cheng WP, et al. Chrysin boosts KLF2 expression through suppression of endothelial cell-derived exosomal microRNA-92a in the model of atheroprotection. Eur J Nutr. 2021 Dec;60(8):4345–55. CrossRef
41. Talebi M, Talebi M, Farkhondeh T, Kopustinskiene DM, Simal-Gandara J, Bernatoniene J, et al. An updated review on the versatile role of chrysin in neurological diseases: chemistry, pharmacology, and drug delivery approaches. Biomed Pharmacother. 2021 Sep 1;141:111906. CrossRef
42. Vedagiri A, Thangarajan S. Mitigating effect of chrysin loaded solid lipid nanoparticles against amyloid β25–35 induced oxidative stress in rat hippocampal region: an efficient formulation approach for Alzheimer’s disease. Neuropeptides. 2016 Aug 1;58:111–25. CrossRef
43. Liu C, Ma X, Zhuang J, Liu L, Sun C. Cardiotoxicity of doxorubicin-based cancer treatment: what is the protective cognition that phytochemicals provide us? Pharmacol Res. 2020 Oct 1;160:105062. CrossRef
44. Krishnamoorthy A, Sevanan M, Mani S, Balu M, Balaji S, Ramajayan P. Chrysin restores MPTP induced neuroinflammation, oxidative stress and neurotrophic factors in an acute Parkinson’s disease mouse model. Neurosci Lett. 2019 Sep 14;709:134382. CrossRef
45. Wang Q, Dong X, Zhang R, Zhao C. Flavonoids with potential anti-amyloidogenic effects as therapeutic drugs for treating Alzheimer’s disease. J Alzheimers Dis. 2021 Jan 1;84(2):505–33. CrossRef
46. Šimic G, Babic Leko M, Wray S, Harrington C, Delalle I, Jovanov-Miloševic N, et al. Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules. 2016 Jan 6;6(1):6. CrossRef
47. Ganguly U, Kaur U, Chakrabarti SS, Sharma P, Agrawal BK, Saso L, et al. Oxidative stress, neuroinflammation, and NADPH oxidase: implications in the pathogenesis and treatment of Alzheimer’s disease. Oxid Med Cell Longev. 2021 Apr 16;2021:7086512. CrossRef
48. Kawahara M, Kato-Negishi M. Link between aluminum and the pathogenesis of Alzheimer’s disease: the integration of the aluminum and amyloid cascade hypotheses. Int J Alzheimers Dis. 2011 Jan 1;2011:276393. CrossRef
49. Goulay R, Mena Romo L, Hol EM, Dijkhuizen RM. From stroke to dementia: a comprehensive review exposing tight interactions between stroke and amyloid-β formation. Transl Stroke Res. 2020 Aug;11(4):601–14. doi: https://doi.org/10.1007/s12975-019-00755-2
50. Qiu T, Liu Q, Chen YX, Zhao YF, Li YM. Aβ42 and Aβ40: similarities and differences. J Pept Sci. 2015 Jul;21(7):522–9. CrossRef
51. Urban AS, Pavlov KV, Kamynina AV, Okhrimenko IS, Arseniev AS, Bocharov EV. Structural studies providing insights into production and conformational behavior of amyloid-β peptide associated with Alzheimer’s disease development. Molecules. 2021 May 13;26(10):2897. CrossRef
52. Steiner H, Fukumori A, Tagami S, Okochi M. Making the final cut: pathogenic amyloid-β peptide generation by γ-secretase. Cell Stress. 2018 Nov;2(11):292–310. CrossRef
53. Bitencourt ALB, Campos RM, Cline EN, Klein WL, Sebollela A. Antibody fragments as tools for elucidating structure-toxicity relationships and for diagnostic/therapeutic targeting of neurotoxic amyloid oligomers. Int J Mol Sci. 2020 Nov 24;21(23):8920. CrossRef
54. Sultana R, Perluigi M, Butterfield DA. Lipid peroxidation triggers neurodegeneration: a redox proteomics view into the Alzheimer disease brain. Free Radic Biol Med. 2013 Sep 1;62:157–69. CrossRef
55. Hur JY. γ-secretase in Alzheimer’s disease. Exp Mol Med. 2022 Apr;54(4):433–46. CrossRef
56. Safieh M, Korczyn AD, Michaelson DM. ApoE4: an emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019 Dec;17(1):64. CrossRef
57. Papiri G, Luzzi S, Marcucci M, Vignini A. Vasoactive neuropeptides and Alzheimer’s disease: a systematic review focusing on calcitonin gene-related peptide. J Integr Neurosci. 2021 Dec 30;20(4):1059–65. CrossRef
58. Brunetti C, Fini A, Sebastiani F, Gori A, Tattini M. Modulation of phytohormone signaling: a primary function of flavonoids in plant–environment interactions. Front Plant Sci. 2018 Jul 20;9:1042. CrossRef
59. Alkahtane AA, Alghamdi HA, Almutairi B, Khan MM, Hasnain MS, Abdel-Daim MM, et al. Inhibition of human amylin aggregation by flavonoid chrysin: an in-silico and in-vitro approach. Int J Med Sci. 2021;18(1):199–206. CrossRef
60. Brás NF, Ashirbaev SS, Zipse H. Combined in silico and in vitro approaches to uncover the oxidation and Schiff base reaction of baicalein as an inhibitor of amyloid protein aggregation. Chemistry. 2022 Feb 21;28(11):e202104240. CrossRef
61. Mahboob A, Senevirathne DKL, Paul P, Nabi F, Khan RH, Chaari A. An investigation into the potential action of polyphenols against human islet amyloid polypeptide aggregation in type 2 diabetes. Int J Biol Macromol. 2023;225:318–50. CrossRef, PMID 36400215.
62. Goyal A, Singh G, Verma A. A comprehensive review on therapeutic potential of chrysin in brain related disorders. CNS Neurol Disord Drug Targets. 2023 Jul 1;22(6):789–800. CrossRef
63. Yalçin S, Yalçinkaya S, Ercan F. In silico detection of inhibitor potential of Passiflora compounds against SARS-Cov-2(Covid-19) main protease by using molecular docking and dynamic analyses. J Mol Struct. 2021 Sep 15;1240:130556. CrossRef
64. Saleem S, Banerjee R, Rajesh Kannan R. Chrysin-loaded chitosan nanoparticle-mediated neuroprotection in Aβ1–42-Induced neurodegenerative conditions in zebrafish. ACS Chem Neurosci. 2022;13(13):2017–34. CrossRef
65. Obaid RJ, Naeem N, Mughal EU, Al-Rooqi MM, Sadiq A, Jassas RS, et al. Inhibitory potential of nitrogen, oxygen and sulfur containing heterocyclic scaffolds against acetylcholinesterase and butyrylcholinesterase. RSC Adv. 2022;12(31):19764–855. CrossRef
66. Zhao J, Huang L, Sun C, Zhao D, Tang H. Studies on the structure-activity relationship and interaction mechanism of flavonoids and xanthine oxidase through enzyme kinetics, spectroscopy methods and molecular simulations. Food Chem. 2020 Sep 1;323:126807. CrossRef
67. Taslimi P, Kandemir FM, Demir Y, Ileritürk M, Temel Y, Caglayan C, et al. The antidiabetic and anticholinergic effects of chrysin on cyclophosphamide-induced multiple organ toxicity in rats: pharmacological evaluation of some metabolic enzyme activities. J Biochem Mol Toxicol. 2019 Jun;33(6):e22313. CrossRef
68. Ekeleme-Egedigwe CA, Famurewa AC, David EE, Eleazu CO, Egedigwe UO. Antioxidant potential of garlic oil supplementation prevents cyclophosphamide-induced oxidative testicular damage and endocrine depletion in rats. J Nutr Intermed Metab. 2019 Dec 1;18:100109. CrossRef
69. Taslimi P, Caglayan C, Gulcin I. The impact of some natural phenolic compounds on carbonic anhydrase, acetylcholinesterase, butyrylcholinesterase, and α-glycosidase enzymes: an antidiabetic, anticholinergic, and antiepileptic study. J Biochem Mol Toxicol. 2017 Dec;31(12):e21995. CrossRef
70. Gregoriou G, Neophytou CM, Vasincu A, Gregoriou Y, Hadjipakkou H, Pinakoulaki E, et al. Anti-cancer activity and phenolic content of extracts derived from Cypriot carob (Ceratonia siliqua L.) pods using different solvents. Molecules. 2021 Aug 19;26(16):5017. CrossRef
71. Sarmah S, Goswami A, Kumar Belwal V, Singha Roy A. Mitigation of ribose and glyoxal induced glycation, AGEs formation and aggregation of human serum albumin by citrus fruit phytochemicals naringin and naringenin: an insight into their mechanism of action. Food Res Int. 2022 Jul 1;157:111358. CrossRef
72. Guo T, Zhang D, Zeng Y, Huang TY, Xu H, Zhao Y. Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer’s disease. Mol Neurodegener. 2020 Dec;15(1):40. CrossRef
73. Cline EN, Bicca MA, Viola KL, Klein WL. The amyloid-β oligomer hypothesis: beginning of the third decade. J Alzheimers Dis. 2018 Jan 1;64(s1):S567–610. CrossRef
74. Straus MR, Bidon MK, Tang T, Jaimes JA, Whittaker GR, Daniel S. Inhibitors of L-type calcium channels show therapeutic potential for treating SARS-CoV-2 infections by preventing virus entry and spread. ACS Infect Dis. 2021 Sep 9;7(10):2807–15. CrossRef
75. Guan PP, Cao LL, Yang Y, Wang P. Calcium ions aggravate Alzheimer’s disease through the aberrant activation of neuronal networks, leading to synaptic and cognitive deficits. Front Mol Neurosci. 2021 Dec 2;14:757515. CrossRef
76. Everett J, Collingwood JF, Tjendana-Tjhin V, Brooks J, Lermyte F, Plascencia-Villa G, et al. Nanoscale synchrotron X-ray speciation of iron and calcium compounds in amyloid plaque cores from Alzheimer’s disease subjects. Nanoscale. 2018;10(25):11782–96. CrossRef
77. Missiroli S, Patergnani S, Caroccia N, Pedriali G, Perrone M, Previati M, et al. Mitochondria-associated membranes (MAMs) and inflammation. Cell Death Dis. 2018 Feb 28;9(3):329. CrossRef
78. Jeremic D, Jiménez-Díaz L, Navarro-López JD. Past, present and future of therapeutic strategies against amyloid-β peptides in Alzheimer’s disease: a systematic review. Ageing Res Rev. 2021 Dec 1;72:101496. CrossRef
79. Breijyeh Z, Karaman R. Comprehensive review on Alzheimer’s disease: causes and treatment. Molecules. 2020 Dec 8;25(24):5789. CrossRef
80. Zhang Y, Xue Z, Hu S, Bai H, Wang J, Wang N. Chrysin inhibits pseudo-allergic reaction by suppressing mitochondrial STAT3 activation via MAS-related GPR family member X2. J Agric Food Chem. 2021 Jun 16;69(23):6569–77. CrossRef
81. Ji Y, Han J, Lee N, Yoon JH, Youn K, Ha HJ, et al. Neuroprotective effects of baicalein, wogonin, and oroxylin A on amyloid beta-induced toxicity via NF-κB/MAPK pathway modulation. Molecules. 2020 Nov 2;25(21):5087. CrossRef
82. Ha SK, Moon E, Kim SY. Chrysin suppresses LPS-stimulated proinflammatory responses by blocking NF-κB and JNK activations in microglia cells. Neurosci Lett. 2010 Nov 26;485(3):143–7. CrossRef
83. Yeo H, Lee YH, Koh D, Lim Y, Shin SY. Chrysin inhibits NF-κB-dependent CCL5 transcription by targeting IκB kinase in the atopic dermatitis-like inflammatory microenvironment. Int J Mol Sci. 2020 Oct 5;21(19):7348. CrossRef
84. Lee A, Kondapalli C, Virga DM, Lewis TL, Koo SY, Ashok A, et al. Aβ42 oligomers trigger synaptic loss through CAMKK2-AMPK-dependent effectors coordinating mitochondrial fission and mitophagy. Nat Commun. 2022;13(1):4444. CrossRef
85. La Torre ME, Villano I, Monda M, Messina A, Cibelli G, Valenzano A, et al. Role of vitamin E and the orexin system in neuroprotection. Brain Sci. 2021 Aug 20;11(8):1098. CrossRef
86. DeRidder L, Sharma A, Liaw K, Sharma R, John J, Kannan S, et al. Dendrimer–tesaglitazar conjugate induces a phenotype shift of microglia and enhances β-amyloid phagocytosis. Nanoscale. 2021;13(2):939–52. CrossRef
87. Subhramanyam CS, Wang C, Hu Q, Dheen ST. Microglia-mediated neuroinflammation in neurodegenerative diseases. Semin Cell Dev Biol. 2019 Oct 1;94:112–20. CrossRef
88. Mozafari N, Ashrafi H, Azadi A. Targeted drug delivery systems to control neuroinflammation in central nervous system disorders. J Drug Deliv Sci Technol. 2021 Dec 1;66:102802. CrossRef
89. Guo Y, Hong W, Wang X, Zhang P, Körner H, Tu J, et al. MicroRNAs in microglia: how do microRNAs affect activation, inflammation, polarization of microglia and mediate the interaction between microglia and glioma? Front Mol Neurosci. 2019 May 10;12:125. CrossRef
90. Behl T, Makkar R, Sehgal A, Singh S, Sharma N, Zengin G, et al. Current trends in neurodegeneration: cross talks between oxidative stress, cell death, and inflammation. Int J Mol Sci. 2021 Jul 11;22(14):7432.
91. Salvador AFM, Kipnis J. Immune response after central nervous system injury. Semin Immunol. 2022 Jan 1;59:101629. CrossRef
92. Bansal R, Singh R. Exploring the potential of natural and synthetic neuroprotective steroids against neurodegenerative disorders: a literature review. Med Res Rev. 2018 Jul;38(4):1126–58. CrossRef
93. Lazarevic M, Mazzon E, Momcilovic M, Basile MS, Colletti G, Petralia MC, et al. The H2S Donor GYY4137 stimulates reactive oxygen species generation in BV2 cells while suppressing the secretion of TNF and nitric oxide. Molecules. 2018 Nov 14;23(11):2966. CrossRef
94. Xiao J, Zhai H, Yao Y, Wang C, Jiang W, Zhang C, et al. Chrysin attenuates experimental autoimmune neuritis by suppressing immuno-inflammatory responses. Neuroscience. 2014 Mar 14;262:156–64. CrossRef
95. An J, Chen B, Kang X, Zhang R, Guo Y, Zhao J, et al. Neuroprotective effects of natural compounds on LPS-induced inflammatory responses in microglia. Am J Transl Res. 2020;12(6):2353–78
96. Guo C, Yang L, Wan CX, Xia YZ, Zhang C, Chen MH, et al. Anti-neuroinflammatory effect of Sophoraflavanone G from Sophora alopecuroides in LPS-activated BV2 microglia by MAPK, JAK/STAT and Nrf2/HO-1 signaling pathways. Phytomedicine. 2016 Dec 1;23(13):1629–37. CrossRef
97. Huat TJ, Camats-Perna J, Newcombe EA, Valmas N, Kitazawa M, Medeiros R. Metal toxicity links to Alzheimer’s disease and neuroinflammation. J Mol Biol. 2019 Apr 19;431(9):1843–68. CrossRef
98. Tang D, Chen X, Kang R, Kroemer G. Ferroptosis: molecular mechanisms and health implications. Cell Res. 2021 Feb;31(2):107–25. CrossRef
99. Ghaderi S, Komaki A, Salehi I, Basir Z, Rashno M. Possible mechanisms involved in the protective effects of chrysin against lead-induced cognitive decline: an in vivo study in a rat model. Biomed Pharmacother. 2023 Jan 1;157:114010. CrossRef
100. Rashno M, Sarkaki A, Ghaderi S, Khoshnam SE. Sesamin: insights into its protective effects against lead-induced learning and memory deficits in rats. J Trace Elem Med Biol. 2022 Jul 1;72:126993. CrossRef
101. Kaur P, Radotra B, Minz RW, Gill KD. Impaired mitochondrial energy metabolism and neuronal apoptotic cell death after chronic dichlorvos (OP) exposure in rat brain. Neurotoxicology. 2007 Nov 1;28(6):1208–19. CrossRef
102. Caruso G, Godos J, Privitera A, Lanza G, Castellano S, Chillemi A, et al. Phenolic acids and prevention of cognitive decline: polyphenols with a neuroprotective role in cognitive disorders and Alzheimer’s disease. Nutrients. 2022 Feb 15;14(4):819. CrossRef
103. Rashno M, Ghaderi S, Nesari A, Khorsandi L, Farbood Y, Sarkaki A. Chrysin attenuates traumatic brain injury-induced recognition memory decline, and anxiety/depression-like behaviors in rats: insights into underlying mechanisms. Psychopharmacology. 2020 Jun;237(6):1607–19. CrossRef
104. Semenovich DS, Plotnikov EY, Titko OV, Lukiyenko EP, Kanunnikova NP. Effects of panthenol and N-acetylcysteine on changes in the redox state of brain mitochondria under oxidative stress in vitro. Antioxidants (Basel). 2021 Oct 27;10(11):1699. CrossRef
105. Van der Pol A, van Gilst WH, Voors AA, van der Meer P. Treating oxidative stress in heart failure: past, present and future. Eur J Heart Fail. 2019 Apr;21(4):425–35. CrossRef
106. Butterfield DA, Halliwell B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci. 2019 Mar;20(3):148–60. CrossRef
107. Makkar R, Behl T, Bungau S, Zengin G, Mehta V, Kumar A, et al. Nutraceuticals in neurological disorders. Int J Mol Sci. 2020 Jun 22;21(12):4424.
108. Zaher MF, Bendary MA, Abd El-Aziz GS, Ali AS. Potential protective role of thymoquinone on experimentally induced Alzheimer rats. Jeddah, Saudi Arabia: King Abdulaziz University; 2020.
109. Aishwarya V, Sumathi T. Chrysin, a natural flavonoid attenuates cognitive dysfunction and neuronal loss associated with amyloid β (25-35)-induced oxidative stress: an experimental model of Alzheimer’s disease. Int J Pharmacogn Phytochem. 2015;7:224–36.
110. Adangale SC, Wairkar S. Potential therapeutic activities and novel delivery systems of chrysin-a nature’s boon. Food Biosci. 2022 Feb 1;45:101316. CrossRef
111. Jung H, Lee SY, Lim S, Choi HR, Choi Y, Kim M, et al. Anti-inflammatory clearance of amyloid-β by a chimeric Gas6 fusion protein. Nat Med. 2022 Sep;28(9):1802–12. CrossRef
112. Medzhitov R. The spectrum of inflammatory responses. Science. 2021 Nov 26;374(6571):1070–5. CrossRef
113. Liu H, Wu X, Luo J, Wang X, Guo H, Feng D, et al. Pterostilbene attenuates astrocytic inflammation and neuronal oxidative injury after ischemia-reperfusion by inhibiting NF-κB phosphorylation. Front Immunol. 2019 Oct 17;10:2408. CrossRef
114. Thakur S, Dhapola R, Sarma P, Medhi B, Reddy DH. Neuroinflammation in Alzheimer’s disease: current progress in molecular signaling and therapeutics. Inflammation. 2023 Feb;46(1):1–17. CrossRef
115. McQuade A, Blurton-Jones M. Microglia in Alzheimer’s disease: exploring how genetics and phenotype influence risk. J Mol Biol. 2019 Apr 19;431(9):1805–17. CrossRef
116. Alam Q, Alam MZ, Mushtaq G, Damanhouri GA, Rasool M, Kamal MA, et al. Inflammatory process in Alzheimer’s and Parkinson’s diseases: central role of cytokines. Curr Pharm Des. 2016;22(5):541–8. CrossRef
117. Rehman MU, Tahir M, Khan AQ, Khan R, Lateef A, Oday-O-Hamiza, et al. Chrysin suppresses renal carcinogenesis via amelioration of hyperproliferation, oxidative stress and inflammation: plausible role of NF-κB. Toxicol Lett. 2013 Feb 4;216(2–3):146–58. CrossRef
118. Simsek H, Akaras N, Gür C, Küçükler S, Kandemir FM. Beneficial effects of chrysin on cadmium-induced nephrotoxicity in rats: modulating the levels of Nrf2/HO-1, RAGE/NLRP3, and caspase-3/Bax/Bcl-2 signaling pathways. Gene. 2023 Jul 30;875:147502. CrossRef
119. Talebi M, Talebi M, Farkhondeh T, Simal-Gandara J, Kopustinskiene DM, Bernatoniene J, et al. Promising protective effects of chrysin in cardiometabolic diseases. Curr Drug Targets. 2022 Apr 1;23(5):458–70. CrossRef
120. Zeinali M, Rezaee SA, Hosseinzadeh H. An overview on immunoregulatory and anti-inflammatory properties of chrysin and flavonoids substances. Biomed Pharmacother. 2017 Aug 1;92:998–1009. CrossRef
121. Borriello M, Iannuzzi C, Sirangelo I. Pinocembrin protects from AGE-induced cytotoxicity and inhibits non-enzymatic glycation in human insulin. Cells. 2019 Apr 26;8(5):385. CrossRef
122. Tan J, Yadav MK, Devi S, Kumar M. Neuroprotective effects of arbutin against oxygen and glucose deprivation-induced oxidative stress and neuroinflammation in rat cortical neurons. Acta Pharm. 2022;72(1):123–34. CrossRef
123. Juan CA, Pérez de la Lastra JM, Plou FJ, Pérez-Lebeña E. The chemistry of reactive oxygen species (ROS) revisited: outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int J Mol Sci. 2021 Apr 28;22(9):4642. CrossRef
124. Bhattacharya T, Soares GA, Chopra H, Rahman MM, Hasan Z, Swain SS, et al. Applications of phyto-nanotechnology for the treatment of neurodegenerative disorders. Materials. 2022 Jan 21;15(3):804.
125. Löscher W, Potschka H, Sisodiya SM, Vezzani A. Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol Rev. 2020 Jul 1;72(3):606–38. CrossRef
126. Bandiwadekar A, Jose J, Khayatkashani M, Habtemariam S, Khayat Kashani HR, Nabavi SM. Emerging novel approaches for the enhanced delivery of natural products for the management of neurodegenerative diseases. J Mol Neurosci. 2022 Mar;72(3):653–76. CrossRef
127. Giacomeli R, de Gomes MG, Reolon JB, Haas SE, Colomé LM, Jesse CR. Chrysin loaded lipid-core nanocapsules ameliorates neurobehavioral alterations induced by β-amyloid1-42 in aged female mice. Behav Brain Res. 2020 Jul 15;390:112696. CrossRef
128. Masetto Antunes M, Godoy G, Masi LN, Curi R, Barbosa Bazotte R. Prefrontal cortex and hippocampus inflammation in mice fed high-carbohydrate or high-fat diets. J Med Food. 2022 Jan 1;25(1):110–3. CrossRef
129. Bruschi ML, de Toledo LD. Pharmaceutical applications of iron-oxide magnetic nanoparticles. Magnetochemistry. 2019 Sep 2;5(3):50. CrossRef
130. Nday CM, Eleftheriadou D, Jackson G. Magnetic chrysin silica nanomaterials behavior in an amyloidogenic environment. Hell J Nucl Med. 2019 Jan-Apr;22:42–50.
131. Tandon A, Singh SJ, Chaturvedi RK. Nanomedicine against Alzheimer’s and Parkinson’s disease. Curr Pharm Des. 2021 Apr 1;27(12):1507–45. CrossRef
132. Ibrahim SS, Abo Elseoud OG, Mohamedy MH, Amer MM, Mohamed YY, Elmansy SA, et al. Nose-to-brain delivery of chrysin transfersomal and composite vesicles in doxorubicin-induced cognitive impairment in rats: insights on formulation, oxidative stress and TLR4/NF-kB/NLRP3 pathways. Neuropharmacology. 2021 Oct 1;197:108738. CrossRef
133. Sharma B, Sharma A. Future prospect of nanotechnology in development of anti-ageing formulations. Int J Pharm Pharm Sci. 2012;4(3):57–66.
134. Chopra H, Dey PS, Das D, Bhattacharya T, Shah M, Mubin S, et al. Curcumin nanoparticles as promising therapeutic agents for drug targets. Molecules. 2021 Aug 18;26(16):4998. CrossRef
135. George MY, El-Derany MO, Ahmed Y, Zaher M, Ibrahim C, Waleed H, et al. Design and evaluation of chrysin-loaded nanoemulsion against lithium/pilocarpine-induced status epilepticus in rats; emphasis on formulation, neuronal excitotoxicity, oxidative stress, microglia polarization, and AMPK/SIRT-1/PGC-1α pathway. Expert Opin Drug Deliv. 2023 Jan 2;20(1):159–74. CrossRef
136. El-Marasy SA, AbouSamra MM, El-Mosallamy AEMK, Emam AN, Mabrok HB, Galal AF, et al. Chrysin loaded nanovesicles ameliorated diabetic peripheral neuropathy. Role of NGF/AKT/GSK-3β pathway. Chem Biol Interact. 2023 Apr 25;375:110402. CrossRef
137. Martins IL, Charneira C, Gandin V, Ferreira da Silva JL, Justino GC, Telo JP, et al. Selenium-containing chrysin and quercetin derivatives: attractive scaffolds for cancer therapy. J Med Chem. 2015 May 28;58(10):4250–65. CrossRef