INTRODUCTION
Neuroinflammation is the immune response in the neuronal tissues of brain in which the resident immune cells–microglia—secretes pro-inflammatory factors on the activation of cytokines to scavenge the pathogenic infiltrators and minimize the damages (Jin-Tao et al., 2015). During neuroinflammation, cytokines and chemokines play a role in mediating the inflammation of the brain tissue (Geeta et al., 2013). This resident immune cells—microglia—sometimes go uncontrolled and lead to prolonged activation in the course of time, thereby releasing enormous amount of harmful neurotoxic compounds. They enhance the apoptosis of the neuronal tissues causing neurodegeneration and neuronal death (Miguel et al., 2011). Damon et al. (2016) reported that neuronal tissue was damaged during the process of neuroinflammation. The initiation of neuroinflammation was reported due to bacterial constituents like lipopolysaccharides and cause of mechanical injury in the brain (Cristina et al., 2012). The inflammation of brain neuronal tissue results in cognitive impairment, neurodegeneration, and neurological diseases (Roisin and McManus Michael, 2017). Oxidative stress induced excessive reactive oxygen species release, elicit cellular damage. Oxidative stress markers such as superoxide dismutase, Glutathione S-transferase, and glutathione peroxidase were altered in lipopolysaccharide-induced neuroinflammation in mice (Doaa et al., 2018). Traumatic brain injuries (TBIs) result in short- and long-term symptoms, including cognitive and emotional difficulties (Ping et al., 2013). Animals with neuroinflammation have problems to diagnose because they lack clear morphological brain deficiencies. Utagawa et al. (2008) observed the morphological changes of neurons and their degeneration in brain of rat exposed to neuroinflammation. Ruth et al. (2018) study showed that the neuroinflammation of brain at the neurodevelopmental stage triggered the adverse risk during the preterm birth.
The weight drop models (WDMs) are fast, reliable, and produce a significant degree of brain injury, neuroinflammation, and histological and behavioral alterations and provide important information on TBI in mice. WDM creates focal brain damage with a cortical contusion, hippocampal and thalamic damage (Niklas and Lars, 2011). The force in WDM quickly accelerates the head into a supporting foam block, resulting in diffuse brain injury without skull fracture. Primary characterization of this model revealed bilateral injury to neurons in the cerebral cortex in the brain (Thomas et al., 2004). Thus, various animal models have been developed to reproduce the neural damage, oxidative stress, and pathologic changes associated with TBI with possible underlying mechanism of neuroinflammation. Nevertheless, several of TBI models are non-physiologic or fail to duplicate the functional deficits observed in animals. In the current study, we developed a model of TBI in mice using a closed-head, weight-drop technique, which characteristically shows significant disruption to the blood–brain barrier, local and systemic inflammation, and functional deficits in behavioral, stress markers, and histological alterations. Quercetin is a potent neuroprotectant with antioxidative and anti-neuroinflammatory properties. Hence, the present study evaluates the protective effects of quercetin on neuropathological injuries of TBI in mice model which almost all equivalent to natural head injuries.
Recent studies have indicated potential of certain plants and their products such as Ashwagandha, Curcumin, and Resveratrol in treating the effects of neuroinflammation (Charbel et al., 2017; Muskan and Gurcharan, 2016; Vincenzo et al., 2018). Quercetin is a flavonoid compound and commonly found in vegetables and fruits—onions, broccoli, tomatoes, potatoes, soya beans, apples, berries, tea, and coffee and also in red wine (Alessandro et al., 2017). Quercetin is mainly present in the form of glycosides at different entities; the derivatives of quercetin are glycosides and ethers: Quercetin 3-O-galactoside, Quercetin 3-O-glucoside, Quercetin 3-O-glucuronide, Quercetin 7-O-glucoside, Quercetin 3-O-diglucoside, and Quercetin 3-methyl ether in plants (Muhammad et al., 2018). Ingested Quercetin with the help of intestinal bacteria triggers glycosidase activity, thereby hydrolyzing to form aglycone and is readily absorbed in the stomach or small intestine (Ju-Suk et al., 2016). Quercetin has got plethora pharmacological importance and known for its antioxidant activity, neurological effects, antiviral activity, anticancer activity, cardiovascular protection, antimicrobial activity, and anti-inflammatory activity (Aneela et al., 2014). Quercetin is known for its anti-inflammatory capacity which reduced the effect of CCl4-induced inflammation in liver tissues (Xi et al., 2018). Quercetin on administration decreased adipose tissues inflammation which was induced by obesity in mice (Jing et al., 2014). Recently, we have reported the neuroprotective and memory enhancing effect of quercetin by inhibiting sodium fluoride-induced neurodegeneration in developing brain of rat (Nageshwar et al., 2017). Another study also reported the antioxidant properties of quercetin against sodium-induced neurodegeneration in brain of rat (Nageshwar et al., 2018). In this study, we investigated the ameliorative effects of Quercetin as anti-inflammatory and antioxidant on neuroinflammation induced by TBI-WDM in mice through behavioral, oxidative parameters, and histological alterations.
MATERIALS AND METHODS
Chemicals
Quercetin was purchased from Sigma Aldrich Company. All other chemicals used in the investigation were of analytical grade.
Animals
The experimentation was performed using mice (Mus musculus). The animals were obtained from National Center for Laboratory Animal Sciences (NCLAS), National Institute of Nutrition (NIN), Jamai Osmania, Hyderabad-500007, Telangana, India. The protocols of the experiments were designed as per the guidelines of Departmental ethical committee, CPCSEA No: 383/01/a/CPCSE. The animals were maintained at standard laboratory conditions. Each animal was maintained in a separate polypropylene cage bedded with 2–3 cm paddy husk. The cage top was covered with a stainless steel grill on 12/12 hours: day/ night cycle, with 20°C–22°C room temperature. Water and food (Standard pellet diet NIN, NCLAS, Hyderabad) are provided through ad libitum.
Quercetin dosage
Quercetin dose (20 mg/kg mass) was standardized as appropriate dosage for animal experimentation. The quercetin (20 mg/kg mass) dose was standardized based on the previous experimental studies (Nabavi et al., 2011; Nageshwar et al., 2017; 2018). The solvent in the ratio of 0.2 ml of ethanol and 0.8 ml of water for 1 ml is used as vehicle for injection of quercetin.
Experimental design
Animals were divided into four groups.
Group-I: Mice without any treatment served as control.
Group-II: TBI caused by weight drop injury (WDI) as the inflammation group.
Group-III: TBI caused by WDI-inflammation along with quercetin (20 mg/kg mass) through intraperitoneal injection.
Group-IV: Mice were treated with quercetin (20 mg/ kg mass) by intraperitoneal injection.
The study period was for 7 days. After 7 days, the behavioral studies were conducted and the mice were sacrificed and brains were dissected out to perform biochemical parameters and histology.
Methods
Traumatic brain injury–weight drop model
The experimental TBI-WDM in mice was maintained as per the protocol described by Marmarou et al. (1994), with necessary modifications. The procedure followed was shown in Figure 1. The metal helmet disk is 6 mm. The weight of the brass taken was 26 g. The mice were first anesthetized by using pentobarbital (40 mg/kg body weight, i.p). The anesthetized mice were brought under the vertical plastic tube and made apart about 3 cm in between the head of the mice against it. The metallic helmet was placed on the head of the mice and the brass weight was dropped into the plastic tube from the fixed point instantly.
 | Figure 1. Traumatic brain injury-weight drop model. [Click here to view] |
Behavioral studies
Rotarod test
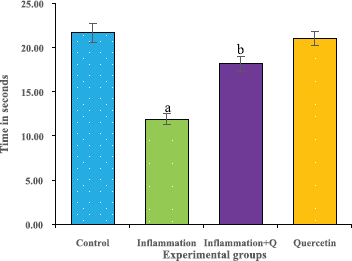
The rotarod test was performed according to the method of Hutter et al. (2012). The rotarod test is widely used to measure the fore and hind limb coordination, motor skills, and continues to be a primary method for the study of motor learning. The time of the instrument (Dolphin™ instruments) adjusted to 0’s and the rotational speed to 20 rpm before the experimentation. The time was noted and the results were analyzed. The results were expressed as time in seconds.
Hot plate test
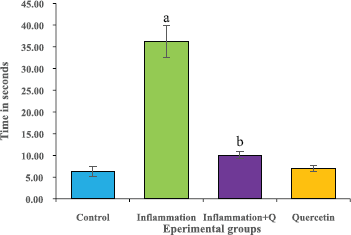
The hot plate test was performed according to the method of Gunn et al. (2011). Rat’s response latency to either a hind-paw lick or a jump on the hot plate (Analgesiometer—Eddy’s Hot Plate) was recorded. In the absence of a response, the animals were removed from the hot plate at 60 seconds (cut off time) and a 60 seconds latency was assigned as the passive response. The results were expressed as time in seconds.
Biochemical estimations
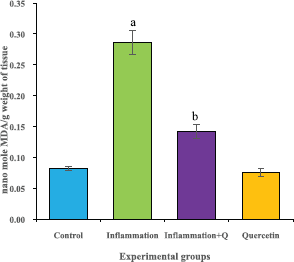
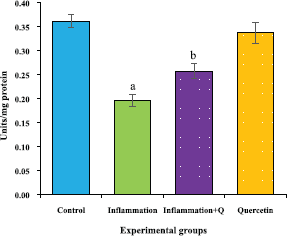
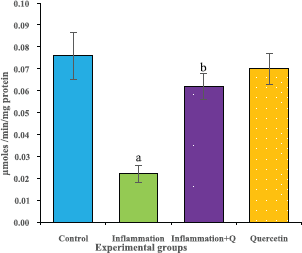
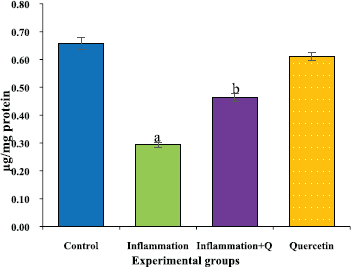
The brain tissue was isolated and homogenized and the supernatant was collected for biochemical estimations. The lipid peroxidation was assessed by measuring thiobarbituric acid reactive substances and was expressed in terms of malondialdehyde (MDA) content, according to the method of Garcia et al. (2005). The results were expressed as nanomole MDA/g weight of tissue. The superoxide dismutase activity was assayed according to the method of Marklund and Marklund (1974). The enzyme activity was expressed as units/mg protein. The catalase activity was assayed by the method of Aebi (1984). One unit of activity is equal to the moles of degraded/minute/mg/protein. The glutathione peroxidase activity was assessed by the method of Flohe and Gunzler (1984). The enzyme activity was expressed as microgram per milligram protein.
Histological studies
Golgi-Cox staining
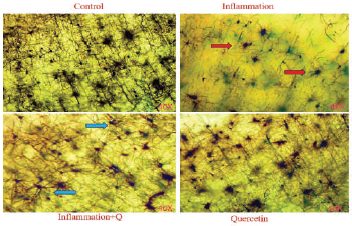
The Golgi-Cox stock solution-fixed brain tissues were sliced at 4–10 μm thickness, mounted on silanized slides, and subjected to Golgi-Cox staining according to the histological method (Gibb and Bryan, 1998). Histological changes were observed using a Lawrence & Mayo microscope (Magnification 40×).
Hematoxylin and Eosin and Congo red staining
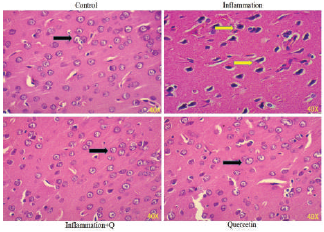
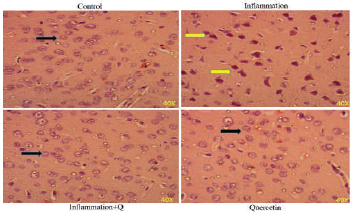
Brain sections were fixed in freshly prepared 10% formalin, processed routinely, and embedded in paraffin. Paraffin sections, 5-μm thick, were prepared and stained with hematoxylin and eosin (Leeson et al., 1985) and Congo red (Romhanyi, 1971) for histological examination. Sections were observed using a Lawrence & Mayo microscope (Magnification 40×).
Statistical Analysis
The results are expressed as the mean ± standard error of the mean (SEM). Comparison of means was conducted using one-way analysis of variance, followed by least significant difference post hoc test to compare means between the different groups. Differences were considered as significant (p < 0.05). Statistical analyses were performed using SPSS version 20 software (USA).