INTRODUCTION
Grapefruit (Citrus Paradisi Macfayden, family Rutaceae) is a non-leafing fruit [1]. The name grapefruit refers to the growing appearance of fruits on trees as clusters. In the 1600s, the first sightings of grapefruit growth were in Jamaica and Barbados. In 661, grapefruit trees grew in South Africa [1]. The origin of grapefruit is the hybridization of pomelo and Citrus maxima with the common orange Citrus sinensis. Grapefruit size is larger than that of orange and smaller than that of pomelo. The grapefruit types were white, pink, and ruby red.
There are varieties of seeded grapefruit [2]; white-colored flesh is seedy; other types vary, including pink and red, and seedless varieties, such as rose fruits, result from mutations [2]. One pound of grapefruit produces approximately 200–250 ml of juice [3]. For daily nutritional needs, people consume grapefruit and its juice from valuable components, including fiber, vitamin C, antioxidants, and phytochemicals, and their nutritional value in many diseases, such as atherosclerotic plaques [4] and cancer [5]. When grapefruit and grapefruit juice (GFJ) were administered concurrently, the bioavailability of many therapeutic agents from different classes differed. This bioavailability modulation either improved drug response or contributed to adverse events [6]. By chance, the effect of GFJ on drug concentration was discovered in 1989; this discovery was part of a study designed to evaluate the interaction of ethanol with felodipine, a calcium channel blocker [7,8]. GFJ was used in this study as a flavor-masking agent and not a major agent. In previous pharmacokinetic studies, the study showed a greater felodipine concentration by many folds compared with felodipine. With these results, the study discovered the effect of GFJ on increasing the plasma felodipine concentration by around fivefold compared with the water used. This study was the starting point for further research to evaluate the effect of GFJ and understand its mechanism of action [8].
The clinical effects and significance of GFJ-drug interactions are highly variable among therapeutic agents within the same and different classes. GFJ affects extensive drug co-administration, including calcium channel blockers such as felodipine, statins such as simvastatin [3], immunosuppressants, benzodiazepines such as midazolam [9], antihistamines such as terfenadine, and many other drugs [10]. These interactions are mainly mediated by intestinal cytochrome P3A4, a CYP enzyme belonging to CYP450 [11]. GFJ consists of phytochemicals mostly responsible for these interactions, including flavonoids and furocoumarins. Naringin (Fig. 1A) was the most abundant flavonoid. Furanocoumarins include bergamottin (Fig. 1B), bergaptol, bergapten, and DHB (Fig. 1C), the most abundant furanocoumarins [12]. This article aims to briefly review the interaction between GFJ and drugs that are CYP3A4 substrates, including a summary of CYP3A4, active components of GFJ, the main mechanism of GFJ-drug interaction, factors that determine the clinical significance of the interaction, examples of adverse events related to the interaction, how to predict and manage the grapefruit interaction, examples of drugs with potential for interaction, and we will discuss the role of the pharmacists.
Humans have the largest cytochrome P450 (CYP) 3A concentration, and this enzyme is responsible for the metabolism of over 60% of all medicines. Because of its very poor substrate specificity, CYP3A4 may be temporarily or permanently inhibited by a wide range of medications. CYP isoenzymes convert some drugs to reactive metabolites capable of irreversibly binding covalently to CYP3A4, and this mechanism-based inhibition of CYP3A4 is characterized by nicotinamide adenine dinucleotide phosphate hydrogen-, time- and concentration-dependent enzyme inactivation [13]. Although the precise structural requirements of CYP3A4 substrates remain unknown, many hallmarks have been established. Among them are a hydrophobic patch that docks into the enzyme’s hydrophobic pocket, hydrogen bond donors that form interactions with active site amino acid residues, activation site amino acid residues that engage with one or more hydrogen bond acceptors, and about 200 Da in the lowest; molecular weight [13,14].
CYTOCHROME P3A4
Cytochrome P, or CYP, is a heme-containing protein, and 450 nm is the maximum absorption at 450 nm in the reduced state in the presence of carbon monoxide. Cytochrome P is a superfamily comprising more than 400 gene families, with members belonging to all biological kingdoms. CYP enzymes are written with the letters “CYP” and an Arabic number referring to the CYP family, followed by a letter referring to the subfamily and an Arabic number referring to the individual gene, isoenzyme, isozyme, or isoform. For example, CYP3A4 represents a CYP enzyme belonging to family 3 [15], subfamily A, and protein 4 in subfamily A. CYP enzymes are classified into families and subfamilies based on their amino acid sequence identity. The family includes enzymes that are 40% or more similar, while subfamilies include enzymes that have more than 55% identical sequences [16–18]. More than 57 documented CYP genes exist in humans, distributed in approximately 18 variant families and 44 subfamilies [16]. CYPs are important in vital life processes because they oxidize endogenous and exogenous compounds, including drugs. In human cells, CYPs are attached to the endoplasmic reticulum or inner mitochondrial membrane [19]. The most abundant isoform of CYP450 is CYP3A4, which is expressed mainly in the liver and intestine [20]. CYP3A4 can reduce or increase the bioavailability and therapeutic efficiency of drugs. If CYP3A4 is inhibited, the plasma level of the drug will increase, and if CYP3A4 is induced, it will decrease. CYP3A4 is the main isoform of the CYP3A subfamily and is an important enzyme for human drug metabolism because of its highly diverse substrate specificity and ability to metabolize compounds of various sizes, shapes, and chemical structures. Thus, CYP3A4 metabolizes approximately 50% or more of currently prescribed drugs. CYP3A4 is expressed mainly in the liver but is also found in high amounts in the intestine [21–23]. Intestinal CYP3A4 mediates biotransformation and contributes to the first-pass metabolism of many prescribed drugs [24]. A pharmacokinetic study showed that the first-pass metabolism of midazolam was 43% in the gut and 44% in the liver [25].
Moreover, similar results for midazolam were reported for verapamil [26,27]. Intestinal metabolism may be greater than that in the liver [26]. This may be due to the anatomical localization of intestinal CYP3A4 [28]. Duodenal, proximal jejunal, and intestinal mucosa, the site of absorption of the drugs dissolved in the intestine, are rich in villi lined by CYP3A4-containing enterocytes [29]. This localization of CYP3A4 is considered the first site for metabolizing orally ingested drugs. In addition, studies found that CYP3A4 protein levels were higher in intestine samples than in liver samples from the same subject, supporting the idea that the entire first-pass metabolism of CYP3A4 substrates occurs in the intestine rather than in the liver [30]. In addition, it is clinically found that oral drugs have raised plasma Cmax and Area under the curve (AUC), not intravenous midazolam, which concludes that GFJ inhibits enteric CYP3A4 and not hepatic [31].
ACTIVE COMPONENTS OF GFJ
GFJ contains phytochemicals, including flavonoids and coumarins (Fig. 1). Naringin is the most abundant flavonoid [32]. Furanocoumarins include bergamottin, bergaptol, bergapten, and DHB, the most abundant furanocoumarins. The first active components in GFJ are flavonoids, mainly naringin (5,7,4′-trihydroxyflavanone-7-O-rhamnoglucoside), which is the main active flavanone glycoside in grapefruit [33] and gives the grapefruit a bitter taste [34]. Many studies have demonstrated that naringin has antioxidant, anti-inflammatory, anti-apoptotic, anti-ulcer, anti-osteoporotic, and anti-carcinogenic properties [35]. Initially, naringin was considered the major component that caused grapefruit-drug interactions. However, later studies showed naringin to be a weak inhibitor of CYP3A4 and showed that administration of isolated, pure naringin in comparable quantities caused less inhibition of intestinal CYP3A4 than administration of GFJ. These studies indicate that other GFJ components inhibit CYP3A4 [36]. Furanocoumarins are secondary metabolites in grapefruits that are defensive against insects, pathogens, and others. The furanocoumarin structure includes the furan group attached to carbons 6 and 7 in the linear type or to carbons 7 and 8 in the angular type. Grapefruit is the major source of furanocoumarins in the Western diet, representing 73% of the furanocoumarin intake from food. The main types of grapefruit furanocoumarins are bergamottin, DHB, and epoxybergamottin [37]. Bergamottin is an intestinal CYP enzyme inhibitor. Furthermore, DHB was the first furanocoumarin to show a strong CYP3A4 inhibition [38]. GFJ most likely contains DHB, which causes interactions via CYP3A4 inhibition. DHB and bergamottin are both irreversible CYP3A4 inhibitors. Researchers have considered furanocoumarins CYP3A4 inhibitors [39]. Studies have shown that GFJ-drug interactions are mainly due to the last three components: naringin, bergamottin, and DHB. There were variations in the number of components between grapefruit types. White grapefruit contained greater amounts of naringin, bergamottin, and DHB than red and pink grapefruit. The highest concentrations were found in albedo and flavedo. Red GFJ contained more bergamottin and DHB than pink juice but a lower concentration of naringin. The lowest concentrations of naringin, bergamottin, and DHB were found in the seeds and pulp of the red grapefruit. These results are consistent with many other studies [40].
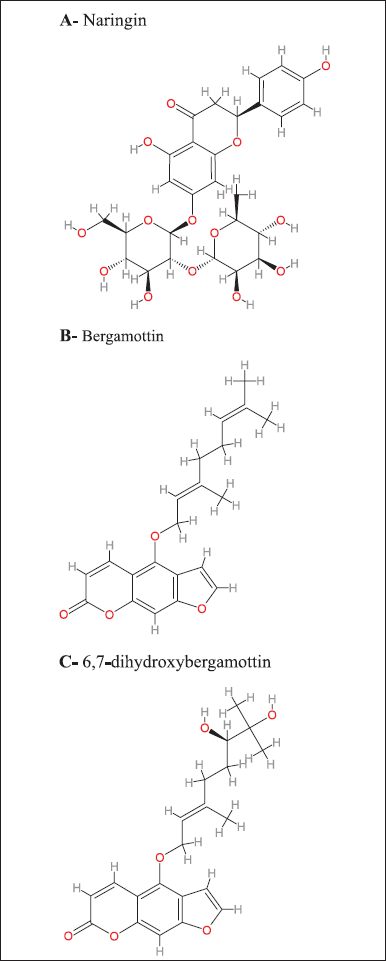 | Figure 1. GFJ compounds, mainly naringin, bergamottin, and DHB inhibit intestinal CYP3A4 enzymes. (A) Naringin. (B) Bergamottin. (C) 6,7-dihydroxybergamottin. [Click here to view] |
CYP3A4 INHIBITION OF GFJ–DRUG INTERACTION
Furanocoumarins decreased intestinal CYP3A4 activity in three ways. The first mechanism is the competitive inhibition of CYP3A4, which is the most common method. This is a reversible mechanism due to the competition between the inhibitor (furanocoumarin) and the substrate of the same CYP enzyme. The substrate requires the same CYP enzyme for metabolism and elimination. Competitive inhibition occurs after the first dose of the inhibitor [39]. The second mechanism of decreased intestinal CYP3A4 activity is irreversible inhibition, which causes the permanent inactivation of isoenzymes. Furanocoumarins are the most potent grapefruit components that cause the irreversible inhibition of CYP3A4. In this method, new CYP3A4 isoenzymes must be generated to restore activity, which requires time; thus, this indicates that the irreversible inhibition method had a longer duration than the reversible inhibition method. The interaction started 30 minutes after drinking one glass of GFJ. The recovery of CYP3A4 requires 2–3 days after the last drinking of GFJ, which explains why the separation between consuming GFJ and substrate drugs should be of sufficient duration, not only a few hours [41]. The third mechanism of decreased intestinal CYP3A4 activity involves the loss of the CYP3A4 enzyme [42].
Published studies have documented that GFJ does not affect drug disposition after intravenous administration or alter liver CYP3A4 activity. Thus, the interaction occurs via inhibition of intestinal CYP3A4 activity without affecting liver CYP3A4 activity. In vivo studies have shown that GFJ inhibits CYP3A4 irreversibly due to the downregulation of intestinal CYP3A4 protein content without variation in intestinal messenger ribonucleic acid levels [41]. The amount of GFJ consumed is one factor that affects the significance of the interaction. One glass of GFJ can inhibit CYP3A4 [42]. The magnitude of this interaction depends on the extent of intestinal CYP3A4 expression, which varies among individuals. These inter-individual variations are difficult to predict, and pharmacological principles must be considered to understand and interpret the variable responses for the same interaction. GFJ-drug interactions significantly affect patients with high intestinal CYP3A4 expression [42].
FACTORS DETERMINE THE CLINICAL SIGNIFICANCE OF GRAPEFRUIT INTERACTION
Determining whether the GFJ-drug interaction is clinically significant depends on the extent of the systemic drug concentration and drug toxicity related to the drug concentration. Additionally, several other factors affect it, such as the oral bioavailability of the affected drug, the type of grapefruit or other fruits consumed, and the inter-individual variations in their responses to the interaction.
Oral bioavailability
Individual variations in the amount of the impact were reliant on intrinsic differences in enteric CYP3A4 protein expression, with people with the greatest baseline CYP3A4 having the largest proportionate rise. GFJ influences medications with intrinsically poor oral bioavailability due to significant pre-systemic metabolism mediated by CYP3A4.
Factors related to GFJ consumption
The most common amount used is one grapefruit, which can produce up to 250 ml of juice, enough to cause interaction. Repeated consumption of GFJ increases this interaction [43–45]. For example, the dose of felodipine in 250 ml of GFJ increased the concentration threefold. The systematic concentration was reduced by fivefold when used in frequent doses with 250 ml of GFJ thrice daily for 6 days.
Effect of the time interval between GFJ and drug administration on their interaction
The temporal gap between the consumption of GFJ and medication administration is crucial in influencing their interaction. For example, when felodipine was administered with 200 ml of GFJ, the maximum pharmacokinetic effect of the interaction occurred when the interval between them was 4 hours; if the interval increased, the effect was reduced; for example, when the interval increased to 10 hours, the effect was half of the maximum effect; and when increased to 1 day, the effect was reduced to 25% of the maximum effect [46]. In addition, the grapefruit type, storage, and patch can affect the magnitude of the interaction, although this has not been studied.
Effect of grapefruit interactions on the patients
GFJ interacts with drugs by inhibiting the cytochrome P450 3A4 (CYP3A4) enzyme, increasing the drug absorbed into the bloodstream. This interaction occurs through post-translational down-regulation, where furanocoumarins bind to the CYP3A4 enzyme, causing it to be broken down more quickly. This decrease in CYP3A4 in the intestinal wall leads to an increase in drug absorption. This interaction is particularly significant for low oral bioavailability drugs like statins, calcium channel blockers, immunosuppressants, protease inhibitors, antihistamines, and sleeping pills (benzodiazepines, zolpidem, zaleplon, and eszopiclone). Another example is the felodipine concentration in 250 ml of GFJ, which varies between patients within 0–8 fold [45]. Patients with a higher quantity of intestinal CYP3A4 had a higher drug concentration and interaction risk; however, there was no clinical assay for intestinal CYP3A4 quantity. Although the patient’s vulnerability is difficult to determine, older people are at a higher risk for drug interactions because they usually use more than a prescribed drug and are less tolerant of high drug concentrations due to physiological reasons [47].
PREDICTION AND MANAGEMENT APPROACH OF THE GFJ-DRUGS INTERACTION
Several factors affect the interaction between GFJ and drugs, making it difficult to classify the interaction as significant or negligible, specifically with inter-individual variations [48,49]. In addition, there are no regulatory guidelines regarding the dietary consumption of grapefruit and GFJ [50]. Thus, a 2016 study included a simple general approach to predict and manage GFJ-drug interactions. The general approach includes the simple principle of expecting the occurrence of significant clinical GFJ-drug interactions, which is as follows: If the drug had three properties, including being given orally, having incomplete bioavailability (very low to intermediate oral bioavailability), and being metabolized extensively by CYP3A4, then it is expected to result in a pharmacokinetic interaction with GFJ, while if the drug did not have all three properties together, there is no expectation of a pharmacokinetic interaction occurring with grapefruit. If a pharmacokinetic interaction between GFJ and the drug is predicted and has any one of the following properties: being used for elderly patients, having inherently low bioavailability, and having overdose toxicity, other CYP3A4 inhibitors, including a cautionary sentence about grapefruit raised its bioavailability. Avoiding GFJ (Seville orange, lime, and pomelo) or using an alternative drug that does not interact with it is recommended. However, if the affected drug lacks all previous properties, there is no recommendation to avoid GFJ and only monitor side effects [48]. To avoid this and similar interactions, patients should avoid drinking GFJ if they are taking any medications that interact with it, and if they do drink GFJ, they should do so at least 2 hours before or after taking their medication.
DRUGS WITH POTENTIAL FOR INTERACTION
Many therapeutic agents are CYP3A4 substrates that are administered concurrently with GFJ. The effects of this combination vary according to the factors affecting it, including metabolism and drug distribution in the body. Most interactions resulted in increased bioavailability owing to CYP3A4 inhibition. Clinically, the interaction effect depends on the pharmacokinetics of the grapefruit, and the interaction may decrease the toxicity, concentration, or bioavailability of the administered drugs. GFJ may decrease the bioavailability of certain drugs, such as celiprolol. This reduction in bioavailability may be due to physiochemical factors, the interaction, and formation of a complex between celiprolol and a component of GFJ, or other reasons that reduce eliprolol bioavailability [42]. Clinical toxicity cases caused by GFJ-drug interactions are rare [51]. This may be because of two reasons. First, drugs interacting with GFJ have a broad therapeutic index (ciclosporin is an exception) owing to the interindividual variability of intestinal CYP3A4 activity. Second, the effect of GFJ is large enough to be a function of intestinal CYP3A4 activity. Toxicity cases may occur due to severe liver disease, where the intestine is the main site of metabolism, or if the activity of intestinal CYP3A4 is lost. In these cases, the standard doses will have a higher systemic exposure, which results in drug toxicity[39]. Table 1 shows examples of drugs that could potentially interact with GFJ.
In addition to previous drugs, other drugs have interacted with GFJ in clinical studies, such as blonanserin (an atypical antipsychotic used in the treatment of schizophrenia and mania) and dapoxetine (a selective serotonin reuptake inhibitor used for the treatment of premature ejaculation). GFJ-dapoxetine interaction was reported in a clinical study in Egypt. The study design included the simultaneous administration of GFJ and dapoxetine. The results showed that consumption of 250 ml of GFJ for 3 days increased plasma concentration and AUC of dapoxetine due to the GFJ-blonanserin interaction [90].
GFJ-blonanserin interaction was reported in a clinical study in China. The study design included co-administration of GFJ and blonanserin concomitantly in healthy subjects. The results showed a prolongation of blonanserin’s half-life, an elevation in its bioavailability, and systematic concentration due to the GFJ-blonanserin interaction [91].
SERIOUS ADVERSE EVENT OF GFJ-DRUGS INTERACTIONS
Clinically, serious adverse events have been reported that are related to GFJ-drug interactions, such as torsade de pointes, rhabdomyolysis, nephrotoxicity, Stevens-Johnson syndrome, and breast cancer. For example, a single-sequence, repeated-measures study included 11 healthy adults. Participants evaluated for amiodarone pharmacokinetics 6 months prior received a single oral dose of 17 mg kg−1 with three glasses of 300 ml grapefruit juice on the same day. GFJ significantly reduced the major metabolite of amiodarone [i.e., N-desethylamiodarone, N-desethylamiodarone (N-DEA)] production in all subjects, increasing AUC and Cmax by 50% and 84%, respectively, compared to water administration. This inhibition of N-DEA production decreased the alterations caused by amiodarone on PR and QTc intervals [67] [NO_PRINTED_FORM]. The second example is the administration of atorvastatin with GFJ given as a single normal amount (e.g., 200–300 ml) or by whole fresh fruit segments), which results in severe rhabdomyolysis with acute renal failure is a known rare adverse effect. In addition, rhabdomyolysis can result from combining simvastatin and GFJ. In a clinical case, the amount of GFJ consumed was one grapefruit daily for 2 weeks [75,92–94]. The third example is the administration of tacrolimus with the consumption of grapefruit marmalade the week before drug administration, which resulted in a significant increase in the drug concentration; the increase was up to 500%, which led to acute renal dysfunction and nephrotoxicity adverse events [95]. The fourth example is the risk of Stevens-Johnson syndrome. A clinical case report in 2019 reported a GFJ-ciprofloxacin interaction, and GFJ was around 1 l for 1 week. This interaction resulted in an elevated systematic concentration of ciprofloxacin, which increases the risk of Stevens-Johnson syndrome [53]. The fifth example is the risk of breast cancer as an adverse event caused by estrogen, especially in menopausal women who consume estrogen and GFJ together [51,96]. In addition, other clinical cases have been reported, including complete heart block by verapamil when consuming a large amount of GFJ before verapamil administration 20 and myelotoxicity by colchicine when consuming 1 l of GFJ daily in the previous 2 months of colchicine administration [70]. In addition, venous thrombosis is caused by ethinylestradiol when administered after consuming one grapefruit daily for only 3 days [97]. In addition to previous cases, in 2019, a clinical case report reported the first clinically significant GFJ-methadone interaction. The patient consumed 500 ml per day of GFJ for 3 days, causing a GFJ-methadone interaction and elevated methadone serum, which ended with opioid toxidrome. The patient suffered from opioid toxidrome, characterized as hypoxic and bradypnea with pinpoint pupils [98]. Previous adverse events cannot be generalized as adverse events of the interaction [99].
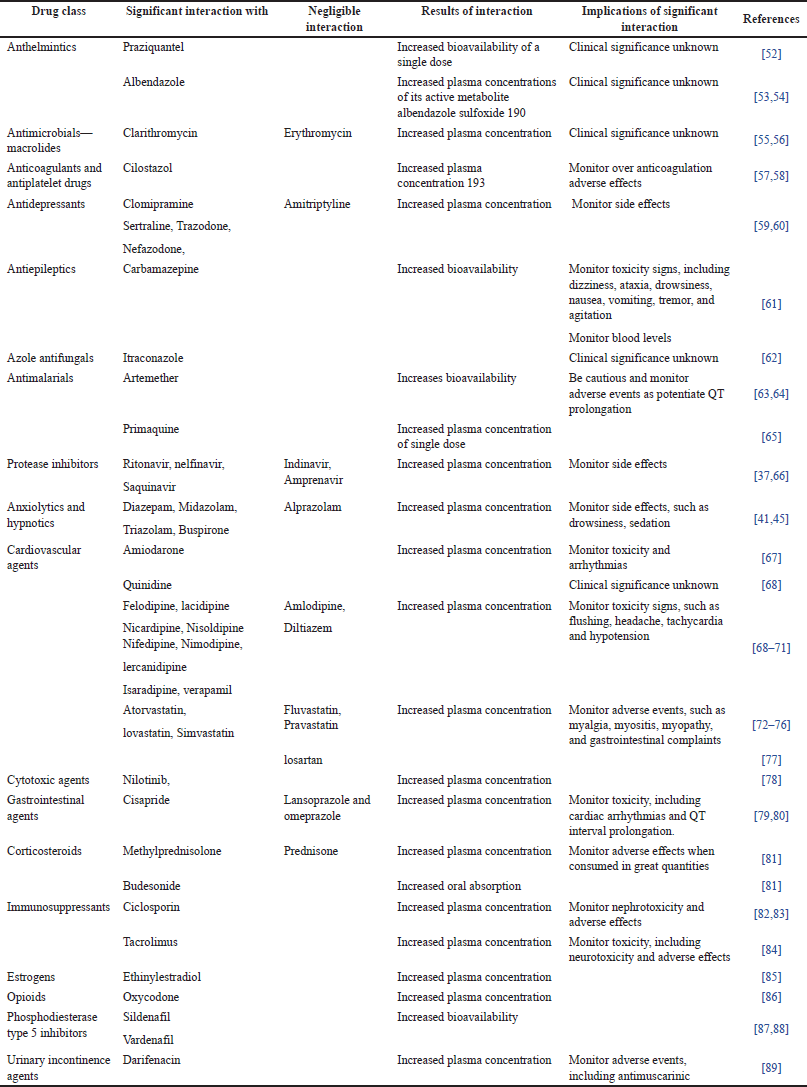 | Table 1. Interactions between GFJ and CYP3A4 substrates. [Click here to view] |
Clinically, there were no significant interactions between GFJ and over-the-counter medications. Although there are interactions with variant levels between GFJ and OTC drugs such as caffeine, dextromethorphan, and chlorpheniramine, this is due to the broad therapeutic index of these agents, thus predicting that the standard dose of OTC agents will not be toxic [42]. Some cases require caution, such as when the patient is older or consumes other prescribed CYP3A4 inhibitors or inducers, dietary supplements, or botanical products. These agents may have an additive effect on CYP3A4 inhibition. However, there is no general advice on avoiding GFJ using OTC agents [84].
ROLE OF PHARMACISTS IN THE MANAGEMENT OF GFJ-DRUG INTERACTION FOR IMPROVED THERAPEUTIC OUTCOME
Patients depend on pharmacists for their reliable medical advice. Therefore, they require accurate and valuable medical information. Thus, pharmacists play a major role in minimizing cases of grapefruit-drug interactions, which are important in reducing the risk of GFJ-drug interactions. Pharmacists should educate patients about grapefruit-drug interactions; thus, they should ask patients about consuming grapefruit, GFJ, or any other juice containing a significant amount when dispensing drugs. Pharmacists should inform patients that most citrus juices, including lemons and oranges, are safe, while sour oranges, such as Seville oranges, can interact with GFJ, and limes may interact with drugs. If patients do not cease consuming grapefruit or GFJ, pharmacists can replace the prescribed drug, which interacts with GFJ, with an alternative drug that has a similar effect without the need to cease the drug. Pharmacists have a strong, extensive pharmacological background with broad medical information about drugs to predict whether the candidate would interact significantly with GFJ. There are some points that pharmacists can consider when predicting or deciding the GFJ-drug interaction, such as the route of drug administration if it is administered orally, the metabolism of the drug if it is mediated by intestinal CYP3A4, the bioavailability of the drug if it has low bioavailability, and the therapeutic index range if it has a narrow therapeutic index. In addition, pharmacists should inform patients about certain points, such as the fact that most drugs do not interact with GFJ. On the other hand, patients should inform physicians or pharmacists if they consume grapefruit or GFJ, especially newly prescribed drugs, and the pharmacist should ask the patient about their daily diet before dispensing both prescribed and OTC drugs. In addition, pharmacists should label dispensed drugs according to the recommendations [42].
CONCLUSION
GFJ has the potential to interact with numerous medicines by suppressing intestinal CYP3A4, resulting in higher drug levels and associated side effects. GFJ contains phytochemicals that may inhibit intestine CYP3A4, an enzyme in drug metabolism. This inhibition may result in higher drug levels, increasing the likelihood of undesirable consequences. However, there have been reports of improved bioavailability of drug-GFJ interactions with no negative consequences.GFJ and medicine interactions are unpredictable and might vary based on the person and the drug. Pharmacists are crucial in teaching patients about the hazards of GFJ-drug interactions.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FUNDING
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Khalil MNA, Farghal HH, Farag MA. Outgoing and potential trends of composition, health benefits, juice production and waste management of the multi-faceted grapefruit Citrus Χ Paradisi: a comprehensive review for maximizing its value. Crit Rev Food Sci Nutr. 2022;62:935–56.
2. Louzada ES, Ramadugu C. Grapefruit: history, use, and breeding. Horttechnology. 2021;31:243–58. CrossRef
3. Fuhr LM, Marok FZ, Fuhr U, Selzer D, Lehr T. Physiologically based pharmacokinetic modeling of bergamottin and 6,7-dihydroxybergamottin to describe CYP3A4 mediated grapefruit-drug interactions. Clin Pharmacol Ther. 2023;114:470–82. CrossRef
4. Kane GC, Lipsky JJ. Drug-grapefruit juice interactions. Mayo Clin Proc. 2000;75:933–42.
5. Richa R, Kohli D, Vishwakarma D, Mishra A, Kabdal B, Kothakota A, et al. Citrus fruit: classification, value addition, nutritional and medicinal values, and relation with pandemic and hidden hunger. J Agric Food Res. 2023;14:100718. CrossRef
6. Bailey DG, Dresser GK. Interactions between grapefruit juice and cardiovascular drugs. Am J Cardiovasc Drugs. 2004;4:281–97.
7. Ducharme MP, Warbasse LH, Edwards DJ. Disposition of intravenous and oral cyclosporine after administration with grapefruit juice. Clin Pharmacol Ther. 1995;57:485–91. CrossRef
8. Hukkinen SK, Varhe A, Olkkola KT, Neuvonen PJ. Plasma concentrations of triazolam are increased by concomitant ingestion of grapefruit juice. Clin Pharmacol Ther. 1995;58:127–31. CrossRef
9. Goosen TC, Cillié D, Bailey DG, Yu C, He K, Hollenberg PF, et al. Bergamottin contribution to the grapefruit juice-felodipine interaction and disposition in humans. Clin Pharmacol Ther. 2004;76:607–17. CrossRef
10. De Castro WV, Mertens-Talcott S, Rubner A, Butterweck V, Derendorf H. Variation of flavonoids and furanocoumarins in grapefruit juices: a potential source of variability in grapefruit juice-drug interaction studies. J Agric Food Chem. 2006;54:249–55. CrossRef
11. Jarrar YB, Al-Essa L, Kilani A, Hasan M, Al-Qerem W. Alterations in the gene expression of drug and arachidonic acid-metabolizing Cyp450 in the livers of controlled and uncontrolled insulin-dependent diabetic mice. Diabetes Metab Syndr Obes. 2018;11:483. CrossRef
12. Garcia-Lor A, Bermejo A, Morales J, Hernández M, Medina A, Cuenca J, et al. Strategies to produce grapefruit-like citrus varieties with a low furanocoumarin content and distinctive flavonoid profiles. Front Plant Sci. 2021;12:640512. CrossRef
13. Zhou S, Chan SY, Goh BC, Chan E, Duan W, Huang M, et al. Mechanism-based inhibition of cytochrome P450 3A4 by therapeutic drugs. Clin Pharmacokinet. 2005;44:279–304.
14. Zhu Q, Chen N, Tian X, Zhou Y, You Q, Xu X. Hematopoietic progenitor kinase 1 in tumor immunology: a medicinal chemistry perspective. J Med Chem. 2022;65:8065–90. CrossRef
15. Bhatia KS, Mahesh AN, Bhatt S. Cytochrome P450. Reference module in biomedical sciences 2023. CrossRef
16. Guengerich FP, Waterman MR, Egli M. Recent structural insights into cytochrome P450 function. Trends Pharmacol Sci. 2016;37:625–40.
17. Nelson DR, Koymans L, Kamataki T, Stegeman JJ, Feyereisen R, Waxman DJ, et al. P450 superfamily: update on new sequences, gene mapping, accession numbers and nomenclature. Pharmacogenetics. 1996 Feb;6(1):1–42. CrossRef
18. Fujikura K, Ingelman-Sundberg M, Lauschke VM. Genetic variation in the human cytochrome P450 supergene family. Pharmacogenet Genom. 2015;25:584–594. CrossRef
19. Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360:1155–62. CrossRef
20. Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17.
21. Paine MF, Khalighi M, Fisher JM, Shen DD, Kunze KL, Marsh CL, et al. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther. 1997;283:1552–62.
22. Kolars JC, Lown KS, Schmiedlin-Ren P, Ghosh M, Fang C, Wrighton SA, et al. Cyp3A gene expression in human gut epithelium. Pharmacogenetics. 1994;4:247–59. CrossRef
23. Rendic S, Guengerich FP. Contributions of human enzymes in carcinogen metabolism. Chem Res Toxicol. 2012;25:1316–83. CrossRef
24. Von Richter O, Burk O, Fromm MF, Thon KP, Eichelbaum M, Kivistö KT. Cytochrome P450 3A4 and P-glycoprotein expression in human small intestinal enterocytes and hepatocytes: a comparative analysis in paired tissue specimens. Clin Pharmacol Ther. 2004;75:172–83. CrossRef
25. Kolars JC, Watkins PB, Merion RM, Awni WM. First-pass metabolism of cyclosporin by the gut. Lancet. 1991;338:1488–90. CrossRef
26. Thummel KE, O’Shea D, Paine MF, Shen DD, Kunze KL, Perkins JD, et al. Oral first-pass elimination of midazolam involves both gastrointestinal and hepatic CYP3A-mediated metabolism. Clin Pharmacol Ther. 1996;59:491–502. CrossRef
27. Shand DG, Hammill SC, Aanonsen L, Pritchett ELC. Reduced verapamil clearance during long-term oral administration. Clin Pharmacol Ther. 1981;30:701–3. CrossRef
28. Von Richter O, Greiner B, Fromm MF, Fraser R, Omari T, Barclay ML, et al. Determination of in vivo absorption, metabolism, and transport of drugs by the human intestinal wall and liver with a novel perfusion technique. Clin Pharmacol Ther. 2001;70:217–27. CrossRef
29. Wacher VJ, Wu CY, Benet LZ. Overlapping substrate specificities and tissue distribution of cytochrome P450 3A and P-glycoprotein: implications for drug delivery and activity in cancer chemotherapy. Mol Carcinog. 1995;13:129–34.
30. Lin JH, Chiba M, Baillie TA. Is the role of the small intestine in first-pass metabolism overemphasized? Pharmacol Rev. 1999;51:135–57.
31. Kupferschmidt HHT, Ha HR, Ziegler WH, Meier PJ, Krähenbühl S. Interaction between grapefruit juice and midazolam in humans. Clin Pharmacol Ther. 1995;58:20–8. CrossRef
32. Shilpa VS, Shams R, Dash KK, Pandey VK, Dar AH, Ayaz Mukarram S, et al. Phytochemical properties, extraction, and pharmacological benefits of naringin: a review. Molecules. 2023;28:5623.
33. Ho PC, Saville DJ, Wanwimolruk S. Inhibition of human CYP3A4 activity by grapefruit flavonoids, furanocoumarins and related compounds. J Pharm Pharm Sci. 2001;4:217–27.
34. Zeng X, Su W, Bai Y, Chen T, Yan Z, Wang J, et al. Urinary metabolite profiling of flavonoids in chinese volunteers after consumption of orange juice by UFLC-Q-TOF-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2017;1061–2:79–88. CrossRef
35. Chtourou Y, Gargouri B, Kebieche M, Fetoui H. Naringin abrogates cisplatin-induced cognitive deficits and cholinergic dysfunction through the down-regulation of AChE expression and INOS signaling pathways in hippocampus of aged rats. J Mol Neurosci. 2015;56:349–62. CrossRef
36. Wang DM, Yang YJ, Zhang L, Zhang X, Guan FF, Zhang LF. Naringin enhances CaMKII activity and improves long-term memory in a mouse model of Alzheimer’s disease. Int J Mol Sci. 2013;14:5576–86. CrossRef
37. Culm-Merdek KE, Von Moltke LL, Gan L, Horan KA, Reynolds R, Harmatz JS, et al. Effect of extended exposure to grapefruit juice on cytochrome P450 3A activity in humans: comparison with ritonavir. Clin Pharmacol Ther. 2006;79:243–54. CrossRef
38. Carbone A, Montalbano A, Spanò V, Musante I, Galietta LJV, Barraja P. Furocoumarins as multi-target agents in the treatment of cystic fibrosis. Eur J Med Chem. 2019;180:283–90. CrossRef
39. Greenblatt DJ, Von Moltke LL, Harmatz JS, Chen G, Weemhoff JL, Jen C, et al. Time course of recovery of cytochrome P450 3A function after single doses of grapefruit juice. Clin Pharmacol Ther. 2003;74:121–9. CrossRef
40. Bourgaud F, Hehn A, Larbat R, Doerper S, Gontier E, Kellner S, et al. Biosynthesis of coumarins in plants: a major pathway still to be unravelled for cytochrome P450 enzymes. Phytochem Rev. 2006;5:293–308.
41. Lundahl J, Regårdh CG, Edgar B, Johnsson G. Effects of grapefruit juice ingestion—pharmacokinetics and haemodynamics of intravenously and orally administered felodipine in healthy men. Eur J Clin Pharmacol. 1997;52:139–45. CrossRef
42. Fukuda K, Guo L, Ohashi N, Yoshikawa M, Yamazoe Y. Amounts and variation in grapefruit juice of the main components causing grapefruit-drug interaction. J Chromatogr B Biomed Sci Appl. 2000;741:195–203. CrossRef
43. Dresser GK, Bailey DG, Carruthers SG. Grapefruit juice-felodipine interaction in the elderly. Clin Pharmacol Ther. 2000;68:28–34. CrossRef
44. Edgar B, Bailey D, Bergstrand R, Johnsson G, Regrdh CG. Acute effects of drinking grapefruit juice on the pharmacokinetics and dynamics on felodipine—and its potential clinical relevance. Eur J Clin Pharmacol. 1992;42:313–7. CrossRef
45. Lown KS, Bailey DG, Fontana RJ, Janardan SK, Adair CH, Fortlage LA, et al. grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99:2545–53. CrossRef
46. Lundahl J, Regårdh CG, Edgar B, Johnsson G. Relationship between time of intake of grapefruit juice and its effect on pharmacokinetics and pharmacodynamics of felodipine in healthy subjects. Eur J Clin Pharmacol. 1995;49:61–7. CrossRef
47. Agosti S, Casalino L, Bertero G, Barsotti A, Brunelli C, Morelloni S. A dangerous fruit juice. Am J Emerg Med. 2012;30:248.e5–8. CrossRef
48. Vieira MLT, Kirby B, Ragueneau-Majlessi I, Galetin A, Chien JYL, Einolf HJ, et al. Evaluation of various static in vitro-in vivo extrapolation models for risk assessment of the CYP3A inhibition potential of an investigational drug. Clin Pharmacol Ther. 2014;95:189–98. CrossRef
49. Mima M, Mallah E, Abudayyih W, Elhajji FD, Salih H, Zakaraya Z, et al. Pharmacokinetics and pharmacodynamics interaction of warfarin in the presence of beverage juices (licorice and pomegranate) in rat plasma by using LC/MS. Latin Am J Pharm. 2017;36:1181–92.
50. Bailey DG, Dresser G, Arnold JMO. Grapefruit-medication interactions: forbidden fruit or avoidable consequences? Can Med Assoc J. 2013;185:309–16.
51. Weber A, Jäger R, Börner A, Klinger G, Vollanth R, Matthey K, et al. Can grapefruit juice influence ethinylestradiol bioavailability? Contraception. 1996;53:41–7. CrossRef
52. Castro N, Jung H, Medina R, González-Esquivel D, Lopez M, Sotelo J. Interaction between grapefruit juice and praziquantel in humans. Antimicrob Agents Chemother. 2002;46:1614–6. CrossRef
53. Nagy J, Schipper HG, Koopmans RP, Butter JJ, Van Boxtel CJ, Kager PA. Effect of grapefruit juice or cimetidine coadministration on albendazole bioavailability. Am J Trop Med Hyg. 2002;66:260–3. CrossRef
54. Awadzi K, Hero M, Opoku NO, Buttner DW, Coventry PA, Prime MA, et al. The chemotherapy of onchocerciasis XVII. A clinical evaluation of albendazole in patients with onchocerciasis; effects of food and pretreatment with ivermectin on drug response and pharmacokinetics. Trop Med Parasitol. 1994;45:203–8.
55. Cheng KL, Nafziger AN, Peloquin CA, Amsden GW. Effect of grapefruit juice on clarithromycin pharmacokinetics. Antimicrob Agents Chemother. 1998;42:927–9.
56. Kanazawa S, Ohkubo T, Sugawara K. The effects of grapefruit juice on the pharmacokinetics of erythromycin. Eur J Clin Pharmacol. 2001;56:799–803. CrossRef
57. Taniguchi K, Ohtani H, Ikemoto T, Miki A, Hori S, Sawada Y. Possible case of potentiation of the antiplatelet effect of cilostazol by grapefruit juice. J Clin Pharm Ther. 2007;32:457–9. CrossRef
58. Abbas R, Chow CP, Browder NJ, Thacker D, Bramer SL, Fu CJ, et al. In vitro metabolism and interaction of cilostazol with human hepatic cytochrome P450 isoforms. Hum Exp Toxicol. 2000;19:178–84. CrossRef
59. Adams CD. Stockley’s drug interactions. Tenth Edition, Stockley’s drug interactions pocket companion 2013. J Med Libr Assoc. 2014;102:221. CrossRef
60. Oesterheld J, Kallepalli BR. Grapefruit juice and clomipramine: shifting metabolitic ratios [10]. J Clin Psychopharmacol. 1997;17:62–3.
61. Garg SK, Kumar N, Bhargava VK, Prabhakar SK. Effect of grapefruit juice on carbamazepine bioavailability in patients with epilepsy. Clin Pharmacol Ther. 1998;64:286–8. CrossRef
62. Kawakami M, Suzuki K, Ishizuka T, Hidaka T, Matsuki Y, Nakamura H. Effect of grapefruit juice on pharmacokinetics of itraconazole in healthy subjects. Int J Clin Pharmacol Ther. 1998;36:306–8.
63. Van Agtmael MA, Gupta V, Van der Wösten TH, Rutten JPB, Van Boxtel CJ. Grapefruit juice increases the bioavailability of artemether. Eur J Clin Pharmacol. 1999;55:405–10. CrossRef
64. Van Agtmael MA, Gupta V, Van Der Graaf CAA, Van Boxtel CJ. The effect of grapefruit juice on the time-dependent decline of artemether plasma levels in healthy subjects. Clin Pharmacol Ther. 1999;66:408–14. CrossRef
65. Cuong BT, Binh VQ, Dai B, Duy DN, Lovell CM, Rieckmann KH, et al. Does gender, food or grapefruit juice alter the pharmacokinetics of primaquine in healthy subjects? Br J Clin Pharmacol. 2006;61:682–9. CrossRef
66. Kupferschmidt HHT, Fattinger KE, Ha HR, Follath F, Krähenbühl S. Grapefruit juice enhances the bioavailability of the HIV protease inhibitor saquinavir in man. Br J Clin Pharmacol. 1998;45:355–9. CrossRef
67. Libersa CC, Brique SA, Motte KB, Caron JF, Guédon-Moreau LM, Humbert L, et al. Dramatic inhibition of amiodarone metabolism induced by grapefruit juice. Br J Clin Pharmacol. 2000;49:373–8. CrossRef
68. Muntingh G. An overview of interactions between grapefruit juice and drugs. SA Pharm J. 2011;78:40–5.
69. Seden K, Dickinson L, Khoo S, Back D. Grapefruit-drug interactions. Drugs. 2010;70:2373–408.
70. Pillai U, Muzaffar J, Sen S, Yancey A. Grapefruit juice and verapamil: a toxic cocktail. South Med J. 2009;102:308–9. CrossRef
71. Uno T, Ohkubo T, Sugawara K, Higashiyama A, Motomura S, Ishizaki T. Effects of grapefruit juice on the stereoselective disposition of nicardipine in humans: evidence for dominant presystemic elimination at the gut site. Eur J Clin Pharmacol. 2000;56:643–9. CrossRef
72. Vaquero MP, Muniz FJS, Redondo SJ, Oliván PP, Higueras FJ, Bastida S. Major diet-drug interactions affecting the kinetic characteristics and hypolipidaemic properties of statins. Nutr Hosp. 2010;25:193–206.
73. Lilja J, Kivisto K, Neuvonen P. Grapefruit juice increases serum concentrations of atorvastatin and has no effect on pravastatin. Clin Pharmacol Ther. 1999;66:118–27. CrossRef
74. Mertens-Talcott SU, Zadezensky I, De Castro WV, Derendorf H, Butterweck V. Grapefruit-drug interactions: can interactions with drugs be avoided? J Clin Pharmacol. 2006;46:1390–416.
75. Dreier JP, Endres M. Statin-associated rhabdomyolysis triggered by grapefruit consumption. Neurology. 2004;62:670. CrossRef
76. Kantola T, Kivistö KT, Neuvonen PJ. grapefruit juice greatly increases serum concentrations of lovastatin and lovastatin acid. Clin Pharmacol Ther. 1998;63:397–402. CrossRef
77. Zaidenstein R, Soback S, Gips M, Avni B, Dishi V, Weissgarten Y, et al. Effect of grapefruit juice on the pharmacokinetics of losartan and its active metabolite E3174 in healthy volunteers. Ther Drug Monit. 2001 Aug [cited 2023 May 22];23(4):369–73. Available from: https://journals.lww.com/drug-monitoring/Abstract/2001/08000/Effect_of_Grapefruit_Juice_on_the_Pharmacokinetics.8.aspx
78. Yin OQP, Gallagher N, Li A, Zhou W, Harrell R, Schran H. Effect of grapefruit juice on the pharmacokinetics of nilotinib in healthy participants. J Clin Pharmacol. 2010;50:188–94. CrossRef
79. Kivistö KT, Lilja JJ, Backman JT, Neuvonen PJ. Repeated consumption of grapefruit juice considerably increases plasma concentrations of cisapride. Clin Pharmacol Ther. 1999;66:448–53. CrossRef
80. Tassaneeyakul W, Tassaneeyakul W, Vannaprasaht S, Yamazoe Y. Formation of omeprazole sulphone but Not 5-hydroxyomeprazole is inhibited by grapefruit Juice. Br J Clin Pharmacol. 2000;49:139–44. CrossRef
81. Seidegård J, Randvall G, Nyberg L, Borgå O. Grapefruit juice interaction with oral budesonide: equal effect on immediate-release and delayed-release formulations. Pharmazie. 2009;64:461–5. CrossRef
82. Paine MF, Widmer WW, Pusek SN, Beavers KL, Criss AB, Snyder J, et al. Further characterization of a furanocoumarin-free grapefruit juice on drug disposition: studies with cyclosporine. Am J Clin Nutr. 2008;87:863–71. CrossRef
83. Edwards DJ, Fitzsimmons ME, Schuetz EG, Yasuda K, Ducharme MP, Warbasse LH, et al. 6’,7’-Dihydroxybergamottin in grapefruit juice and seville orange juice: effects on cyclosporine disposition, enterocyte CYP3A4, and P-glycoprotein. Clin Pharmacol Ther. 1999;65:237–44. CrossRef
84. Shimomura S, Wanwimolruk S. Drug interactions with grapefruit juice. Pharm Times. 2010;5:2–6. CrossRef
85. Fingerová H, Oborná I, Petrová P, Budíková M, Jezdínský J. Does grapefruit juice increase the bioavailability of orally administered sex steroids?. Ceska Gynekol. 2003;68:117–21.
86. Nieminen TH, Hagelberg NM, Saari TI, Neuvonen M, Neuvonen PJ, Laine K, et al. Grapefruit juice enhances the exposure to oral oxycodone. Basic Clin Pharmacol Toxicol. 2010;107:782–8. CrossRef
87. Lee M, Min DI. Determination of sildenafil citrate in plasma by high-performance liquid chromatography and a case for the potential interaction of grapefruit juice with sildenafil citrate. Ther Drug Monit. 2001;23:21–6. CrossRef
88. LEVITRA [cited 2023 May 22].
89. SmPC bupropion prolonged release tablets—summary of product characteristics. [cited 2023 May 22]. Available from: https://www.medicines.org.uk/emc/product/5129/smpc#gref
90. Abdlekawy KS, Donia AM, Elbarbry F. Effects of grapefruit and pomegranate juices on the pharmacokinetic properties of dapoxetine and midazolam in healthy subjects. Eur J Drug Metab Pharmacokinet. 2017;42:397–405. CrossRef
91. Shang DW, Wang ZZ, Hu HT, Zhang YF, Ni XJ, Lu HY, et al. Effects of food and grapefruit juice on single-dose pharmacokinetics of blonanserin in healthy Chinese subjects. Eur J Clin Pharmacol. 2018;74:61–7. CrossRef
92. Karch AM. The grapefruit challenge: the juice inhibits a crucial enzyme, with possibly fatal consequences. Am J Nurs. 2004;104:33–5.
93. Mazokopakis EE. Unusual causes of rhabdomyolysis. Intern Med J. 2008;38:364–7. CrossRef
94. Abdel-Halim H, Dayyih WA. Pomegranate juice-drug interactions: pharmacokinetic parameters studied using different liquid chromatographytechniques. Sapporo Med J. 2020;54:1–8.
95. Peynaud D, Charpiat B, Vial T, Gallavardin M, Ducerf C. Tacrolimus severe overdosage after intake of masked grapefruit in orange marmalade. Eur J Clin Pharmacol. 2007;63:721–2.
96. Schubert W, Cullberg G, Edgar B, Hedner T. Inhibition of 17 beta-estradiol metabolism by grapefruit juice in ovariectomized women. Maturitas. 1994 Dec;20(2–3):155–63. CrossRef. PMID: 7715468.
97. Goldbart A, Press J, Sofer S, Kapelushnik J. Near fatal acute colchicine intoxication in a child. A case report. Eur J Pediatr. 2000;159:895–7. CrossRef
98. Ershad M, Cruz MD, Mostafa A, McKeever R, Vearrier D, Greenberg MI. Opioid toxidrome following grapefruit juice consumption in the setting of methadone maintenance. J Addict Med. 2020;14:172–4. CrossRef
99. Grande LA, Mendez RD, Krug RT, Verschuyl EJ. Attention-grapefruit! Lancet. 2009;373:1222. CrossRef