INTRODUCTION
Capsicum species are cultivated for their culinary and medicinal purposes, with documented use in ancient literature from various regions worldwide. The cultivation of chili peppers, a member of the Capsicum genus, dates back to 7500 BC and has played a significant role in human cuisine throughout history. Excavations in South Western Ecuador have revealed evidence of chili pepper cultivation more than 6,000 years ago [1]. Chili peppers, which are members of the Solanaceae family and Capsicum genus, are taken globally as vegetables and spices due to their pungent aroma and the presence of health-promoting metabolites/compounds. These fruits contain a range of favorable chemical compounds, including capsaicinoids, carotenoids (pro-vitamin A), flavonoids, essential oils, vitamins (C and E), as well as several antifungal, antibacterial compounds (hydroxycinnamic and hydroxybenzoic acids), and mineral elements. These compounds contribute to the medicinal value and nutritional benefits associated with Capsicum fruits. The Capsicum genus encompasses over 200 varieties, exhibiting significant variations in fruit size, shape, flavor, and sensory heat. One of the domesticated Capsicum species worldwide, the five major ones are Capsicum annuum, Capsicum frutescens, Capsicum chinense, Capsicum baccatum, and Capsicum pubescens [2]. These species are commonly used as culinary spices and are also employed in various medicinal applications within Indian, Native American, and traditional Chinese medicine (TCM). They have been traditionally utilized for treating ailments such as arthritis, rheumatism, stomach aches, skin rashes, and even dog/snake bites and wounds. Various ethnic communities in northeastern India have also traditionally utilized it to treat these human illnesses. In Nagaland, hot infusions are used to treat toothaches and muscular aches, while Capsicum species, particularly the naga chili, are used to tone up body muscles after strenuous exercise [3]. In Eastern Nicaragua, a decoction of C. chinense fruit is applied topically to give relief in postpartum abdominal and backpain, fever, body aches, pulmonary disorders (cold, coughs, and so on), skin rashes, and soreness [3]. Because of its bronchodilation effect, C. chinense has also been proven to be useful in the symptomatic treatment of asthma. In certain gastrointestinal disorders, consuming tiny amounts of C. chinense pepper pods on a regular basis is quite useful [4]. Capsaicin can act as a topical analgesic, providing relief from pain associated with arthritis, muscle aches, and migraines. It may boost metabolism and reduce appetite, potentially aiding in weight management efforts. In addition, capsaicin may improve blood circulation and reduce the risk of heart disease. Capsaicin exhibits antimicrobial activity against certain bacteria and fungi, potentially aiding in wound healing and infection prevention [3]. Capsicum annuum, C. chinense, and C. frutescens are particularly rich in phytochemicals known for their antioxidant properties. These include carotenoids, capsaicinoids, and phenolic compounds, with notable flavonoids such as quercetin and luteolin. Capsaicin, a lipophilic alkaloid, serves as the principal component responsible for the pungent sensation associated with these peppers. Capsicum peppers are native to and widely available in several countries including Sri Lanka, Bangladesh, India, Mexico, Australia, the United Kingdom, and the United States. In India, particularly in the northeastern region such as Assam, Capsicum peppers are extensively cultivated and consumed, and they are known by various names such as Bhut joloia, Bih Jolokia, and Naga Jolokia. The hottest chili pepper found in Assam, known as bhut joloia or Ghost Chili, is a hybrid derived from a cross between C. chinense and C. frutescens. It is also commonly referred to as Naga Jolokia, Ghost Chili, Ghost Pepper, or simply Naga Chili. The cultivation of C. chinense is prominent in the Indian state of Assam, and other northeastern Indian states such as Nagaland and Manipur [5]. The Scoville heat unit (SHU) is a measurement of pungency or spiciness in chili peppers and other substances. It is a widely used scale to compare the heat levels of different pepper varieties. Indian capsicums, which belong to the Capsicum species, can have varying levels of pungency, as measured in SHU. For example, the SHU value for Indian capsicums, also known as chiltepin peppers, ranges from 100,000 to 200,000 SHU. In 2007, C. chinense gained recognition in the Guinness World Records as the world’s hottest chili pepper, with a SHU rating of 1,001,304 [6]. On the other hand, the jalapeno pepper (C. annuum, J, which originated in Jalapa, Mexico, is mildly hot with a heat rating of 5,000 SHU and is popular among American consumers [7]. The Scotch Bonnet (SB) or Habanero pepper (C. chinense, SB), with a heat rating of 250,000–300,000 SHU, is cultivated in the United States and Jamaica for its distinctive spiciness and flavor [5,8].
The objective of this work is to provide a comprehensive overview of the applications of C. chinense in pharmacy and its potential benefits in managing various diseases. The paper delves into the role of capsaicin, the chief constituent of C. chinense that underlies the therapeutic effects in pain management, arthritis, gastrointestinal disorders, cardiovascular diseases, obesity, diabetes, and cancer. In addition, this work describes recent advancements in developing efficacious formulations for therapeutic effect which will cater the source of information for the formulation scientists.
PLANT DESCRIPTION
Capsicum chinense is primarily a self-pollinated plant (Fig. 1), but when insect populations are high, cross-pollination can occur to some extent, reaching up to 10%. When grown under suitable conditions, it behaves as a semi-perennial herb. In the northeastern states of India, it typically reaches a height of 50–130 cm, although it can grow taller under semi-perennial conditions. The stem of the plant is green, and the nodes exhibit pigmentation with anthocyanin, resulting in a dark color. The flowers of C. chinense are pendant and have creamy white corollas, occasionally displaying a hint of pale green. They bloom in clusters, with 1–2 flowers per node, but typically produce no more than two fruits per node once they reach maturity. The filaments of the flowers are purple, while the anthers exhibit a blue coloration. The elongated fruits of C. chinense have an undulating surface and measure approximately 5–9 cm in length and 2.5–3.0 cm in diameter. Table 1 gives a detailed description of C. chinense plant. These fruits come in a variety of colors, ranging from mild green to brilliant crimson to blazing orange. Capsicum chinense fruits contain 4–5 predominant capsaicinoids, which are the most significant phytoconstituents found in the fruit. Capsaicinoids are characterized as the acid amide of vanillyl amide and C9–C11 branched fatty acids. Capsaicin, dihydrocapsaicin (DHC), norhydrocapsaicin, homocapsaicin, and homodihydrocapsaicin are five naturally occurring capsaicinoids reported. In a single season, a plant produces around 15–20 full-sized fruits and 10–14 smaller fruits as shown in Figure 2. The color changes observed in C. chinense fruits during maturation are as follows [9,10].
 | Figure 1. Capsicum chinense plant. [Click here to view] |
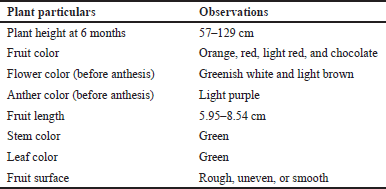 | Table 1. Description of C. chinense plant. [Click here to view] |
a) Young light green-colored fruits transition to orange when they reach maturity.
b) Young dark green-colored fruits transform into dark red as they mature.
c) Young green-colored fruits turn light red when they reach maturity.
d) Young dark green-colored fruits change to dark chocolate color at maturity.
These color transformations as shown in Figure 3 indicate the ripening process of C. chinense fruits and are characteristic of different varieties within the species.
CULTIVATION OF C. CHINENSE
Chili cultivation is extensively practiced in the northeastern states of India, including Assam, Nagaland, and Manipur. In this region, two planting seasons are followed: Kharif and Rabi. Kharif cultivation primarily takes place in the hilly states, commencing from February to March, with harvesting carried out from May to June. Table 2 enlist some specifications for cultivating C. chinense [11,12].
For C. chinense to retain its quality and shelf life, postharvest handling is crucial. Research suggests that storage conditions, such as temperature and packaging, can affect the weight loss, vitamin C content, and overall quality of chili peppers [15]. In addition, postharvest diseases such as Black Mold and Grey Mold can affect the quality of bell peppers, which belong to the same genus as C. chinense [16,17]. For postharvesting handling, it is suggested that the fruits should be sorted according to quality, size, and color and were then washed with fresh water to get rid of dirt and debris. However, it is also recommended to eliminate the ill or damaged fruits. It is well known that the presence of moisture supports microorganisms’ growth, thus, to avoid this the fruits need to be dried completely and stored in somewhere dry and cool place. Fruit deterioration can occur if it is kept in an area with high humidity or direct sunshine. Fruit from the C. chinense plant can be kept for several weeks [16,17]. Fruit should be packaged in dry and clean containers. To promote airflow, use cardboard boxes or perforated plastic bags. Take extra care when moving the fruit to prevent harm. To safeguard the fruit while it is being transported, use padded boxes or containers.
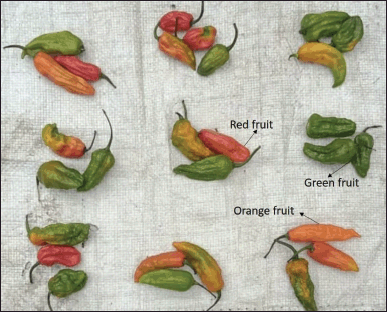 | Figure 2. Capsicum chinense fruit showing different colors as per maturity (green—immature and unripened fruit; orange—partially matured and ripened fruit; and red—completely matured and ripened fruit). [Click here to view] |
 | Figure 3. Dried Capsicum chinense fruit (red and chocolate in color; for retaining therapeutic efficacy avoid storing them in high humidity or direct sunshine). [Click here to view] |
CHEMICAL COMPOSITION OF C. CHINENSE AND ITS RECEPTOR INTERACTION
The spiciness or heat of C. chinense is predominantly derived from a substance called capsaicin, which is primarily found inside the placenta or flesh of the chili fruit. Chemically classified as a phenolic compound, capsaicin exhibits remarkable potency, providing a strong flavor even when highly diluted. Capsicum fruits also contain a group of five distinct and highly pungent compounds called capsaicinoids. These capsaicinoids, namely capsaicin, DHC, nordihydrocapsaicin, homocapsaicin, and homodihydrocapsaicin are created by chili peppers as secondary metabolites. Table 3 enlist the chemical structure of various capsaicinoids present in capsicum. They are believed to serve as repellents against certain mammals and fungi. Chemically, capsaicinoids are classified as branched-chain fatty acid amides of vanillyl amine and typically range from C9 to C12 in length. The placental epidermal tissue of the pepper stores and produces capsaicinoids. Among the capsaicinoids present in capsicum, capsaicin, and DHC make up over 80% of their composition. The content of capsaicinoid, particularly capsaicin, is often used as an indicator of the heat or pungency level of capsicum, ensuring both functional and nutritional quality. Capsicum chinense contains approximately 5.36% capsaicin and DHC [18,19]. Moreover, capsicum fruits are an excellent source of essential nutrients such as ascorbic acid known as vitamin C (an essential antioxidant that supports the immune system and overall health), numerous minerals, or natural colorants such as anthocyanins as well as carotenoids, flavonoids (quercetin, kaempferol, luteolin, and apigenin), triterpenoids (β-amyrin, α-amyrin, ursolic acid, and oleanolic acid), and steroids (β-sitosterol, stigmasterol, and campesterol) [20]. Capsicum chinense is rich in carotenoids, including beta-carotene, which is a precursor of vitamin A. Carotenoids contribute to the vibrant colors of the peppers and have antioxidant properties.
 | Table 2. Specifications for the cultivation of C. chinense. [Click here to view] |
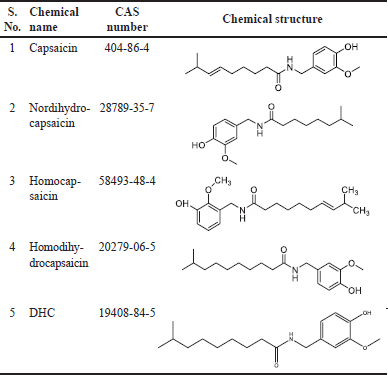 | Table 3. Chemical structure of various capsaicinoids present in capsicum. [Click here to view] |
Upon consumption, capsaicin diffuses through the lingual epithelium of the oral cavity and specifically binds to a receptor called the transient receptor potential vanilloid 1 (TRPV1), also known as the vanilloid receptor [21]. Most nerve cells that are sensitive to pain and heat activate this receptor. Acting as a nonspecific calcium channel, capsaicin activates the TRPV1 receptor, causing it to open after which the initial influx of calcium triggers the release of neurotransmitters, leading to sensations of warmth when capsaicin concentrations are low and burning pain when concentrations are higher [22]. Prolonged activation of the receptor results in the depletion of substance P, a neurotransmitter involved in pain transmission, and subsequent desensitization, which reduces the responsiveness to capsaicin [23].
PHYSICOCHEMICAL PROPERTIES OF CAPSAICIN
The primary aromatic component of chili peppers is capsaicin, a naturally occurring proto-alkaloid. It is characterized as an off-white, crystalline solid with lipophilic properties. The chemical, also known as trans-8-methyl-N-vanillyl-6-nonenamide, has no color or smell and is lipophilic in nature. While it is not water soluble, it can dissolve in ethanol, acetone, and fatty oils. The melting point of capsaicin ranges from 62°C to 65°C. Structurally, capsaicin belongs to the vanilloid family, which includes compounds such as zingerone (found in ginger), eugenol, vanillin (derived from vanilla), bay leaves, and cloves. These vanilloids share common features such as an aromatic ring and a long hydrophobic chain with a polar amide group. It is worth noting that capsaicin can also be found in the fruits of other Capsicum plant species. The extraction of capsaicin in solution was first achieved by Bucholtz in 1816, who successfully isolated the spicy component of peppers [24].
TRADITIONAL MEDICINAL USE
While the primary use of capsicum is in culinary applications for adding flavor and heat to dishes, it has also been traditionally utilized for medicinal and cultural purposes. Here are some traditional uses of capsicum.
Asthma
In North America, C. annuum fruits and leaves part are blended with other herbs to give relief from asthma [3]. Capsaicin, the fiery component has been found to potentially have benefits in reducing asthma symptoms. Studies have shown that extract containing capsaicin can dilate blood vessels and alleviate chronic congestion. However, it is important to note that capsaicin should be consumed in smaller amounts regularly for these potential benefits to be realized [25,26].
Gastro-intestinal abnormalities
Dried fruits of C. chinense are widely used in the northeast region of India for getting relief from stomach pain. Similarly, in Taiwan and the Batanes Islands, the fruits of C. frutescens are used in stomach aches and diarrhea [3]. Consuming capsaicin-containing capsicum pulp has been shown to increase saliva and gastric juice output. In addition, it protects the mucous membrane against physical and chemical damage. Capsaicin is frequently used to achieve these effects in tiny doses [27].
Toothache and muscle pain
In traditional remedies of Orissa (India), treating tooth pain and pain in the muscles includes warm infusions made from capsicum fruits. The heat generated by the capsaicin can help relieve external pain in the muscles [3,28]. In addition, the leaves of C. chinense are traditionally used for treating ailments such as headaches [29]. Capsaicin-sensitive cells are thought to have peripheral ends that, when activated, can trigger the discharge of various inflammatory mediators neuropeptides, including Calcitonin gene-related peptide (CGRP).
Eliminating the pus from boils
In Cuba, Fiji, and Assam (India), to make it easier to eliminate pus from boils, a thin layer of a finely powdered paste made from delicate C. chinense leaves is put on the area [3].
Arthritis
Capsicum chinense, along with other ingredients such as garlic, ginger, betel nut leaf, sesame, and salt, is commonly used to treat arthritis in older individuals. The preparation involves grinding these ingredients into a fine paste, which is then heated. The heated paste is applied as a patch directly over the painful joints and left in place for 60 minutes. After removing the paste, the area is cleaned with lukewarm water and then massaged with oil. This therapy is typically repeated daily, and within 10 days, it often begins to provide relief from arthritis symptoms. It is important to note that individual experiences may vary, and consulting a healthcare professional is advisable before trying any treatment approach [30].
Pain reliever
Topical application of capsicum extract is recommended as a useful adjunct treatment for various conditions, including rheumatoid arthritis, osteoarthritis (OA), neuralgias, diabetic neuropathy, and several painful and inflammatory skin conditions brought on by cancers, surgery, or other trauma in Southern Spain, Eastern Cuba, and Southern Italy [3]. Recent research conducted by Jolayemi and Ojewole [31] has demonstrated that capsaicin exhibits a dose-dependent anti-inflammatory effect comparable to the inflammation-reducing ability of diclofenac. This highlights the medicinal use of capsaicin as a therapeutic option for managing inflammation and associated symptoms.
Gastrointestinal benefit
The gastrointestinal tract contains various types of sensory nerves, including capsaicin-sensitive sensory nerves. These nerves are essential for preserving the gastrointestinal mucosa’s health. The presence of capsaicin in C. chinense can contribute to the strength and integrity of the mucosal lining of the gastrointestinal tract. By supporting the upkeep of the gastrointestinal mucosa, capsaicin in C. chinense may help promote a healthy digestive system [32] and is traditionally used in the Amazon and northeast region of India to get relief [3].
Cardiovascular activity
In southern and central Italy, capsicum extract is used as cardiotonic to improve the contraction of heart muscles as well as to maintain blood pressure [3]. Capsaicin has been found to possess properties that can potentially lower the risk of cardiovascular disorders. It may prevent platelet aggregation and clotting factors VIII and IX from doing their function, both of which contribute to the development of blood clots. By crossing the plasma membrane of platelets, capsaicin can change its fluidity, affecting platelet function. Studies suggest that capsaicinoids, including capsaicin, have possible beneficial effects on heart function, offering potential treatment options for conditions such as coronary heart disease, myocardial infarction, hypertension, and atherosclerosis.
One specific effect of capsaicin is its capability to enhance the resistance of low-density lipoprotein (LDL) to oxidation [33]. LDL oxidation is an important step in the development of atherosclerosis. LDL oxidation can be demonstrated to be both delayed and slowed down by capsaicin. Regular consumption of chili over four weeks was observed to reduce the resistance of blood lipoproteins to decomposition in both men and women. These findings suggest that capsaicin may have a positive impact on cardiovascular health by promoting the stability of LDL particles and potentially reducing the risk of atherosclerosis-related complications.
Cancer prevention
Peppers play a significant role in the production of secondary metabolites with a variety of pharmacological characteristics. For example, traditional medicines from ancient civilizations (such as Chinese, Mayan, and Caribbean) have included ingredients from the genera of Piper, Capsicum, and Pimenta in their formulations for treating cancer [34]. Studies have indicated that consuming C. chinense or ghost pepper may have potential benefits in preventing cancer. Capsaicin, the active compound in C. chinense, has been found to exhibit anticancer properties by inhibiting the growth of cancer cells. It can interfere with the metabolism of cancer cells and induce their destruction. Specifically, capsaicin has shown promising effects for eliminating the spread of breast cancer cells to other cells as well as killing cancer cells in the prostate gland. In research conducted in laboratories on breast cancer, capsaicin was found to prevent the spread of the disease’s cells. For prostate cancer, both using in vitro (inside the laboratory) and in vivo (in living organisms) capsaicin can stop prostate cancer cells from proliferating. According to these results, capsaicin could help prevent as well as treat both breast and prostate cancer.
Furthermore, it has been discovered that DHC, another capsaicinoid present in C. chinense, can induce a process called autophagy in human colon cancer cells. Autophagy is a cellular mechanism that involves the degradation by itself or regeneration of the components of cells, and its activation can potentially inhibit the growth of cancer cells. While these findings are promising, it is significant to remember that further investigation is required to completely comprehend the mechanisms and potential therapeutic applications of capsaicin and capsaicinoids in cancer prevention and treatment [35].
Weight loss
Capsicum has been traditionally used to reduce fat in China, as it has been used in various therapeutic customs in TCM [36]. Indeed, there is evidence to suggest that capsaicin can have a positive impact on metabolism and weight management. Studies have indicated that capsaicin can increase the rate of energy expenditure and enhance fat oxidation. By increasing thermogenesis, capsaicin may contribute to burning stored fat and potentially aid in weight loss efforts [36,37].
Furthermore, capsaicin has been found to have appetite-suppressing effects. It may help reduce feelings of hunger and decrease calorie intake, which can support weight management goals. In addition, some research suggests that capsaicin can reduce cravings for fatty, sweet, and salty foods, which may contribute to overall reduced food consumption. It is important to note that while capsaicin may have potential benefits for metabolism and appetite control, it should not be considered a magic solution for weight loss [38]. It can be viewed as one component of a comprehensive weight management strategy that includes a balanced diet and regular physical activity.
Inhibition of bacterial growth
In Eastern Nicaragua, fever and vaginal infection are cured by applying capsicum extract obtained from the decoction of fruits and leaves [3]. Studies have suggested that capsaicin may possess antimicrobial properties, including activity against Helicobacter pylori (H. pylori), a bacterium known to cause gastric ulcers and associated with certain gastrointestinal disorders [39]. In particular, capsaicin has shown potential in inhibiting both metronidazole-resistant and metronidazole-sensitive strains of H. pylori in laboratory settings.
The findings indicate that capsaicin could be a potential alternative or adjunct treatment for antibiotic-resistant H. pylori infections, providing an option for patients who prefer nonsynthetic antibiotics or who have developed resistance to conventional antibiotics. However, it is significant to emphasize that additional research is required to completely comprehend the efficacy and safety of capsaicin as a therapeutic agent against human H. pylori infections [39,40].
THE TRPV1/VR1 CAPSAICIN RECEPTOR
There is indeed substantial evidence supporting the discovery of a capsaicin receptor and a capsicum receptor, which dates back to the late 1980s. A novel cation channel gated by capsaicin has been uncovered through patch clamp research, triggering substantial endeavors toward isolating, identifying, and cloning this membrane protein. In 1997, the David Julius group successfully replaced it, referred to be the “capsaicin receptor” or “vanilloid receptor 1” (VR1) at that time. The IUPHAR Nomenclature Committee has revised the naming of this receptor due to its structural resemblance to the “transient receptor potential vanilloid 1” (TRPV1). Hence, it is now commonly referred to as TRPV1. Regarding its endogenous chemical ligands, there has been an ongoing debate. Initially, anandamide or lipoxygenase products were proposed as potential ligands for TRPV1. However, the exact nature of the endogenous ligands is still a subject of investigation. Recent research suggests that N-oleoyl dopamine or its structural variants may also act as putative endogenous ligands for the TRPV1 capsaicin receptor. These ligands have a lower counteracting effect on cannabinoid receptors compared to anandamide and other arachidonoyl amides. It is vital to highlight that further study is required to completely understand the specific ligands and mechanisms associated with TRPV1, as well as its broader physiological functions and implications [41].
PHARMACOKINETICS
The skin readily absorbs topical capsaicin. When used topically, capsaicin rapidly reaches its maximum cutaneous concentrations. Compared to preparations using propylene glycol or mineral oil, these amounts are higher in isopropyl formulations. The half-life of capsaicin is around 24 hours. In a 12-subject trial, the topical administration of 3% solutions of capsaicin—consisting of 55% capsaicin, 35% hydro capsaicin, and 10% other analogs—in three various vehicle preparations—mineral oil, propylene glycol containing 20% alcohol, and 70% isopropyl alcohol—was assessed. At 1 minute of application, capsaicinoids have been found in the stratum corneum, and a steady state was quickly attained. In comparison to the participants who received mineral oil or propylene glycol formulations, the participants who got the 70% isopropyl alcohol formulation had roughly three times the greatest concentration. This demonstrated that the capsaicinoids were more relatively soluble in the bigger volume of alcohol. In all three formulations, the half-life of capsaicin was approximately 24 hours. After being administered intravenously to mice, capsaicin is broadly disseminated throughout the brain, spinal cord, and liver. By way of cytochrome p450 enzymes, capsaicinoids are converted to macrocyclic, alkyl dehydrogenated, omega- and omega-1 hydroxylated products. The principal metabolite of capsaicin is DHC. The kidney removes DHC and its metabolites. The kidneys also eliminate capsaicin [42].
INTERACTIONS
Adenosine prevents capsaicin from attaching to its receptor; however, it is unclear what therapeutic significance this interaction has. Capsaicin produces mucosal vasodilation, which can change how other medications are absorbed when used topically or orally at the same time. However, it has little effect on the transfer of water and electrolytes across the jejunal mucosa [43].
MECHANISM OF ACTION
Although it has been demonstrated that capsaicin lowers the quantity of substance P (a neurotransmitter that delivers signals of pain to the brain) linked to inflammation, this is not thought to be the primary mechanism for pain alleviation. The “defunctionalization” of nociceptor fibers caused by capsaicin’s ability to cause a topical hypersensitivity reaction on the skin is thought to be the substance’s mode of action. Many causes, including a transitory decrease of membrane potential, a lack of neurotrophic substance transport that results in an altered phenotypic, and reversible nerve fiber terminal retraction of the outermost layer and superficial layer, contribute to this change in pain mechanisms [44].
PHARMACOLOGICAL ACTION OF C. CHINENSE
Anti-arthritic activity
A nanovesicle external product developed with the semipurified capsaicinoids herbal extract of C. chinense outperformed a commercially available formulation of capsaicin in terms of anti-arthritic efficacy in rat models. During the monitoring period, it also decreased joint pain and swelling. In both animal and human models, the nanovesicle formulation demonstrated improved toleration and acceptability. This strong favorable outcome and less irritating effect indicate that it may be a single of the alternatives for production growth for anti-arthritic therapy [45].
Gastric ulcer protective activity
Ranitidine and the ethanolic extract of C. chinense Jacq were tested for their ability to prevent aspirin-induced stomach ulcers in albino mice. According to the research, ranitidine and C. chinense Jacq both prevent ulcers in a comparable way [46].
Anticancer activity
Research by Amruthraj et al. [38,47], on the ability of capsaicinoids produced from C. chinense to treat cancer showed that HepG2 cell viability is decreased when exposed to acetonitrile extract. In addition, the extract’s capsaicinoids reduced the development of nitric oxide, lipid peroxidation (LPO), and lactate dehydrogenase depending on the dose. Consequently, the research established both the anti-inflammatory potential and the anticancer properties of the capsaicinoids in acetonitrile extract by controlling the release of free radicals [38,47]. Recently in a study, it was found that defensin γ-thionin, a group of small, cysteine-rich, basic, antimicrobial plant proteins from C. chinense is cytotoxic to the K562 leukemia cells with an IC50 = 290 μg/ml (50.26 μM) which clearly signifies that apart from capsaicin other phyto constituents of C. chinense also poses therapeutic potential [48].
Antioxidant and anti-inflammatory activities
Capsaicinoids, which are found in hot peppers, have major medical uses. Capsicum chinense DHC and capsaicin (C) levels in comparison to those of the SB and Jalapeno (C. annuum), two popularly consumed spicy peppers (C. chinense). Capsicum chinense had a C and DHC content of 5.36%, it is 338 times greater than SB and 18 times higher than Jalapeno. In the same research, the researchers also separated pure forms of capsaicin (C) and DHC and calculated the inhibitory amounts of the cyclooxygenase (COX-1 and -2) and LPO enzymes. The analysis of capsaicin and DHC in C. chinense has been reported for the first time, along with a comparison of the hot peppers’ capsaicinoids content with those in Jalapeno and SB varieties and their COX and LPO inhibitory properties [7].
SOME LATEST RESEARCH ON CAPSAICIN-BASED FORMULATIONS
There are various formulations reported in the literature that contain capsaicin. The resulting capsaicin-based formulation showed promising results with respect to the pharmacodynamic activity. One of the formulations is emulgel which is a novel formulation, and it is the mixture of emulsion and gel. The advantages of emulgel like the ease of spreading, greaseless, long shelf life, and so on, over other systems make it a suitable system for the delivery of various drugs. Therefore, in a recent study, Rompicherla et al. [49] developed a transemulgel by combining capsaicin and carbopol 934 with aqueous aloe vera gel. They assessed the prepared formulation with respect to pH, viscosity, in vitro diffusion, spreadability, in vitro and ex vivo studies. In addition, a skin irritancy test was also performed on rat skin. They found that the formulation showed a pH of 6.1 ± 0.1 which ensures nonirritancy of the formulation as it is compatible with the skin pH of skin. The formulation showed good spreadability (20.33 g) and enhanced permeation characteristics when applied topically. The skin irritancy test indicated that the formulation containing capsaicin did not cause any irritation. They concluded that these topical gel formulations have the potential to serve as an alternative to oral formulations for relieving OA pain while reducing systemic exposure [49].
Similarly, in another study, Burki et al. [50] also developed and characterized dexibuprofen-capsaicin emulgel for transdermal drug delivery, with enhanced anti-inflammatory and analgesic properties. They prepared the emulgel formulations and subjected them to various evaluations, including ex vivo tests, FTIR analysis, physical inspection, stability assessment, spreadability measurement, rheological behavior analysis, viscosity determination, and drug content analysis. They also performed pharmacodynamic activity (analgesic and anti-inflammatory) of the prepared formulation on Sprague-Dawley rats. The result of their study showed that dexibuprofen-capsaicin emulgel exhibited satisfactory physical appearance and stability, with pH values ranging between 5.5 and 6.0, conductivity ranging between 73 and 76 s/m, spreadability between 12 and 17 g cm/s, and drug contents of 102.84% ± 0.53 for capsaicin and 94.09% ± 0.41 for dexibuprofen. They also found that capsaicin levels were 86.956% ± 1.46 (with 100 mg menthol), 76.687% ± 1.21 (with 75 mg menthol), and 65.543% ± 1.71 (without menthol) after 6.5 hours after performing in the in vitro release assay. The improved emulgel effectively prevented rat paw edema induced by carrageenan. Moreover, they also observed that the dexibuprofen-capsaicin emulgel exhibited superior analgesic activity compared to a commercially available diclofenac sodium emulgel [50].
In another study by Goci et al. [51], prepared a hydrogel containing capsaicin extract. The objective of their study was to develop a hydrogel formulation utilizing carbopol that incorporates capsaicin and evaluate its in vitro release as well as its antibacterial and antifungal activities. They extracted capsaicin using a 98% ethanol extraction process from C. annuum fruits and identified the capsaicin present in the extract using high-performance liquid chromatography (HPLC). The results of in vitro investigations of the formulation indicated 50% of the capsaicin release within a 52-hour period. Furthermore, they also showed that the formulation based on carbopol significantly enhanced the antibacterial properties of capsaicin against various types of bacteria and fungi that were examined in the study. Their study suggested that the carbopol-based formulation can effectively augment the antimicrobial effects of capsaicin against a broad range of microorganisms [51].
Apart from the gel, nanocarriers such as nanocrystals, nanoliposomes, nanoparticles, nanoemulsion, and so on, are also formulated by various researchers. The use of nano-crystallization as a strategy to increase the solubility, dissolution rate, and consequently bioavailability is a novel approach to address the problem associated with poorly soluble drugs. Since capsaicin is also a poorly soluble drug, Khan et al. [52] formulated capsaicin nanocrystals with the objective of enhancing the water solubility, dissolution, and adhesiveness to the skin’s epidermal layer. They subjected the formulations to various physicochemical, in vitro, ex vivo, and in vivo analyses. The development of capsaicin nanocrystals via high-speed homogenization was successfully demonstrated by X-ray diffraction (XRD). According to in vitro studies, 89.94% ± 1.9% of the drug was released in 24 hours. Similar to this, after 12 hours, drug permeation was 68.32% ± 1.83%, drug retention in the skin was 16.13% ± 1.11%, and drug retention on the skin was 9.12% ± 0.14%. The anti-inflammatory activity of the nanocrystals was greater than that of the commercial medication (Dicloran®). The results of their study demonstrated that enhancing capsaicin’s solubility rate has the potential to pave the way for the development of far more cost-effective dosage forms, which will yield the same or better pharmacological benefits than current products, but at significantly lower doses [52].
Another group of researchers focused on the anticancer activity of capsaicin and to evaluate it prepared a nanoliposomes model which supposed to improve capsaicin’s pharmacokinetic qualities. Al-Samydai et al. [53], prepared nanoliposomes using the thin-film method, and the properties of prepared formulations were investigated, followed by qualitative and quantitative assessments of encapsulation effectiveness and drug loading using HPLC at various lipid/capsaicin ratios. 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide (MTT; cell viability assay) was utilized to calculate IC50. They found that capsaicin-loaded nanoliposomes exhibited optimal shape, particle size, zeta potential, and stability. Capsaicin-loaded nanoliposomes demonstrated significantly improved anticancer efficacy against the cancer cell lines examined (p < 0.001), with higher selectivity against cancer cells when compared to capsaicin alone. When compared to capsaicin, the encapsulated capsaicin nanoliposomes improved pharmacokinetic characteristics, increasing antitumor efficacy and selectivity. Their proposed model appears to have the potential for creating capsaicin formulations for cancer prevention and treatment [53].
Anantaworasakul et al. [54,55] also developed a lipid-based nanoparticle that retained a significant amount of capsaicin (0.25%) from an extract of capsicum oleoresin. They prepared two types of lipid-based particles (solid lipid nanoparticles and nanostructured lipid carriers). This study concentrated on the selective capture of capsaicin from oleoresin in amorphous chili extract-loaded nanostructured lipid carriers with entrapment efficiencies of 85.27% ± 0.12% and loading capacities of 8.53% ± 0.01%. Based on the results of an in vitro skin irritant test, the reduced skin irritation of the concentrated capsaicin NLCs can be due to their zero-order kinetics, which extended capsaicin release, and their substantially faster transdermal penetration [54]. In another study, they reported that a nanomaterial lipid-based carrier for noninvasive capsaicin (extracted from C. chinense) delivery was produced in a size range of 106–156 nm by high-shear homogenization at a high intensity of 10,000 rpm. Compared to the free chili formulation, the NLC_C and Gel NLC_C formulations displayed higher levels of physical and chemical stabilities. Research on drug release and swine biopsies showed that the NLCs had a prolonged release period and significantly penetrated the outer layer of the skin, dispersing in the dermis more effectively than the free compounds. It was demonstrated that the chili extract-loaded NLCs could be used to distribute capsaicin transdermally while reducing skin irritation, which is the main reason why patients do not comply [55].
Capsaicin-loaded nanoemulsions were prepared by Nigam et al. [56] in a reported literature. Before the preparation of formulation, they extracted capsaicin utilizing liquid–liquid extraction techniques and solid–liquid extraction procedures. They also characterized the extracts and reported that dried chilies plant extracts have more alkaloid and capsaicinoid content and higher yields than their fresh counterparts. Oleic acid, labrasol, and tween 20 were used to prepare o/w nanoemulsion. The zeta potential, PDI, and particle size of the produced capsaicin nanoemulsion were measured. They also performed in vitro, cytotoxicity, and cell viability studies on the prepared emulsion. They observed above 90% cell viability with the prepared capsaicin nanoemulsion. They concluded that the dissolution of capsaicin was enhanced after encapsulation in nanoemulsion and this system of capsaicin-loaded nanoemulsion can serve as a viable carrier for the delivery of capsaicin to enhance its analgesic effects [56].
Capsaicin is used in plant biotechnology to control phytopathogens. However, its low solubility limits its use. Therefore, Sánchez-Arreguin et al. [57] prepared nanoparticles to address this issue. The aim of their research was to investigate the effect of high doses of capsaicin on the formation of nanoparticles as well as their inhibitory effect on the growth of Rhodotorula mucilaginosa yeast. They prepared bovine serum albumin (BSA)-capsaicin nanoparticles with varying concentrations of capsaicin. Rhodotorula mucilaginosa cell cultures were treated with nanoparticles and their effects on cell viability were measured. They discovered that higher capsaicin concentrations changed a number of physicochemical properties. These changes were likely brought on by modifications made to the hydrophobicity sites of the albumin during nanostructuration. The ratios of capsaicin to BSA were 0, 16.2, 32.5, 48.7, and 65.0 g/mg of BSA. Due to the nano-structural characteristics of the capsaicin in the BSA molecules, the nanoparticles responded differently throughout the absorption process. The results showed that R. mucilaginosa cells are stimulated to proliferate and multiply at a concentration of only 16.2 g/mg capsaicin, suggesting that the impact of the encapsulated capsaicin is concentration dependent. Yeast cultures only showed a substantial death and growth inhibition rate at the maximum concentration of capsaicin (65.0 g/mg). A hormesis effect was visible in R. mucilaginosa cells in response to the dose of applied nanoparticles. They concluded that BSA-capsaicin nanoparticles are an advance in nanobiotechnology that could be a beneficial tool for controlling pathogen fungus [57].
Capsaicin has a wide range of therapeutic applications; however, it has a poor pharmacokinetic profile, is rapidly eliminated by the body, and has toxic metabolites with a short half-life. Nanoencapsulation, a method that optimizes the pharmacokinetics of drugs and chemicals and allows them to target specific organs, is an essential strategy for dealing with this problem. Therefore, in another study, De Freitas et al. [58], also formulated capsaicin-loaded albumin nanoparticles using the desolvation-coacervation process and optimized using factorial design. They reported that the prepared nanoparticles had an average diameter of approximately 200 nm with encapsulation efficiency of 98.3% ± 7.4% and exhibited a quasi-spherical shape. The in vitro release assay revealed a biphasic profile, wherein there was a rapid release of capsaicin during the initial 12 hours, followed by a decreased release rate thereafter. This suggests a controlled release behavior of capsaicin from the nanoparticles over time.
In addition, in a concentration- and time-dependent in vitro assay, the nano-encapsulated capsaicin demonstrated potent antioxidant activity. Their study indicated that the capsaicin-loaded albumin nanoparticles exhibited strong antioxidant properties, which could have potential implications for various applications in antioxidant therapy [58].
According to the ethnobotanical study, C. chinense may have anti-arthritic properties, but its usability is limited by its topical side effects. The semipurified capsaicinoids extract of C. chinense was developed as a topical formulation using an ethosomal nanovesicle method in a study by Sarwa et al. [45], which improved the effectiveness. They characterized the developed formulation for various physicochemical characteristics, permeation, and penetration tests, and optimized it utilizing surface response methods. On the basis of Freund’s full adjuvant-induced chronic arthritis model, the experimental formulations were evaluated. Their research revealed that ethosomal nanovesicles made with semipurified capsaicinoids extract had better anti-arthritic activity in rats than Thermagel (a commercially available capsaicin formulation) in terms of reducing joint swelling and pain over the course of the observation period. Better toleration and acceptability were demonstrated by nanovesicle formulation in both animal and human models. The semipurified capsaicinoids extract of C. chinense-loaded ethosomal nanovesicle carrier showed substantial favorable results with a reduced irritating effect, indicating that it could be one of the options for formulation development in anti-arthritic therapy [45].
Similarly, the same group of researchers, Sarwa et al. [59], developed a novel nanolipid approach based on elastic phospholipid vesicles to encapsulate a semi-purified extract of C. chinense for topical drug delivery application. The notion was that by enclosing the irritant extract in elastic nanolipid vesicles, the skin would be more receptive to the formulation and the vesicles would be able to penetrate deeper and more quickly. Different physicochemical parameters were used to characterize the prepared formulations. As in the previous study, Freund’s adjuvant-induced arthritis model was used to evaluate the effectiveness of the formulation. They also conducted a Phase I clinical trial to test the novel formulations on volunteers from the human population and confirmed them to be acceptable. According to their study, this method has a great deal of potential for topical delivery of the C. chinense bioactive and may open the door to its use in clinical settings [59].
CONCLUSION
The review highlights the significant therapeutic potential of C. chinense in medicine, specifically focusing on its applications in managing various diseases. The phytochemical composition of C. chinense, particularly the active compound capsaicin, exhibits a range of pharmacological properties such as analgesic, anti-inflammatory, antioxidant, antimicrobial, and anticancer activities. These properties contribute to its potential efficacy in the management of pain, arthritis, gastrointestinal disorders, cardiovascular diseases, obesity, infection, diabetes, and cancer.
The available evidence from experimental research findings supports the use of C. chinense and capsaicin in the treatment and management of these diseases. However, it is essential to conduct further research to determine the optimal dosage, formulation, and long-term safety of C. chinense-based therapies. By harnessing the unique properties of C. chinense and capsaicin, new opportunities arise for the development of innovative formulations and interventions in the field of pharmacy.
Despite the promising therapeutic effects, potential drug interactions and adverse effects should be taken into consideration. Therefore, proper caution and monitoring are necessary when incorporating C. chinense into pharmaceutical interventions.
In summary, C. chinense holds immense potential as a valuable resource in managing various diseases, offering a promising avenue for future research and the development of pharmaceutical interventions. Continued investigation into the therapeutic applications of C. chinense will further contribute to its integration into evidence-based healthcare practices, ultimately benefiting patients and improving their quality of life.
LIST OF ABBREVIATIONS
C. chinense: Capsicum chinense; DHC: dihydrocapsaicin; H. pylori: Helicobacter pylori; LDL: low-density lipoprotein; NLC- Nano Lipid Carrier; LPO: lipid peroxidation; OA: osteoarthritis; PDI- Polydispersity index; SB: Scotch Bonnet; SHU: Scoville heat unit; TCM: traditional Chinese medicine; TRPV1: transient receptor potential vanilloid 1; VR1: vanilloid receptor 1.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVAL
This study does not involve experiments on animals or human subjects.
INFORMED CONSENT
All authors have their consent for publication.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Chile History [Internet]. [cited 2023 Nov 2]. Available from: https://www.secospice.com/chile_history.php
2. Sanatombi K, Sharma GJ. Capsaicin content and pungency of different Capsicum spp. cultivars. Not Bot Horti Agrobot Cluj Napoca. 2008;36(2):89–90.
3. Meghvansi MK, Siddiqui S, Khan MH, Gupta VK, Vairale MG, Gogoi HK, et al. Naga chilli: a potential source of capsaicinoids with broad-spectrum ethnopharmacological applications. J Ethnopharmacol [Internet]. 2010 Oct;132(1):1–14. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0378874110005817
4. Rudrapal M, Kumar Sarwa K. Capsicum: chemistry and medicinal properties of indigenous indian varieties. In: Capsicum [Internet]. London, UK: IntechOpen; 2020. Available from: https://www.intechopen.com/books/capsicum/-em-capsicum-em-chemistry-and-medicinal-properties-of-indigenous-indian-varieties
5. Sarwa K, Mazumder B, Rudrapal M, Debnath M, Kumar A, Verma V, et al. Capsaicinoids content of some indigenous Capsicum varieties of Assam, India. J Nat Sci Res. 2013;3:112–6.
6. Talukdar J, Saikia AK, Borah P. Survey and detection of the diseases of Bhut Jolokia (Capsicum chinense Jacq.) in Assam. J Crop Weed. 2015;11:186–92.
7. Liu Y, Nair MG. Capsaicinoids in the hottest pepper Bhut Jolokia and its antioxidant and antiinflammatory activities. Nat Prod Commun. 2010 Jan;5(1):91–4. CrossRef
8. Tiwari A, Kaushik MP, Pandey KS, Dangi RS. Adaptability and production of hottest chilli variety under Gwalior agro-climatic conditions. Curr Sci. 2005;88(10):1545–6.
9. Kumar Sarwa K, Kiran J, Sahu J, Rudrapal M, Debnath M. A short review on Capsicum chinense Jacq. J Herb Med Toxicol. 2012;6(2):7–10.
10. Vázquez-Espinosa M, Olguín-Rojas J, Fayos O, González-De-Peredo AV, Espada-Bellido E, Ferreiro-González M, et al. Influence of fruit ripening on the total and individual capsaicinoids and capsiate content in Naga Jolokia Peppers (Capsicum chinense Jacq.). Agron. 2020 Feb 8 [cited 2023 Dec 3];10(2):252. Available from: https://www.mdpi.com/2073-4395/10/2/252/htm
11. Castro Alves VC, Pinto NOF, Penha MFA, Gomes BL, Reifschneider FJB, Garruti DS. Aroma-active compounds of Capsicum chinense Var. Biquinho. In: Flavour science [Internet]. Amsterdam, The Netherlands: Elsevier; 2014. pp 567–71. Available from: https://linkinghub.elsevier.com/retrieve/pii/B9780123985491001045
12. Vuerich M, Petrussa E, Filippi A, Cluzet S, Fonayet JV, Sepulcri A, et al. Antifungal activity of chili pepper extract with potential for the control of some major pathogens in grapevine. Pest Manag Sci [Internet]. 2023 Jul 16;79(7):2503–16. Available from: https://onlinelibrary.wiley.com/doi/10.1002/ps.7435
13. Days to harvest: for each subspecies [Internet]. [cited 2023 Nov 2]. Available from: https://pepperseeds.ca/index.php?information_id=14&route=information%2Finformation
14. Arora R, Gill NS, Chauhan G, Rana AC. An overview about versatile molecule capsaicin. Int J Pharm Sci drug Res. 2011;3(4):280–6.
15. Tiamiyu QO, Adebayo SE, Ibrahim N. Recent advances on postharvest technologies of bell pepper: A review. Heliyon [Internet]. 2023 Apr;9(4):e15302. Available from: https://linkinghub.elsevier.com/retrieve/pii/S2405844023025094
16. Maskey B, Bhattarai R, Bhattarai G, Shrestha NK. Post-harvest quality of fresh Akabare Chili (Capsicum chinese) as affected by hydrocooling, package modification and storage temperature. Int J Food Prop [Internet]. 2021 Jan 1;24(1):163–73. Available from: https://www.tandfonline.com/doi/full/10.1080/10942912.2020.1865399
17. Adewoyin O, Famaye A, Ipinmoroti R, Ibidapo A, Fayose F. Postharvest handling methods, processes and practices for pepper. In: Capsicum—current trends and perspectives [Internet]. London, UK: IntechOpen; 2023. Available from: https://www.intechopen.com/chapters/83289
18. Salgado-Roman M, Botello-Álvarez E, Rico-Martínez R, Jiménez-Islas H, Cárdenas-Manríquez M, Navarrete-Bolaños JL. Enzymatic treatment to improve extraction of capsaicinoids and carotenoids from chili (Capsicum annuum) fruits. J Agric Food Chem. 2008;56(21):10012–8. CrossRef
19. Thoennissen NH, O’kelly J, Lu D, Iwanski GB, La DT, Abbassi S, et al. Capsaicin causes cell-cycle arrest and apoptosis in ER-positive and-negative breast cancer cells by modulating the EGFR/HER-2 pathway. Oncogene. 2010;29(2):285–96. CrossRef
20. Gayathri N, Gopalakrishnan G, Sekar T. Phytochemical screening and antimicrobial activity of Capsicum chinense Jacq. [Internet]. Int J Adv Pharm. 2016 [cited 2023 Dec 3];5:12–20. Available from: https://core.ac.uk/reader/335078010
21. Yang ZH, Wang XH, Wang HP, Hu LQ, Zheng XM, Li SW. Capsaicin mediates cell death in bladder cancer T24 cells through reactive oxygen species production and mitochondrial depolarization. Urology. 2010;75(3):735–41. CrossRef
22. Báez S, Tsuchiya Y, Calvo A, Pruyas M, Nakamura K, Kiyohara C, et al. Genetic variants involved in gallstone formation and capsaicin metabolism, and the risk of gallbladder cancer in Chilean women. World J Gastroenterol. 2010;16(3):372. CrossRef
23. Zhou Z, Peng J, Wang CJ, Li D, Li TT, Hu CP, et al. Accelerated senescence of endothelial progenitor cells in hypertension is related to the reduction of calcitonin gene-related peptide. J Hypertens. 2010;28(5):931–9. CrossRef
24. Ilie M, Caruntu C, Tampa M, Georgescu SR, Matei C, Negrei C, et al. Capsaicin: physicochemical properties, cutaneous reactions and potential applications in painful and inflammatory conditions (Review). Exp Ther Med. 2019;916–25. CrossRef
25. McCarty MF, DiNicolantonio JJ, O’Keefe JH. Capsaicin may have important potential for promoting vascular and metabolic health: Table 1. Open Heart [Internet]. 2015 Jun;2(1):e000262. Available from: https://openheart.bmj.com/lookup/doi/10.1136/openhrt-2015-000262
26. Munjuluri S, Wilkerson DA, Sooch G, Chen X, White FA, Obukhov AG. Capsaicin and TRPV1 Channels in the cardiovascular system: the role of inflammation. Cells [Internet]. 2021 Dec 22;11(1):18. Available from: https://www.mdpi.com/2073-4409/11/1/18
27. Kono Y, Kubota A, Taira M, Katsuyama N, Sugimoto K. Effects of oral stimulation with capsaicin on salivary secretion and neural activities in the autonomic system and the brain. J Dent Sci [Internet]. 2018 Jun;13(2):116–23. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1991790217301320
28. Epstein JB, Marcoe JH. Topical application of capsaicin for treatment of oral neuropathic pain and trigeminal neuralgia. Oral Surg Oral Med Oral Pathol [Internet]. 1994 Feb;77(2):135–40. Available from: https://linkinghub.elsevier.com/retrieve/pii/0030422094902755
29. Buntinx L, Vermeersch S, de Hoon J. Development of anti-migraine therapeutics using the capsaicin-induced dermal blood flow model. Br J Clin Pharmacol [Internet]. 2015 Nov 6;80(5):992–1000. Available from: https://bpspubs.onlinelibrary.wiley.com/doi/10.1111/bcp.12704
30. Roy A. Bhut jolokia (Capsicum chinense Jaqc): a review. Int J Pharm Sci Res. 2016;7(3):882–9.
31. Jolayemi AT, Ojewole JAO. Comparative anti-inflammatory properties of capsaicin and ethyl-aAcetate extract of Capsicum frutescens Linn [Solanaceae] in rats. Afr Health Sci. 2013;13(2):357–61. CrossRef
32. Holzer P, Schluet W, Lippe IT, Sametz W. Involvement of capsaicin-sensitive sensory neurons in gastrointestinal function. Acta Physiol Hung. 1987 [cited 2023 Nov 2];69(3-4):403–11. Available from: https://pubmed.ncbi.nlm.nih.gov/3310522/
33. Baruah S, Zaman MK, Rajbongshi P, Das S. A review on recent researches on Bhut jolokia and pharmacological activity of capsaicin. Int J Pharm Sci Rev Res. 2014;24(2):89–94.
34. Cunha MR, Tavares MT, Fernandes TB, Parise-Filho R. Peppers: a “hot” natural source for antitumor compounds. Molecules [Internet]. 2021 Mar 10;26(6):1521. CrossRef
35. Lin YT, Wang HC, Hsu YC, Cho CL, Yang MY, Chien CY. Capsaicin induces autophagy and apoptosis in human nasopharyngeal carcinoma cells by downregulating the PI3K/AKT/mTOR pathway. Int J Mol Sci. 2017 Jun 23;18(7):1343. CrossRef
36. Zheng J, Zheng S, Feng Q, Zhang Q, Xiao X. Dietary capsaicin and its anti-obesity potency: from mechanism to clinical implications. Biosci Rep [Internet]. 2017 Jun 30;37(3):BSR20170286. CrossRef
37. Zhang W, Zhang Q, Wang L, Wang P, Qing Y, Sun C. The effects of capsaicin intake on weight loss among overweight and obese subjects: a systematic review and meta-analysis of randomised controlled trials. Br J Nutr [Internet]. 2023 Nov 14 [cited 2023 Nov 2];130(9):1645–56.
38. Amruthraj NJ, Raj JPP, Lebel LA. Polar aprotic extraction of capsaicinoids from Capsicum chinense Bhut jolokia fruit for antimicrobial activity. Int J Biol Pharm Res. 2013;4(12):959–64.
39. Tayseer I, Aburjai T, Abu-Qatouseh L, AL-Karabieh N, Ahmed W, Al-Samydai A. In vitro anti-Helicobacter pylori activity of capsaicin. J Pure Appl Microbiol [Internet]. 2020 Mar 31;14(1):279–86. CrossRef
40. Das S, Deka S, Gohain K. A preclinical study on gastric ulcer protective activity of world’s hottest chilli Capsicum frutenscenes. J Clin Diagn Res. 2008;2:1024–7.
41. Szolcsányi J. Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides. 2004;38(6):377–84. CrossRef
42. Hayman M, Kam PCA. Capsaicin: A review of its pharmacology and clinical applications. Curr Anaesth Crit Care. 2008;19(5–6):338–43. CrossRef
43. Hammer J, Hammer HF, Eherer AJ, Petritsch W, Holzer P, Krejs GJ. Intraluminal capsaicin does not affect fluid and electrolyte absorption in the human jejunum but does cause pain. Gut. 1998;43(2):252–5. CrossRef
44. O’Neill J, Brock C, Olesen AE, Andresen T, Nilsson M, Dickenson AH. Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacol Rev. 2012 Oct;64(4):939–71. CrossRef
45. Sarwa KK, Das PJ, Mazumder B. A nanovesicle topical formulation of Bhut Jolokia (hottest capsicum): a potential anti-arthritic medicine. Expert Opin Drug Deliv. 2014;11(5):661–76. CrossRef
46. Mathur R, Dangi RS, Dass SC, Malhotra RC. The hottest chilli variety in India. Curr Sci. 2000;79(3):287–8.
47. Amruthraj NJ, Preetam JPR, Saravanan S, Lebel LA. In vitro studies on anticancer activity of capsaicinoids from Capsicum chinense against human hepatocellular carcinoma cells. Int J Pharm Pharm Sci. 2014;6(4):254–8.
48. Flores-Alvarez LJ, Jiménez-Alcántar P, Ochoa-Zarzosa A, López-Meza JE. The antimicrobial peptide γ-thionin from Habanero Chile (Capsicum chinense) induces caspase-independent apoptosis on human K562 chronic myeloid leukemia cells and regulates epigenetic marks. Molecules [Internet]. 2023 Apr 23;28(9):3661. CrossRef
49. Rompicherla NC, Joshi P, Shetty A, Sudhakar K, Amin HIM, Mishra Y, et al. Design, formulation, and evaluation of aloe vera gel-based capsaicin transemulgel for osteoarthritis. Pharmaceutics. 2022;14(9):1812. CrossRef
50. Burki IK, Khan MK, Khan BA, Uzair B, Braga VA, Jamil QA. Formulation development, characterization, and evaluation of a novel dexibuprofen-capsaicin skin emulgel with improved in vivo anti-inflammatory and analgesic effects. AAPS PharmSciTech. 2020;21:1–14. CrossRef
51. Goci E, Haloci E, Di Stefano A, Chiavaroli A, Angelini P, Miha A, et al. Evaluation of in vitro capsaicin release and antimicrobial properties of topical pharmaceutical formulation. Biomolecules. 2021;11(3):432. CrossRef
52. Khan BA, Rashid F, Khan MK, Alqahtani SS, Sultan MH, Almoshari Y. Fabrication of capsaicin loaded nanocrystals: physical characterizations and in vivo evaluation. Pharmaceutics. 2021;13(6):841. CrossRef
53. Al-Samydai A, Alshaer W, Al-Dujaili EAS, Azzam H, Aburjai T. Preparation, characterization, and anticancer effects of capsaicin-loaded nanoliposomes. Nutrients. 2021;13(11):3995. CrossRef
54. Anantaworasakul P, Chaiyana W, Michniak-Kohn BB, Rungseevijitprapa W, Ampasavate C. Enhanced transdermal delivery of concentrated capsaicin from chili extract-loaded lipid nanoparticles with reduced skin irritation. Pharmaceutics. 2020;12(5):463. CrossRef
55. Anantaworasakul P, Anuchapreeda S, Yotsawimonwat S, Naksuriya O, Lekawanvijit S, Tovanabutra N, et al. Nanomaterial lipid-based carrier for non-invasive capsaicin delivery; manufacturing scale-up and human irritation assessment. Molecules [Internet]. 2020 Nov 27;25(23):5575. CrossRef
56. Nigam K, Gabrani R, Dang S. Nano-emulsion from capsaicin: formulation and characterization. Mater Today Proc. 2019;18:869–78. CrossRef
57. Sánchez-Arreguin A, Carriles R, Ochoa-Alejo N, López MG, Sánchez-Segura L. Generation of BSA-capsaicin nanoparticles and their hormesis effect on the Rhodotorula mucilaginosa yeast. Molecules. 2019;24(15):2800. CrossRef
58. De Freitas GBL, De Almeida DJ, Carraro E, Kerppers II, Martins GAG, Mainardes RM, et al. Formulation, characterization, and in vitro/in vivo studies of capsaicin-loaded albumin nanoparticles. Mater Sci Eng C. 2018;93:70–9. CrossRef
59. Sarwa K, Mazumder B, Suresh P, Kaur C. Topical analgesic nanolipid vesicles formulation of capsaicinoids extract of Bhut Jolokia (Capsicum chinense Jacq): pharmacodynamic evaluation in rat models and acceptability studies in human volunteers. Curr Drug Deliv [Internet]. 2016 Jun 18 [cited 2023 Jul 17];13(8):1325–38. Available from: https://pubmed.ncbi.nlm.nih.gov/27306849/