INTRODUCTION
Transient receptor potential vanilloid 1 (TRPV1) is a non-selective ligand-gated cation channel, first identified at the end of the 1990s [1]. It belongs to the TRPV subfamily of the transient receptor potential channel superfamily [2]. It is a homotetrameric protein, widely expressed in human tissues [2], mainly in the neuronal tissue including afferent nerve fibers of the dorsal root and trigeminal ganglion, and vagal nodose ganglia where it has a role in sensing heat and pain [3]. In addition, it is also expressed in non-neuronal cells including the vascular smooth muscle cells, where its activation mediates vasoconstriction of the arteries [4], and in the gingival epithelial cells, where its stimulation promotes cell proliferation [5].
TRPV1 is a key nociceptive channel functionally involved in signal transduction in multiple processes [6]. It can be activated by variable chemical and physical stimuli including divalent cations such as Ca+2, heat, capsaicin, and inflammatory mediators including bradykinin [3,7,8]. Moreover, diacylglycerol [9] and signaling lipids such as phosphoinositide 4,5-bisphosphate [10], and endogenous cannabinoids could activate TRPV1 [11]. Phosphorylation of TRPV1 enhances sensitivity to chemical and thermal stimuli, while dephosphorylation results in its desensitization which is a calcium-dependent mechanism [12]. TRPV1 activation is not only involved in chronic pain and peripheral neuropathy, but also in other physiological conditions including inflammation, apoptosis, cough, and obesity [12]. Therefore, targeting the TRPV1 channel by inhibition or modulation has recently been highlighted as a potential therapeutic strategy in several preclinical models and clinical studies of chronic pain, inflammation, cancer, metabolic disorders, neuropathic, postoperative, and musculoskeletal pain [13–15].
Bibliometric analysis is a comprehensive and effective approach to reviewing the available and prominent publications in a particular field of research [16]. It is based on utilizing mathematical and statistical methods to quantitatively analyze current research and to predict the research trend in a specific research area [16,17]. A few bibliometric studies have been conducted to analyze the progress in TRPV1 research particularly in the fields related to its role in pain and inflammation [18–20]. To the best of our knowledge, no systematic or bibliometric analysis has been performed specifically on TRPV1 inhibition or modulation. This study aims to explore the research trend and hotspots in the field of inhibiting TRPV1 and/or developing TRPV1 antagonists through conducting a bibliometric analysis. The findings would shed light on the significance of TRPV1 inhibition in different physiological and pathological conditions and the new directions for future research. In addition, they would provide a comprehensive evaluation of the current research status and identify the pioneer scholars and their collaborations in this field.
MATERIAL AND METHODS
Search strategy and refine the retrieved publications
A comprehensive search for related publications was conducted using the Scopus database. The data search was carried out on the 8th of August 2023, using author keywords, abstracts, and titles search strategy during the period from 2003 until 2022. The search query employed the following string:(TITLE-ABS-KEY (TRPV1 OR “TRPV1” OR trpv-1) OR TITLE-ABS-KEY (transient AND receptor AND potential AND vanilloid 1) OR TITLE-ABS-KEY (vanilloid AND receptor* 1) AND TITLE-ABS-KEY (inhib* OR modul* OR block* OR antagonist*)) AND PUBYEAR > 2002 AND PUBYEAR < 2023 AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”)) AND (LIMIT-TO (SRCTYPE, “j”)) AND (LIMIT-TO (LANGUAGE, “English”)) AND (LIMIT-TO (PUBSTAGE, “final”)). Only research articles and reviews published between 2003 and 2022 in peer-reviewed journals were included. Documents in press, letters, notes, editorials, and other publication types were excluded. In addition, books, book chapters, and conference proceedings were also excluded. The publication language was set to English only.
Data extraction
The total retrieved publications were extracted from the Scopus database on the same search day to avoid any potential bias due to the daily updating of the database. The obtained data were extracted in comma separated value format for processing and conducting bibliometric analysis.
Bibliometric analyses and visualization
Microsoft Office Excel 365 was used to account for the annual publications, total citations, and publication type. The most current version of the Visualization of Similarities (VOSviewer 1.6.19) (www.vosviewer.com) [16] was used to analyze and visualize research trends in the scientific literature. The extracted publications were imported to VOSviewer to perform the network visualization map and cluster analysis. Visual representations of the relationships between countries, authors, cited authors, journals, and keywords were conducted. In addition, cluster density maps were created to visualize the distribution of keywords. Biblioshiny software, part of the Bibliometrix package, was employed to further analyze authors’ keywords [16,21,22]. This approach would provide information to identify commonalities and focus areas by investigating patterns in the authors’ utilization of keywords.
In analyzing authors’ keywords, all the extracted words were manually checked, and the synonymous or related keywords were merged into one related word (i.e., cannabinoid and cannabinoids were considered one term “cannabinoids”) using a thesaurus file. Such manipulation was achievable by VOSviewer and Biblioshiny and would enhance the accuracy and validation of the analysis.
RESULTS
General data and annual publication and citation output
A total of 7,470 relevant publications were retrieved in the field of TRPV1 inhibition from 2003 to 2022 based on the used search terms. Overall, the number of publications was increasing over the determined period, indicating a steady upward trend. For example, the number of published documents in 2022 increased by four times compared to that in 2003. However, there was a slight decrease in the number of published documents between 2021 and 2022 (Fig. 1A). The publications’ citations increased from 11,338 to 23,206 citations over the period from 2003 to 2011, respectively (Fig. 1A). On the other hand, from 2011 to 2022, there was an obvious decrease in the total citations of the published documents to achieving only 2,185 total citations in 2022 (Fig. 1A). The combined pattern of annual publications and total citations between 2003 and 2022 is demonstrated in Figure 1A. Research articles account for approximately 80% of the total relevant publications (5,991 out of a total of 7,470), (Fig. 1B), indicating that attention has been given to conducting original studies in the research of TRPV1 inhibition. Moreover, analysis of research subject areas indicated that biochemistry, genetics, and molecular biology were the most research areas covered in the field of TRPV1 inhibition, with 3,359 publications, accounting for 44.97% of the total publications (Table 1). The second top research area was medicine (38.85%) followed by pharmacology, toxicology, and pharmaceutics (29.77%). It is worth mentioning that more than one article was classified in more than one research field, resulting in a total percentage greater than 100%.
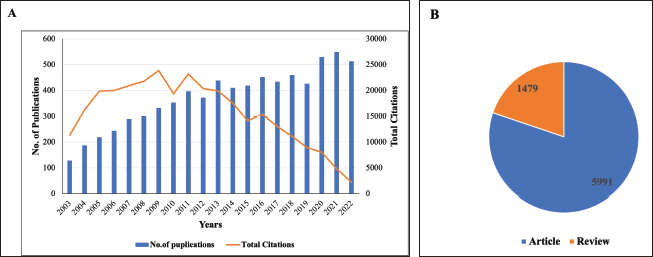 | Figure 1. Global pattern of the TRPV1 inhibition research from 2003 to 2022. (A) Merged bar chart of chronological distribution of publications and total citations of the publications per year. (B) A pie chart presents the number of extracted publication types. [Click here to view] |
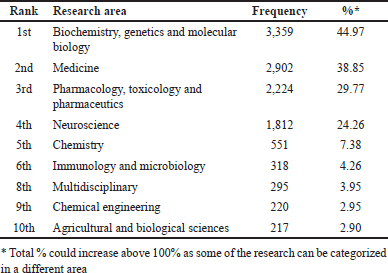 | Table 1. The top research areas of publications on TRPV1 inhibition. [Click here to view] |
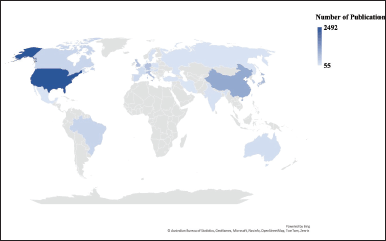 | Figure 2. The geographical distribution of the 27 countries that participated in the publication related to TRPV1 inhibition. [Click here to view] |
Distribution of publications across countries and international collaboration
Geographically, the retrieved publications were distributed over 140 countries. Setting a threshold of a minimum of 50 publications per country, 27 countries met this threshold. A heat map presents the distribution of the identified 27 countries (Fig. 2). In addition, Table 2 lists the top ten countries ranked by the number of publications. The United States of America (USA) had the highest publication output with 2,495 publications (33.36% of 7,470 publications). Therefore, it emerged as the leading contributor to the field of TRPV1 inhibition research. As shown in Table 2, the USA was followed by China with 1,048 publications (14.03%), then Japan (757, 10.13%). Publications originating from the USA, the United Kingdom (UK), and Italy had the most significant scientific impact and achieved the highest total citations (141,511, 38,594, and 34,446, respectively). Calculating the average citations per publication indicated that publications from the UK (665 documents) achieved the highest scientific impact (58.04) followed by Italy (57.60), then by the USA (56.79), (Table 2). Despite this, the USA had the strongest total link strength (TLS = 1,211), indicating its significant contribution and collaboration with other countries in this field, (Table 2).
Analysis of authors and co-cited authors
A total of 23,875 authors contributed to the publication of the retrieved documents. Of these authors, 65 (productive scholars) contributed at least 25 publications in the field of TRPV1 inhibition. Table 3 presents the ten most active authors and co-cited authors in this field. As presented in the table below, Di Marzo, V. (Vincenzo Di Marzo), a professor of biochemistry at Laval University, Canada, had the highest number of publications and citations (130 publications and 12,170 citations). His research mainly focuses on endocannabinoids and targeting transient receptor potential (TRP) channels. As for the co-cited authors, Julius, D. (David Julius), a professor of Physiology at the University of California, USA, was ranked first with 8,510 co-citations, followed by Di Marzo, V. (6,161 co-citations). Julius, D. is the Nobel Prize laureate in Physiology in 2021 for his research on the characterization of the TRPV1 and molecular mechanism of pain and heat sensations.
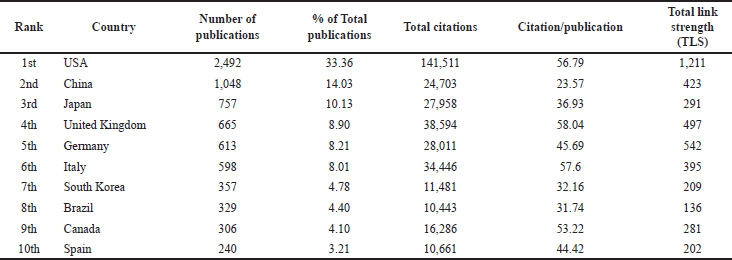 | Table 2. The top 10 active countries in publishing documents on TRPV1 inhibition. [Click here to view] |
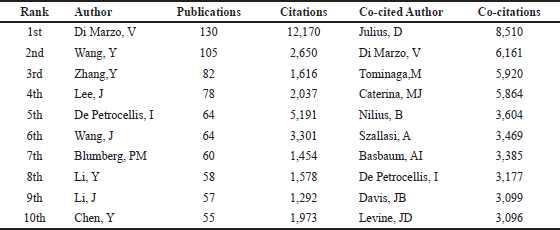 | Table 3. The Top 10 most productive authors and co-cited authors in research of TRPV1 inhibition. [Click here to view] |
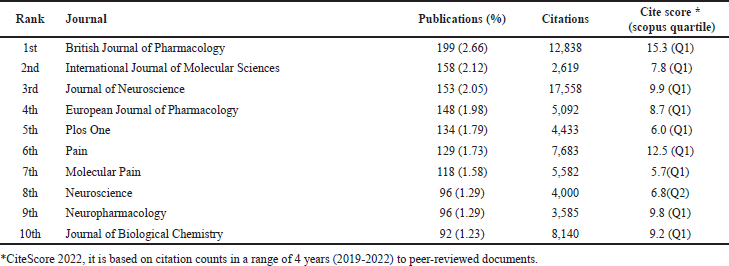 | Table 4. The top 10 active publishing journals in the field of TRPV1 inhibition. [Click here to view] |
Analysis of contributing journals
The 7,470 retrieved documents were published in a total of 1,428 Scopus-indexed, peer-reviewed journals. Among these, only 42 journals have published 30 or more documents. Table 4 shows the top 10 prolific and active journals. British Journal of Pharmacology was the most prolific and productive in this field with 199 published documents (2.66% out of the total retrieved documents) and 12,838 citations, followed by the International Journal of Molecular Sciences with 158 (2.12%) documents and 2,619 citations, and then by Journal of Neuroscience with 153(2.05%) documents and 17,558 citations. Nine of the top 10 journals are classified as Q1 by the Scopus database
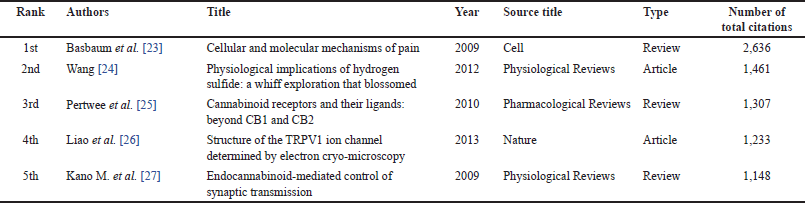 | Table 5. The top 5 cited documents with total citations. [Click here to view] |
Analysis of co-cited documents
The retrieved documents have a total citation count of 350,425 with an average of 469 citations per document. Moreover, 1,846 documents have been cited 10 times or more. The five most-cited documents and their total citations are presented in Table 5. The most cited document is a review of cellular and molecular mechanisms of pain, published in 2009 by Basbaum et al., in Cell Journal [23]. It achieved 2,636 total citations from (2009 to August 2023) with an annual citation normalization of 188 citations per year, indicating its impact on providing an essential theory foundation in the field.
Bibliometric mapping
International collaboration
VOSviewer software was used to generate network visualization maps and analyze international collaboration and partnership. Figure 3 shows the collaboration map for 27 featured countries each having a minimum of 50 publications in the field of TRPV1 inhibition. Countries are presented as nodes, with the larger nodes representing a high number of publications. The three largest nodes, respectively, represented the USA, China, and Japan.
The 27 featured countries were categorized into five clusters based on their extent of collaborations. Cluster 1 (represented by red) comprises eight countries which are Brazil, Canada, Denmark, France, Italy, Spain, Sweden, and the UK. Cluster 2 (green) comprises the following seven countries: Austria, Germany, Hungary, Mexico, Netherlands, Poland, and Switzerland. Six countries comprise Cluster 3 (blue), including the USA, China, and Japan. Belgium, India, Iran, the Russian Federation, and Turkey are the five countries that makeup Cluster 4 (colored in yellow). One country was included in cluster 5 (purple) which is Australia.
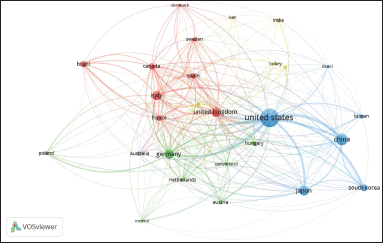 | Figure 3. International collaboration map related to the field of TRPV1 inhibition. Countries are presented as nodes, with the larger nodes representing a high number of publications. [Click here to view] |
Intellectual networking of co-authorship and co-cited authors
To explore the influential researcher and potential collaborators, the collaborative authorship and co-cited authors network maps are created and presented in Figure 4. Authors who published 25 or more papers were only included in the analysis. Among the identified 23,875 contributing authors, 65 met this criterion and were grouped into nine clusters. Interestingly, the author who had the highest number of publications and citations in the field of TRPV1 inhibition, Di Marzo, V., a professor of biochemistry at Laval University, Canada, collaborated with 6 other authors including Appendino, G. De Petrocellis, I. Maccarrone, M. Maione, S. Petrosino S., and Rossi, F. (cluster 2, consisting of seven authors, represented in green color), Figure 4A. It is noticed that authors from the same cluster cooperated relatively closely with each other. However, there was a minor collaboration between separate clusters (Fig. 4A). Furthermore, authors achieved a minimum of 1,000 citations (co-cited authors) were included in the network analysis. Out of 376,441 co-cited authors in the retrieved documents, only 75 co-cited authors were cited more than 1,000 times. These authors were grouped in four clusters as illustrated in the visual map in Figure 4B. The red cluster represented the highly co-cited authors who had a significant contribution to the field. Indeed, the cluster consisted of the scientists who first discovered the TRPV1, a capsaicin- and heat-activated ion channel. These include the Nobel Prize awarded, David Julius (Julius, D) and his colleagues Michael J Caterina (Caterina, M.J.), Markoto Tominaga (Tominaga, M.), and Mark A. Schumacher (Schumacher M.A.) (Fig. 4B).
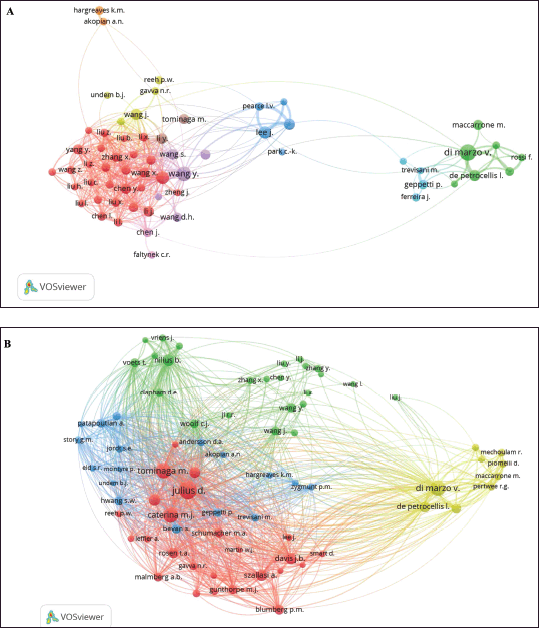 | Figure 4. Network visualization map of co-authorship and co-cited authors related to the field of TRPV1 inhibition. (A) A network visualization map of author-author collaboration in the retrieved documents. Sixty-five authors who published a minimum of 25 publications were included in the map. The authors were grouped into nine clusters. (B) A network visualization map of co-cited authors. Seventy-five co-cited authors with a minimum of 1,000 citations were included in the analysis, and they were grouped into four clusters. The larger nodes represent a high number of citations. [Click here to view] |
Analysis of author-keywords and hotspots forecasting
To identify the research hotspot in the field of TRPV1 inhibition and the emerging trends during the determined period, the author keywords were analyzed, and represented in different maps including visualization, conceptual structure, and thematic maps which were created using two different bibliometric tools including VOSviewer and Biblioshiny. The analysis of author keywords was conducted using a thesaurus file to consider and remove synonyms. In addition, only author keywords with a minimum occurrence of 50 times were included in the analysis. Among 12,073 identified author keywords, only 43 were selected and recognized as highly frequent author keywords. The top 10 most frequently occurring author keywords are presented in Table 6. TRPV1 achieved the highest occurrence with 1,544 times followed by capsaicin (706) and then by pain (648). Occurrence analysis and visualization maps of author keywords are shown in Figure 5A and B. The network visualization map illustrates the most frequent keywords as 43 circular nodes, Figure 5A. These nodes represent author keywords connected according to the focus of the research topic. The node size represents the frequency and the strength of connections. In addition, the Top 3 high-frequency keywords; TRPV1, capsaicin, and pain were located and highlighted in the middle of the density map (Fig. 5B), reflecting the research hotspots in this field. The identified author keywords were grouped into six separate clusters, each of them containing several keywords connected and colored in a specific color (Fig. 5A and Supplementary Table 1). These clusters provide insights into the main research areas and hot topics in the field of TRPV1 inhibition. Cluster 1 (red color) includes 10 terms focused on the endocannabinoid system (Supplementary Table 1). At the same time, Cluster 2 (green color) contains 10 keywords related to pain, nociception, and analgesia. Cluster 3 (blue color) includes nine keywords about TRP channels and calcium. Cluster 4 (yellow color) is represented by five keywords related to cancer, inflammation, cytokines, apoptosis, and oxidative stress. Cluster 5 (purple color) includes five words focused on neurogenic inflammation, sensory neurons, and migraine. Finally, four keywords were included in cluster 6 (light blue color) and related to electrophysiology, TRPV1 antagonists, and capsaicin, which is a TRPV1 activator. Supplementary Table 1 presents the details about the keywords included in each cluster.
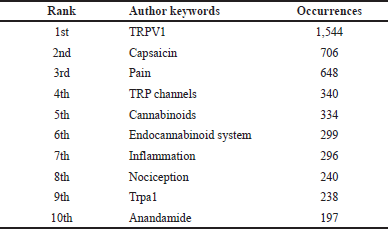 | Table 6. The top 10 highly frequent author keywords. [Click here to view] |
A conceptual structural map was generated to further examine and validate the clustering of author keywords (Fig. 5C). This map revealed the presence of four groups summarizing the connection between the identified author keywords. Interestingly, groups presented in the conceptual structural map (Fig. 5C) were aligned closely with the clusters identified in the network visualization map established by the VOSviewer (Fig. 5A and B). Therefore, the results obtained from both VOSviewer and Biblioshiny, two bibliometric software programs, provide a remarkable similarity. Moreover, a thematic map based on the extracted author keywords was established using Biblioshiny software (Fig. 5D). The thematic analysis presents an overview of the status of the TRPV1 inhibition research field. In addition, it provides knowledge about the potential of future research development regarding thematic areas within the field. The thematic map is divided into four quadrants; the upper right quadrant (Q1), represents driving or main themes (motor), the upper left quadrant (Q2) represents the very specialized and highly developed themes (niche), the lower left quadrant (Q3) which is emerging or declining themes, and lower right quadrant (Q4) representing underlying and foundational themes (basic). As shown in Figure 5D, the thematic analysis identified four themes (clusters) of the extracted author keywords.
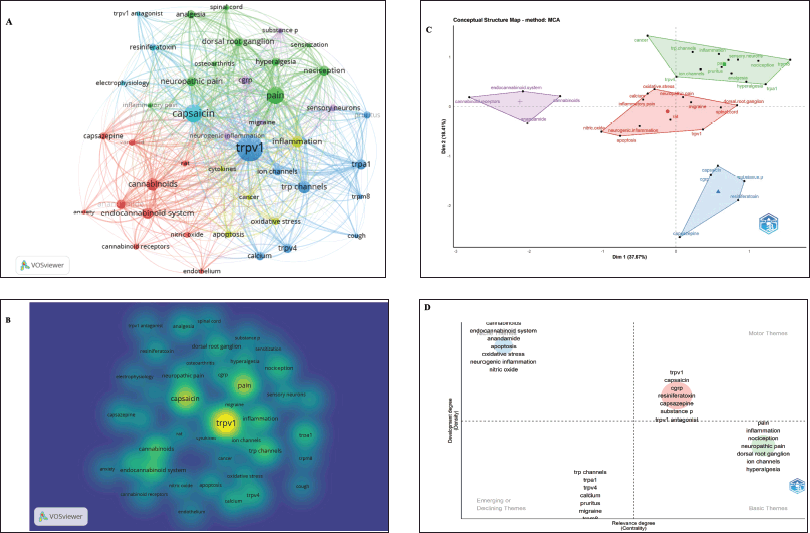 | Figure 5. The occurrence analysis and visualization maps of author keywords. Keywords with a minimum occurrence of 50 times were included in the analysis. (A) Network visualization map of author keywords created by VOS viewer; the map shows the co-occurrence of 43 keywords grouped in six clusters. The larger nodes represent high-occurrence keywords. (B) Density visualization map of the author keywords obtained by VOS viewer. (C) Conceptual structure map created by Bibloshiny software. The selected keywords were grouped into four conceptual groups. (D) Thematic map of author keywords analyses obtained using Bibloshiny software. The thematic analysis presents four clusters (themes) of authors’ keywords. The themes are characterized according to two properties including density and centrality. Density is represented on the vertical axis, while centrality is presented on the horizontal axis. Density measures the cohesiveness among the nodes and centrality expresses the degree of correlation between different topics. [Click here to view] |
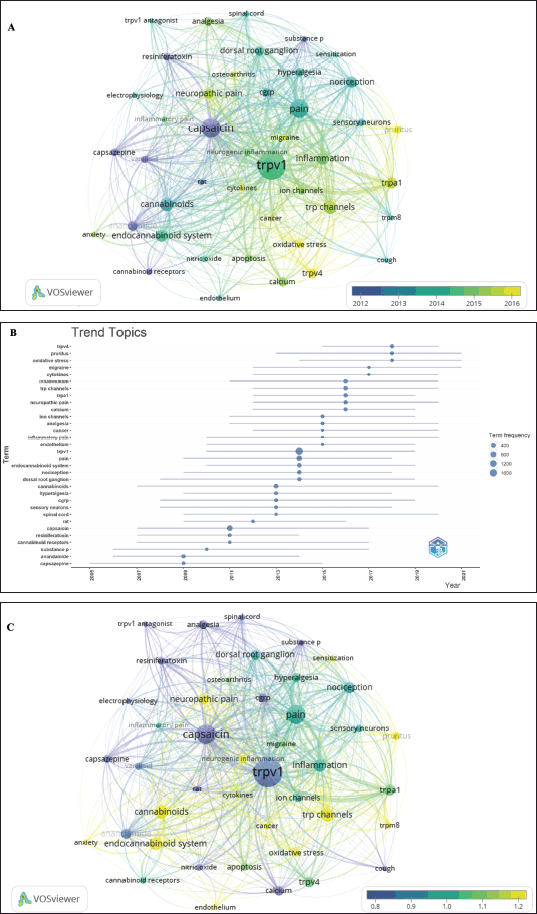 | Figure 6. The overlay visualization maps of author keywords considering publication year and citations. Keywords with a minimum occurrence of 50 times were included in the analysis. (A) Overlay visualization map of the highest occurrence of author keywords with average publication year by VOS viewer. (B) Overall research trends considering the author keyword over a period. (C) Overlay visualization map of the highest occurrence of author keywords with average normalized citations by VOS viewer. [Click here to view] |
A theme including keywords such as capsaicin, capsazepine, and TRPV1 antagonist is the main theme of the research field. While, theme containing cannabinoids, the endocannabinoid system, oxidative stress, neurogenic inflammation, and nitric oxide is a well-developed theme and structuring of the research field. It is worth mentioning that the research field of the active and highly cited author (Di Marzo, V) was mainly endocannabinoid and targeting TRP channels (Section “Analysis of authors and co-cited authors”). Keywords including TRP channels, calcium, pruritus, and migraine are grouped in a theme located in Q3, representing emerging or declining themes. Scholars in this field might further investigate TRP channels, particularly TRPV1, as a therapeutic strategy for pruritus and migraine. Finally, the foundational and basic theme consisted of keywords related to pain and its types such as neuropathic pain, hyperalgesia, and inflammation.
Furthermore, the analysis of author keywords underwent another assessment using two approaches. The first approach involved creating a normalized overlay of author-keywords clusters based on the average publication year, as shown in Figure. 6A. The color-coding of author-keyword clusters in the figure was based on the dates of their publications, with yellow representing recently published keywords. Keywords such as oxidative stress, cytokines, pruritus, cancer as well and TRPV4 were relatively new terms related to the publication in the field of TRPV1 inhibition (Fig. 6A). The second approach employed Biblioshiny software to explore the trend topics by plotting terms (keywords) versus publication years (Fig. 6B). In line with the first approach, the trend topic plot indicates that cluster words “TRPV4,” “pruritus,” “oxidative stress,” “ cytokines,” and “inflammation” were the most studied in the last 7 years (Fig. 6B), indicating a new role of TRPV channels in cancer and inflammation. On the other hand, cluster words “TRPV1,” “pain,” and “endocannabinoid system,” were frequently evolved in research documents before 2015. Overall, these investigations showed how the author keywords representing topic trends have evolved within the retrieved publications in recent years.
Given that older publications usually achieve more citations with time, the citations were normalized, by VOSviewer, based on the time of publication and an overlay visualization map of the highest occurrence of author keywords with average normalized citations (Fig. 6C). Terms including “TRP channels,” “ endocannabinoid system,” “ cannabinoids,” and “neuropathic pain” received the highest normalized citations compared to the other author keywords (Fig. 6C). Normalizing the citations based on their time of publication would help in addressing the potential bias generated by the varying publication dates of the documents.
DISCUSSION
TRPV1 is an ion channel protein widely expressed in sensory neurons and heat-sensitive nociceptors [1]. It plays an important role in sensing and transmitting pain and heat signals. Since being discovered, the number of research publications studying this protein has been increasing annually. Moreover, academic attention has been focused on targeting the TRPV1 channel and assessing the therapeutic potential of TRPV1 antagonists for pain and inflammation management [28–30]. This study presented a comprehensive bibliometric analysis of the most recent and relevant publications on TRPV1 inhibition and modulation. It illustrated trends in this research filed over time between 2003 and 2022. In addition, the current analysis would provide insight into the TRPV1 inhibition research hotspots and domains and facilitate the identification of the leading scholars and institutions for possible new collaboration for developing novel therapeutic agents. A few bibliometric studies have been carried out to analyze the progress in TRPV1-related research and these studies extracted the research articles from the Web of Science core collection database [18–20]. On the other hand, the current study retrieved relevant publications related to the scope of TRPV1 inhibition and modulation from Scopus, the largest robust database that provides abstracts and citations of peer-reviewed literature and offers advanced search options.
Bibliometric analysis is a useful tool for analyzing and visualizing a huge number of scientific documents, contributing countries, core authors, active journals, citation scores, and keyword clusters, as well as the periods of citation. Using two bibliometric tools, VOSviewer and Biblioshiny, the present study added a layer of valuable systematic information about the global research trend in the field of TRPV1 inhibition and modulation.
The results revealed a significant increase in publications in this field over the determined period. In total 7,470 scientific papers were obtained from 1,428 Scopus-indexed journals. Almost 80% of the published papers were research articles, indicating active scientific research in the field of TRPV1 inhibition and targeting. Moreover, this area of research has a significant scientific impact. The retrieved publications achieved 350,425 citations, with an average of 46.9 citations per document. The highly cited publications were published from 2007 to 2013, (Fig. 1 and Table 3), and among them is a review by Basbaum et al. [23]). This review was published in a cell journal (Impact factor (IF) = 64.5) and received the highest number of citations. Interestingly, the corresponding author of this review was David Julius, the highly co-cited author in the retrieved publications, and the first researcher who contributed to the identification of the TRPV1 protein in 1997 [1,31].
Based on an analysis of the geographic distribution of the retrieved publications, researchers from the USA contributed the highest number of publications, with 2,492 publications (33.3%), followed by researchers from China, with 1,048 publications (14%), and then by Japan (757 publications, 10%). In line with previous bibliometrics findings [18], these three top countries in the field of TRPV1 inhibition research were grouped in the same cluster (Fig. 3), suggesting a mutual collaboration between different researchers from these countries. Furthermore, analysis of the scientific impact of the contributing countries, indicated that papers retrieved from the UK had the highest scientific impact (58.04 citations per paper), followed by Italy (57.60 citations per paper). While the USA was in the third place with 56.79 citations per paper (Table 2). Although the UK and Italy published only 665 and 598 papers, respectively, these papers discussed important findings as they achieved the highest scientific impact demonstrated by their high citation count and average citations by paper.
Interestingly among the 23,875 authors who contributed to the publication in the field of TRPV1 inhibition, only 65 were considered productive scholars as they have published at least 25 publications. Vincenzo Di Marzo, a current professor of biochemistry at Laval University, Canada, was the most productive author with the highest number of papers and citations (130 publications and 12,170 citations). His research mainly focuses on endocannabinoid systems and targeting TRP channels. Among his publications was a research article entitled “Effects of cannabinoids and cannabinoid-enriched cannabis extracts on TRP channels and endocannabinoid metabolic enzymes” [32]. In this study, Di Marzo’s research team provided scientific evidence on the effect of cannabinoid derivatives and cannabis extracts on the TRPV1 channel. These compounds were found to stimulate and desensitize human TRPV1, supporting their potential roles as analgesic, anti-inflammatory, and anti-cancer agents [32]. As for co-cited author analysis, David Julius, a professor of Physiology at the University of California, USA, and Noble laureate, was ranked the first among the top co-cited authors, with 8,510 co-citations. Julius, D. and his colleagues were the first to use expression cloning to identify the TRPV1 ion channel. He is the corresponding author of the highly co-cited publications among the retrieved documents [1]. These influential authors are potential collaborators, and their publications provided a significant impact and insights on the future of TRPV1 inhibition and modulation research.
The research related to TRPV1 inhibition and modulation was mainly published in pharmacology, pain, and neuroscience journals. British Journal of Pharmacology (Impact factor (IF) 2022 = 7.3) is the leading international journal publishing in pharmacology, and was ranked the first in the number of publications (n = 199). While the Journal of Neuroscience (IF 2022 = 5.3) achieved the highest citations in the field of TRPV1 inhibition (17,558 citations). This journal is a multidisciplinary journal and publishes documents on a wide range of nervous system-related topics. The published research articles and reviews in this journal have an essential scientific influence in the field of TRPV1 inhibition.
In the academic field, keywords are very important and reflect research trends and hotspots in any research scope. In the current study, two different bibliometric tools were used to explore the co-occurrence of author keywords in the retrieved documents. A total of 43 keywords were identified more than 50 times in the retrieved publications. As expected, the word TRPV1 was the most frequent keyword, followed by capsaicin, and then pain. Looking for the history of TRPV1 ion channel discovery, it was called the first capsaicin receptor [1]. Capsaicin is the main pungent compound in chili peppers. It selectively activates TRPV1 and triggers the burning pain sensation [1,31]. Although it is a TRPV1 agonist, capsaicin has paradoxically long been used as an analgesic [33]. Several topical therapeutic agents containing capsaicin are available in the market for pain relief including capsaicin cream (Zostrix) and high-concentration capsaicin patches (Qutenza) [2]. This paradoxical effect is explained by the mechanism of TRPV1 desensitization mediated by prolonged exposure to capsaicin [34].
The identified author keywords were grouped into six clusters by VOSveiwer analysis and overlapped in four main conceptual clusters by biblioshiny, and both software provided related and similar findings. The obtained clusters indicated the main research interest and the focus of the retrieved documents. Cannabinoids, endocannabinoids, and other keywords including anandamide, anxiety, vanilloid, capsazpine, endothelium, and nitric oxide were clustered together and represented in the well-developed theme. Endocannabinoids are involved in the modulation of various physiological processes, including pain, inflammation, depression, and anxiety [35–37]. Endocannabinoids are endogenous cannabinoids that mediate the activity of the TRPV1 channel in pain regulation [36]. Several previous studies indicated the association of the endocannabinoid system with various pain types including chronic pain, inflammatory pain, neuropathic pain, and migraine [38–40]. Moreover, an association between the endocannabinoid system and pain-anxiety comorbidity has recently been highlighted in Alzheimer’s disease [35]. This indicates a new direction of TRPV1 inhibition research and the possible role of the TRPV1 channel in mediating pain-associated psychological problems including anxiety and depression.
TRPV1 channel is also expressed in cardiovascular tissue such as vascular endothelium, regulating vascular function and contributing to endothelium-dependent vasodilation [41]. Activation of TRPV1 by capsaicin was proved in animal models to enhance endothelium-dependent relaxation by increasing nitric oxide production in endothelial cells [42,43]. These findings were validated in vitro using mouse vascular endothelial cells. Interestingly the capsaicin-mediated endothelium relaxation effect was abolished by TRPV1 inhibitor capsazepine, confirming the essential role of TRPV1 in this physiological process [43]. The research trends and academic interest in the role of TRPV1 in cardiovascular disease have recently been investigated by a bibliometric analysis published by Zhang et al. [20]. Moreover, new research has focused on the role of TRPV1 in the modulation of lipids metabolism and as a potential target to treat various metabolic disorders including hyperlipidemia and other cardiovascular diseases [2].
Among the identified author keywords were apoptosis, cancer, cytokines, inflammation, and oxidative stress. These keywords formed a separate cluster, suggesting a line of research related to the role of TRPV1 in this scope. TRPV1-mediated signaling is involved in the modulation of several cellular processes. It has been proven that TRPV1 is overexpressed in cancer and is involved in cell proliferation, migration, and survival of tumor cells [44–46]. A line of evidence has shown that inhibition of TRPV1 through nanoparticle-mediated TRPV1 channel blockade enhances thermo-immunotherapy via inhibiting heat shock factor 1-mediated dual self-defense pathways in cancer cells [47]. Moreover, studies have provided evidence on the role of TRPV1 in cancer chemotherapy, particularly in overcoming resistance and alleviating pain. TRPV1 inhibition potentiates the tumor’s susceptibility to chemotherapy, such as cisplatin, by blocking the autophagy-mediated hyperactivation of the epidermal growth factor receptor signaling pathway [48]. Moreover, TRPV1 channel activation is believed to be involved in the stimulation of the itch sensation [49]. Therefore, there is a great interest in inhibiting TRPV1 channels as a target for pruritus treatment, in particular the development of topical preparation to avoid the hyperthermia associated with oral formulations. In this bibliometric analysis, pruritus was one of the author’s keywords that were involved in recent publications and located in the emerging theme in the thematic map.
Despite the number of published bibliometric analyses about TRPV1, the current study is the first to investigate the research trends TRPV1 inhibition and modulation research field. The previous studies mainly focused on the analysis of the publications included in the Web of Science core collection database. In this study, we considered Scopus, the largest robust database that provides abstracts and citations of peer-reviewed literature and offers advanced search options. Moreover, two bibliometric tools were employed in this study to analyze the retrieved documents. In addition, the VOSviewer thesaurus file was created to merge the related keywords. This strategy would help to standardize the findings and prevent the replication of the same words in the visualization map, thus providing comprehensive and valid results. The search on the retrieved publications was conducted in August 2023. The timeline for the included publication was between (2003–2022). Therefore, very recent publications were not included in this analysis. In addition, the analysis included only publications written in the English language which might affect the overall research trends.
CONCLUSION
In conclusion, this study emphasizes the research trends and hotspots in the TRPV1 inhibition and modulation research field. It provides a bibliometric analysis of the articles and reviews published in this area including their publication year, contributing authors, regional distributions, publishing journals, and citations. The publication in this field was increasing during the investigated period and most of the publications were research articles highlighting a great interest in experimental, original, and clinical research in this field. Our results showed that the USA occupied the first position in the number of publications. Despite that Julius, D.’s research group, who first discovered the TRPV1 protein, Di Marzo V. from the University of Laval, Canada, was the core and active author in the retrieved documents. However, both are future potential collaborators in this research field.
TRPV1 is a multimodal nociceptor that could be activated and/or allosterically modulated by different stimuli. Its inhibition is potentially involved in various pathological conditions. Analysis of the keywords shed light on the directions of future research in the field of TRPV1 inhibition. In this study, the most frequently mentioned and replicated author keywords were TRPV1, pain, and capsaicin. Bibliometric analysis indicated a new role of TRPV1 inhibition in cancer, inflammation, oxidative stress, pruritus, osteoarthritis, migraine, endocannabinoid system, nitric oxide, and endothelium relaxation. The role of TRPVI inhibition in these conditions should be translated into developing therapeutic strategies and discovering novel pharmaceutical formulas.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the international committee of medical journal editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
USE OF ARTIFICIAL INTELLIGENCE (AI)-ASSISTED TECHNOLOGY
The authors declares that they have not used artificial intelligence (AI)-tools for writing and editing of the manuscript, and no images were manipulated using AI.
PUBLISHER’S NOTE
All claims expressed in this article are solely those of the authors and do not necessarily represent those of the publisher, the editors and the reviewers. This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–24. CrossRef
2. Abdalla SS, Harb AA, Almasri IM, Bustanji YK. The interaction of TRPV1 and lipids: insights into lipid metabolism. Front Physiol. 2022;13:1066023. Epub 20221215. CrossRef
3. Iftinca M, Defaye M, Altier C. TRPV1-targeted drugs in development for human pain conditions. Drugs. 2021;81(1):7–27. CrossRef
4. Kark T, Bagi Z, Lizanecz E, Pásztor ET, Erdei N, Czikora A, et al. Tissue-specific regulation of microvascular diameter: opposite functional roles of neuronal and smooth muscle located vanilloid receptor-1. Mol Pharmacol. 2008;73(5):1405–12. Epub 20080206. CrossRef
5. Takahashi N, Matsuda Y, Yamada H, Tabeta K, Nakajima T, Murakami S, et al. Epithelial TRPV1 signaling accelerates gingival epithelial cell proliferation. J Dent Res. 2014;93(11):1141–7. Epub 20140929. CrossRef
6. Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, et al. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288(5464):306–13. CrossRef
7. Kwon DH, Zhang F, Suo Y, Bouvette J, Borgnia MJ, Lee SY. Heat-dependent opening of TRPV1 in the presence of capsaicin. Nat Struct Mole Biol. 2021;28(7):554–63. Epub 2021/07/10. CrossRef
8. Caterina MJ, Pang Z. TRP channels in skin biology and pathophysiology. Pharmaceuticals (Basel, Switzerland). 2016;9(4):77. Epub 2016/12/17. CrossRef
9. Woo DH, Jung SJ, Zhu MH, Park CK, Kim YH, Oh SB, et al. Direct activation of transient receptor potential vanilloid 1(TRPV1) by diacylglycerol (DAG). Mol Pain. 2008;4:42. Epub 20081001. CrossRef
10. Senning EN, Collins MD, Stratiievska A, Ufret-Vincenty CA, Gordon SE. Regulation of TRPV1 ion channel by phosphoinositide (4,5)-bisphosphate: the role of membrane asymmetry. J Biol Chem. 2014;289(16):10999–1006. Epub 2014/03/07. CrossRef
11. Muller C, Morales P, Reggio PH. Cannabinoid ligands targeting TRP channels. Front Mole Neurosci. 2018;11:487. Epub 2019/01/31. CrossRef
12. Aghazadeh Tabrizi M, Baraldi PG, Baraldi S, Gessi S, Merighi S, Borea PA. Medicinal chemistry, pharmacology, and clinical implications of TRPV1 receptor antagonists. Med Res Rev. 2017;37(4):936–83. Epub 20161215. CrossRef
13. Koivisto A-P, Belvisi MG, Gaudet R, Szallasi A. Advances in TRP channel drug discovery: from target validation to clinical studies. Nat Rev Drug Discov. 2022;21(1):41–59. CrossRef
14. Harb AA, Bustanji YK, Almasri IM, Abdalla SS. Eugenol reduces LDL cholesterol and hepatic steatosis in hypercholesterolemic rats by modulating TRPV1 receptor. Sci Rep. 2019;9(1):14003. CrossRef
15. El-Hammadi MM, Small-Howard AL, Jansen C, Fernández-Arévalo M, Turner H, Martín-Banderas L. Potential use for chronic pain: Poly(Ethylene Glycol)-Poly(Lactic-Co-Glycolic Acid) nanoparticles enhance the effects of cannabis-based terpenes on calcium influx in TRPV1-Expressing cells. Int J Pharm. 2022;616:121524. Epub 2022/02/02. CrossRef
16. van Eck NJ, Waltman L. Visualizing bibliometric networks. Ding Y, Rousseau R, Wolfram D, editors. Measuring Scholarly Impact: Methods and Practice. Cham: Springer International Publishing; 2014. pp 285–320.
17. Donthu N, Kumar S, Mukherjee D, Pandey N, Lim WM. How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. 2021;133:285–96. CrossRef
18. Wang S, Wang W, Ye X. Bibliometric analysis of global research on transient receptor potential vanilloid 1 in the field of pain. J Pain Res. 2023;16:1517–32. Epub 2023/05/17. CrossRef
19. Xu P, Shao RR, He Y. Bibliometric analysis of recent research on the association between TRPV1 and inflammation. Channels (Austin, Tex). 2023;17(1):2189038. Epub 2023/03/16. CrossRef
20. Zhang L, Xu Y, Ma Y, Xie T, Liu C, Liu Q. Research trends in transient receptor potential vanilloid in cardiovascular disease: bibliometric analysis and visualization. Front Cardiovasc Med. 2023;10:1071198. Epub 2023/03/14. CrossRef
21. Bustanji Y, Shihab KHA, El-Huneidi W, Semreen MH, Abu-Gharbieh E, Alzoubi KH, et al. Analysis and mapping of global scientific research on human monkeypox over the past 20 years. Veterinary world. 2023;16(4):693–703. Epub 2023/05/26. CrossRef
22. Bustanji Y, Taneera J, Semreen MH, Abu-Gharbieh E, El-Huneidi W, Faris MA-IE, et al. Gold nanoparticles and breast cancer: a bibliometric analysis of the current state of research and future directions. OpenNano. 2023;12:100164. CrossRef
23. Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–84. Epub 2009/10/20. CrossRef
24. Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92(2):791–896. Epub 2012/04/27. CrossRef
25. Pertwee RG, Howlett AC, Abood ME, Alexander SPH, Marzo VD, Elphick MR, et al. International union of basic and clinical pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62(4):588. CrossRef
26. Liao M, Cao E, Julius D, Cheng Y. Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature. 2013;504(7478):107–12. CrossRef
27. Kano M, Ohno-Shosaku T, Hashimotodani Y, Uchigashima M, Watanabe M. Endocannabinoid-mediated control of synaptic transmission. 2009;89(1):309–80. doi: 10.1152/physrev.00019.2008.
28. Uniyal A, Gadepalli A, Tiwari V, Allani M, Chouhan D, et al. Unlocking the potential of TRPV1 based siRNA therapeutics for the treatment of chemotherapy-induced neuropathic pain. Life Sci. 2022;288:120187. Epub 2021/12/03. CrossRef
29. Lee H, Ahn S, Ann J, Ha H, Yoo YD, Kim YH, et al. Discovery of dual-acting opioid ligand and TRPV1 antagonists as novel therapeutic agents for pain. Eur J Med Chem. 2019;182:111634. Epub 2019/09/01. CrossRef
30. Kim C, Ann J, Lee S, Sun W, Blumberg PM, Frank-Foltyn R, et al. Discovery of 2-(3,5-difluoro-4-methylsulfonaminophenyl)propanamides as potent TRPV1 antagonists. Bioorg Med Chem Lett. 2018;28(14):2539–42. Epub 2018/06/10. CrossRef
31. Bautista DM. Spicy science: David Julius and the discovery of temperature-sensitive TRP channels. Temperature (Austin, Tex). 2015;2(2):135–41. Epub 2016/05/27. CrossRef
32. De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. British J Pharm. 2011;163(7):1479–94. Epub 2010/12/24. CrossRef
33. Arora V, Campbell JN, Chung MK. Fight fire with fire: neurobiology of capsaicin-induced analgesia for chronic pain. Pharm Ther. 2021;220:107743. Epub 2020/11/13. CrossRef
34. Sanz-Salvador L, Andrés-Borderia A, Ferrer-Montiel A, Planells-Cases R. Agonist- and Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J Biol Chem. 2012;287(23):19462–71. Epub 2012/04/12. CrossRef
35. Blanton H, Reddy PH, Benamar K. Chronic pain in Alzheimer’s disease: endocannabinoid system. Exp Neurol. 2023;360:114287. Epub 2022/12/02. CrossRef
36. Lowe H, Toyang N, Steele B, Bryant J, Ngwa W. The endocannabinoid system: a potential target for the treatment of various diseases. Int Journal of molecular sciences. 2021;22(17). Epub 2021/09/11. CrossRef
37. Fitzgibbon M, Finn DP, Roche M. High times for painful blues: the endocannabinoid system in pain-depression comorbidity. Int J Neuropsychopharmacol. 2015;19(3):pyv095. Epub 2015/09/06. CrossRef
38. Maldonado R, Baños JE, Cabañero D. The endocannabinoid system and neuropathic pain. Pain. 2016;157 Suppl 1:S23–S32. Epub 2016/01/20. CrossRef
39. Anand P, Whiteside G, Fowler CJ, Hohmann AG. Targeting CB2 receptors and the endocannabinoid system for the treatment of pain. Brain Res Rev. 2009;60(1):255–66. Epub 2009/01/20. CrossRef
40. Greco R, Demartini C, Zanaboni AM, Francavilla M, De Icco R, Ahmad L, et al. The endocannabinoid system and related lipids as potential targets for the treatment of migraine-related pain. Headache. 2022;62(3):227–40. Epub 2022/02/19. CrossRef
41. Earley S, Brayden JE. Transient receptor potential channels in the vasculature. Physiological reviews. 2015;95(2):645–90. Epub 2015/04/03. CrossRef
42. Yang D, Luo Z, Ma S, Wong WT, Ma L, Zhong J, et al. Activation of TRPV1 by dietary capsaicin improves endothelium-dependent vasorelaxation and prevents hypertension. Cell Metab. 2010;12(2):130–41. Epub 2010/08/03. CrossRef
43. Wang Q, Zhang C, Yang C, Sun Y, Chen K, Lu Y. Capsaicin alleviates vascular endothelial dysfunction and cardiomyopathy via TRPV1/eNOS pathway in diabetic rats. Oxid Med Cell Longev. 2022;2022:6482363. Epub 2022/05/24. CrossRef
44. Waning J, Vriens J, Owsianik G, Stüwe L, Mally S, Fabian A, et al. A novel function of capsaicin-sensitive TRPV1 channels: involvement in cell migration. Cell Calcium. 2007;42(1):17–25. Epub 2006/12/23. CrossRef
45. Zhang SS, Ni YH, Zhao CR, Qiao Z, Yu HX, Wang LY, et al. Capsaicin enhances the antitumor activity of sorafenib in hepatocellular carcinoma cells and mouse xenograft tumors through increased ERK signaling. Acta Pharm Sinica. 2018;39(3):438–48. Epub 2017/12/01. CrossRef
46. Li L, Chen C, Chiang C, Xiao T, Chen Y, Zhao Y, et al. The impact of TRPV1 on cancer pathogenesis and therapy: a systematic review. Int J Biol Sci. 2021;17(8):2034–49. Epub 2021/06/17. CrossRef
47. Li T, Jiang S, Zhang Y, Luo J, Li M, Ke H, et al. Nanoparticle-mediated TRPV1 channel blockade amplifies cancer thermo-immunotherapy via heat shock factor 1 modulation. Nat Commun. 2023;14(1):2498. CrossRef
48. Oh SJ, Lim JY, Son MK, Ahn JH, Song K-H, Lee H-J, et al. TRPV1 inhibition overcomes cisplatin resistance by blocking autophagy-mediated hyperactivation of EGFR signaling pathway. Nature Communications. 2023;14(1):2691. CrossRef
49. Lee KP, Koshelev MV. Upcoming topical TRPV1 anti-pruritic compounds. Dermatol Online J. 2020;26(9):3. Epub 2020/10/16.
Supplementary Material
 | Supplementary Table 1. The keywords included in the clusters by VOS viewer. [Click here to view] |