INTRODUCTION
Emerging infections, drug resistance, and oxidative stress-mediated diseases have made it inevitable to search for new antioxidants and antimicrobials. In this context, plants could be used as safe and efficacious therapeutic options; several plants have been reported with diverse biological properties and therapeutic potential [1–6]. Among various plant families, Asteraceae is the most prominent family of the plant kingdom. It includes 1,701 accepted genera and over 24,000 species [7,8], comprising ~10% of the flowering plants. Most family members are annual or perennial herbs; some tropical forms include shrubs, vines, and trees [9]. These can be categorized into ornamental plants (Tagetes, Chrysanthemum, Calendula, Ageratum), wild plants (Brachyscome, Arctium, Boltonia), noxious weeds (Taraxacum, Carduus, Cirsium), and economically important plants (Lactuca, Cynara, Helianthus, Artemisia). The characteristic feature of this family is its inflorescence, called calathium or capitulum [10].
Asteraceae’s genus Cirsium (thistle) is an annual, biennial or perennial herb. It comprises approximately 378 recognized species of spiny, perennial, biennial, or rarely yearly [8]. The species are found throughout the northern hemisphere (North America, North Africa, Asia, and Eurasia), from subtropical to boreal latitudes [11,12]. The plants of Cirsium genus are mainly utilized for the treatment of leukemia and peptic ulcer in folklore medicine [13], epistaxis, eye infections, metrorrhagia, syphilis [14], gonorrhea, skin sores, bleeding piles, diabetes, and hemostasis, therefore, making them safe and effective medicine [15–17]. Numerous phytochemicals such as flavonoids, phenolic acids, polyacetylenes, acetylenes, phenylpropanoids, sterols, and terpenoids contribute to these medicinal qualities of Cersium species [18]. Among various species, Cirsium vulgare and Cirsium arvense are considered noxious weeds [8,19].
Cirsium arvense (L.) Scop. is one of the world’s most troublesome and persistent weeds. It is native to Europe and the northern hemisphere but was also introduced to North America in the 1600s and the southern hemisphere [20]. It is often found in grasslands and riparian habitats. Even as a weed, C. arvense is known for its medicinal properties [21,22]. For example, plant decoction is a remedy for epistaxis, gastrointestinal disorders, hemorrhage, hypertension, metrorrhagia, pyogenic infections, scabies, ulcers, and skin diseases [23,24]. Additionally, C. arvense was reported as an antimicrobial and antioxidant agent, and its potential is attributed to the presence of flavonoids, alkaloids, steroids, and saponins [21,25–27].
Previously published review articles were primarily focused on the consequences of C. arvense’s spread as a perennial weed in European countries, as well as control measures [28]. Further, reviews are available on medicinal properties, phytochemical and pharmacological studies of the genus Cirsium, and different species [29]. So, in this review, we endeavored to study C. arvense’s ethnomedicinal uses, phytochemistry, and pharmacology. The primary goal of this review is to explore C. arvense as an economically significant plant that can provide a way to bioprospect this weed and open new doors for future research in different areas.
TAXONOMIC DESCRIPTION AND GEOGRAPHICAL DISTRIBUTION OF C. ARVENSE
Herb, dioecious, perennial, up to 160 cm tall. Stem erect, unwinged, branched above. Leaves petiolate, petioles narrowly winged, lamina 3–30 × 1–6 cm, oblong to elliptic, margins plane to revolute, entire, spinulose, main spines 1–7 mm, abaxial faces glabrous to densely grey-tomentose, adaxial green, glabrous to thinly tomentose. Inflorescence capitulum, terminal, corymbose; involucre narrowly ovoid. Phyllaries imbricate, in 5–7 rows, lacking wings and scarious appendage. Corolla reddish purple or rarely white; female florets 1.6–2.4 cm; male florets 1.5–1.8 cm. Fruits achene, yellowish. Pappus bristles dirty white, 2.5–3.5 cm [19,30,31]. Taxonomical features of C. arvense are shown in Figure 1.
Cirsium arvense grows in diverse habitats (ranging from moist places to grasslands, mountain slopes, flooded lands, disturbed sites, etc.) at 100–4,300 m [31]. Its native range is Temperate Eurasia, Northwest Africa. It has been introduced into North America, South America, Africa, Europe, Asia, Australia, and other regions, as shown in Figure 2 [8].
TRADITIONAL USES OF C. ARVENSE
In light of existing literature, several studies have reported the ethnomedicinal and culinary uses of C. arvense. A brief overview of the ethnomedicinal uses of C. arvense has been depicted in Table 1.
Ethnomedicinal uses
Various ethnobotanical studies have recognized the therapeutic and health-promoting uses of the whole plant of C. arvense [32]. In North America, C. arvense (whole plant) is used as a remedy against cirrhosis, lipoma, liver cancer, and gout [33,34]. Further, a decoction of the plant is used for the treatment of gastrointestinal disorders, hypertension, hemorrhage, lung troubles, epistaxis, hematemesis, ulcers, scabies, metrorrhagia, pyogenic infections, and various types of skin diseases [23,24,35–39]. The infusion or extract of the whole plant is used for the cure of mouth infections by North American Indian tribals and is considered to be useful as an astringent, diuretic, and health-promoting tonic [21,27,40].
Also, root decoction is used as an anthelmintic, astringent, diuretic, tonic, and remedy against hepatic disorders and intestinal worms [23,41,42]. David [41] documented the use of syrup from the roots to alleviate cough, while root juice is used to cure diabetes and jaundice and against snake bites [34,43–46]. The roots paste mixed with Amaranthus spinosus is given in case of indigestion [47]. Leaf juice and tea are employed for treating tuberculosis, piles, eye pain, skin-related problems, wounds, and urogenital diseases [41,44,45,48]. Subsequently, leaves paste is applied to heal boils [30]. Leaves are chewed to relieve toothache and sore throat because of their anti-inflammatory properties [21,27]. A mixture of the roots and leaves is used for oral disorders, toothache, diarrhea, dysentery, tuberculosis, and hepatic disorders [41,42,45,49]. The tincture of the leaves and flowers has been recommended against dermatitis [50].
Culinary uses
The foliage of C. arvense and aromatic seeds are used as food [37,53] due to their significant content of vitamins, minerals, and fibers [21,27]. The raw or cooked soft roots are eaten with other vegetables and used as drought food [54,55]. The peeled stem is cooked like Asparagus, while the leaves are used raw or cooked [54].
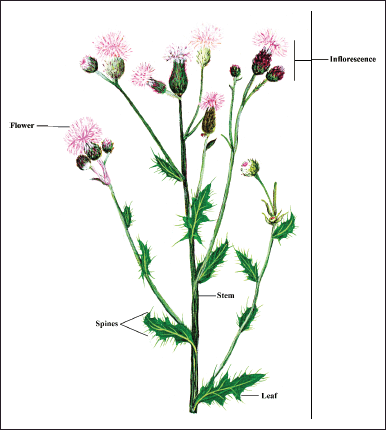 | Figure 1. Salient botanical features of C. arvense. (Source: Patanjali Herbal Museum, Haridwar, India). [Click here to view] |
PHYTOCHEMISTRY OF C. ARVENSE
Cirsium arvense contains carotenoids, alkaloids, flavonoids, phenols, tannins, terpenoids, and glycosides [27,56]. Different flavonoids such as kaempferol-3-O-β-D-glucopyranoside, hispidulin-7-O-β-D-glucopyranoside, quercetin-3-O-β-D-glucopyranoside, luteolin-5-O-β-D-glucopyranoside and phenolic acid like caftaric, protocatechuic, and neochlorogenic acid have been reported in C. arvense by Popova [57], Khan et al. [58] and Ashmita et al. [59] observed the presence of α-tocopherol, 9,12,15-octadecatrienoic acid, hispidulin, and tracin C. arvense. The plant also contains flavones (acacetin and apigenin), caffeic acid, chlorogenic acid, enicin, protocatechualdehyde, rutin, stigmasterol, taraxasterol, and triterpenes [36,60,61]. The aerial parts and young inflorescence have alkaloids, choline, glucoside, and saponins [52,62]. Roots contain phytotoxic compounds, whereas leaves have flavones and cyanide-glycoside [60,61]. The C. arvense flower’s methanolic extract contains triterpenoids (α and β-amyrin), sterols (γ-sitosterol, stigmasterol), and olean-12-en-3-ol, acetate [63]. Some other constituents like 1,2-benzenedicarboxylic acid; mono(2-ethylhexyl) ester; 10-octadecenoic acid, methyl ester; 2-pentadecanone; 6,10,14-trimethyl,2H-1-benzopyran, 6,7-dimethoxy-2-2-dimethyl,3,5-ditert-butyl-4-hydroxyacetophenone, 6,7-dimethoxycoumarin, 9,12-octadecadienoic acid (Z,Z)-,methyl ester, citronellol, acacetin, arvense A-B, camphor, ciryneol C, dihydroxy-6,7-dimethoxyflavone 4′-glucoside, ergoline-8-carboxylic acid, 10-methoxy-methyl-,methyl ester, heneicosane, heptadecanoic acid, 16-methyl-,methyl ester, hexadecanoic acid, nonadecane, pectolinarigenin-7-O-glucopyranoside, phytol, and scopoletin have also been reported in C. arvense [64]. The chemical structures of some of the representative phytoconstituents are highlighted in Figure 3.
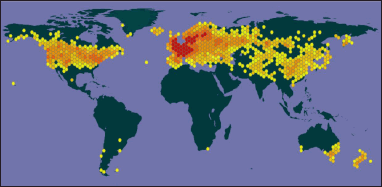 | Figure 2. Map showing the geographical distribution of C. arvense (Source: https://www.gbif.org/). [Click here to view] |
PHARMACOLOGICAL PROFILE OF C. ARVENSE
The plant contains many phytoconstituents that have shown potential towards various bacterial strains, cancer cells, fungi, and also against free radicals. The validation of ethnomedicinal information by utilizing evidence-based pharmacological studies is necessary. Toxicity studies should support biological activities to assure the safety and efficacy of herbal medicine. This weed is not much explored; only a few studies, like antimicrobial, antioxidant, and antiproliferative are available in light of existing literature.
Antioxidant activity
Antioxidants are the molecules that can scavenge free radicals or reactive oxygen species (ROS) like superoxide (O2−), hydroxyl radical (•OH), hydrogen peroxide (H2O2), and others. ROS are produced in a cell due to biochemical reactions and can adversely affect nucleic acids, lipids, and proteins, resulting in oxidative stress and multiple diseases [65]. Antioxidants are crucial for inhibiting oxidative reactions and removing ROS or neutralizing harmful effects of ROS in the body [66]. In this context, the crude extract from leaves, flowers, and roots of C. arvense displayed in vitro antioxidant activity against 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical, superoxide anion radical, and also in ferric reducing antioxidant power assay [67]. The ethanol extract of C. arvense aerial parts exhibited antioxidant activity in ferrous ion (Fe++) chelating assay, DPPH, H2O2, O2− and nitric oxide radical scavenging assays with IC50 values of 92, 118, 142, 110, and 100 μg/ml, respectively [27]. On the other hand, the aqueous extract from C. arvense leaves exhibited antioxidant activity with total antioxidant status of 2.74 m/ml [68]. The crude methanol extract of C. arvense inflorescence and leaves and its fractions (chloroform, diethyl ether, ethyl acetate, and n-butanol) were also evaluated for antioxidant activity. With a total antioxidant status of 1.76–2.69 mM/l, all fractions demonstrated antioxidant activity. The inflorescences’ butanol and leaves’ ethyl acetate fractions were observed to be the most active [69]. Cirsium arvense is reported to have antioxidant potential, but further studies (in vitro and in vivo) are warranted to validate this potential.
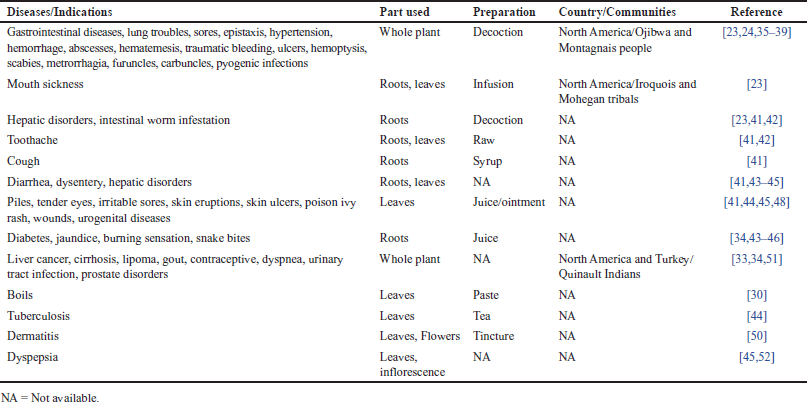 | Table 1. Ethnomedicinal uses of C. arvense. [Click here to view] |
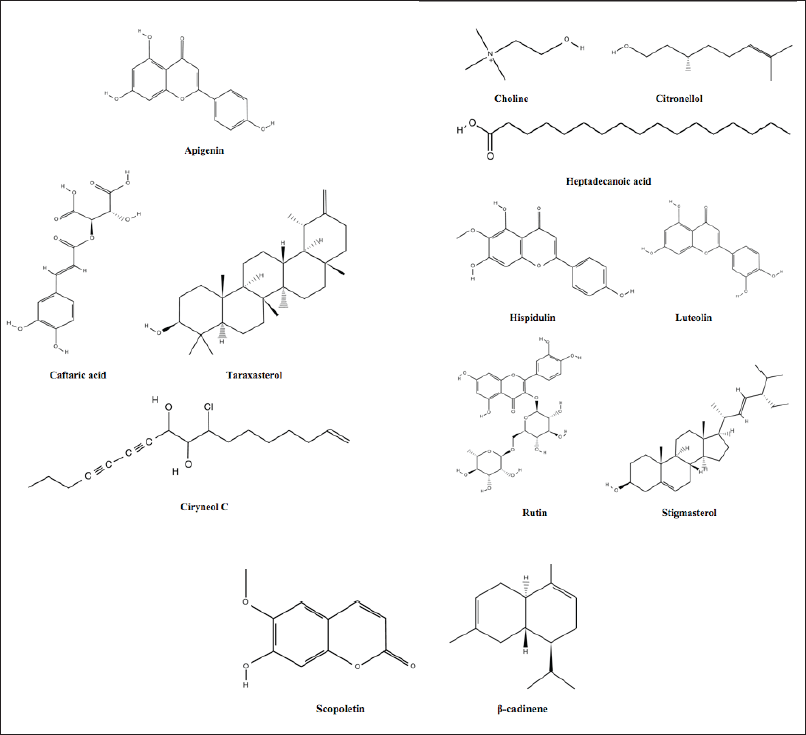 | Figure 3. Representative chemical composition of C. arvense. [Click here to view] |
Antiproliferative activity
In vitro antiproliferative activity of different extracts (chloroform, n-hexane, aqueous methanol, and water) of C. arvense herb and roots (10 μg/ml) was evaluated against A431 (skin epidermoid carcinoma), HeLa (cervix epithelial adenocarcinoma), and MCF7 (breast epithelial adenocarcinoma) cells using 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide assay. All fractions exhibited antiproliferative activity with 2.88%–21.15% inhibition against all tested cell lines [70].
Antimicrobial activity
Different extracts of C. arvense plant parts have been evaluated by various researchers for antimicrobial activity. The aqueous extract of C. arvense leaves exhibited antimicrobial activity against Staphylococcus aureus with minimum inhibitory concentration (MIC) 12.5 mg/ml), Bacillus subtilis (MIC 50 mg/ml), Pseudomonas aeruginosa (MIC 50 mg/ml), and Candida albicans (MIC 1.56 mg/ml) [68]. Similarly, chloroform, n-butanol, n-hexane, and ethyl acetate fractions (100 μl) of C. arvense methanol extract were evaluated against Gram positive (S. aureus and Micrococcus luteus), Gram negative bacterial strains (Escherichia coli, Klebsiella pneumoniae, Enterobacter sp. and P. aeruginosa) and fungus (Aspergillus niger). The chloroform fraction was observed to be most active against S. aureus with an inhibition zone diameter (IZD)15 mm, followed by Enterobacter sp. (IZD 14 mm), M. luteus (IZD 13 mm), E. coli (IZD 10 mm), and others [21]. The ethanolic extract (200, 250, and 500 μg/disc) from C. arvense aerial parts was evaluated against Streptococcus pyogenes, S. aureus, Staphylococcus epidermidis, Shigella boydii, Shigella sonnei, Shigella flexneri, Streptococcus agalactiae, E. coli and Enterococcus faecalis. The extract at 500 μg/disc inhibited all bacterial strains except S. epidermidis, S. agalactiae, and E. faecalis, where maximum activity was observed towards S. pyogenes (13.6 mm) [71].
The compounds hispidulin, tracin, 9,12,15-octadecatrienoic acid, α-tocopherol, and luteolin (1,000 μg/ml) from the C. arvense were screened for anti-microbial activity against bacteria (E. coli, B. subtilis, S. flexneri, S. aureus, Salmonella typhi, and P. aeruginosa) and fungi (C. albicans, C. glabrata, Trichophyton longifusus, Aspergillus flavus, Fusarium solani, and Microsporum canis). All tested compounds showed activity against tested microbial strains with IZD ranging between 9 and 34 mm. Tracin was observed to be most effective against B. subtilis. In contrast, luteolin and α-tocopherol were effective against M. canis with IZD 13–36 mm, whereas hispidulin was highly active against F. solani. Ashmita et al. [59] also observed the antimicrobial activity of compounds arvense A-B. All these studies support the antimicrobial potential C. arvense; however, most studies have only presented qualitative data, and quantitative studies with MIC are still required.
Mechanistic insights into antibacterial potential
Antibiotic resistance has grown to be a serious global concern. Drug-resistant infections are mainly brought on by the improper use and overuse of antibiotics [72]. Antibacterial drugs disrupt bacterial membranes and inhibit DNA, RNA, and protein synthesis [73]. Bacterial strains are constantly devising new mechanisms through many processes to adapt and withstand antibiotics’ lethal or biostatic effects [74]. Efflux pump (groups of transporter proteins) hyperactivity contributes to drug resistance; it extrudes drugs from cells to the external environment and reduces the antibiotic concentration inside [75–78]. Figure 4 displays antibiotic resistance mechanisms and a suggested strategy (based on existing literature) accentuating the antibacterial activity of C. arvense’s phytoconstituents.
The enzymatic resistance mechanism involves a range of bacterial enzymes generated against distinct antibiotics, which cause structural modifications of antibiotics by hydrolysis or transferring functional groups, decreasing their efficiency [78]. In addition, bacteria acquire resistance via porin channel impairment (outer membrane protein alteration), thereby reducing the uptake of antibiotics [76,78]. The mutation in bacterial DNA and biofilm formation can also confer antimicrobial resistance [73,76,78,79].
The utilization of herbal remedies against bacterial strains resistant to antibiotics has recently grown. Many plants possess antibacterial chemicals that can work alone or with antibiotics [80]. Likewise, to other medicinal plants, C. arvense aerial parts contain antibacterial compounds like hispidulin, luteolin, and tracin, which might help manage antibiotic resistance. Additionally, acacetin, apigenin, and citronellol are the active constituents observed in C. arvense, have already been reported in the literature as antimicrobials [81–83]. Therefore, these compounds from C. arvense, alone or in combination with antibiotics, can manage drug resistance by inhibiting hyperactivity of the efflux pump, drug-inactivating enzymes, cell wall protein alteration, DNA, RNA, and protein synthesis.
Toxicity study
The ethanol extract of aerial parts of C. arvense showed toxicity against brine shrimp (Artemia salina) with LC50 51 μg/ml in comparison to standard vincristine sulfate (LC50 0.44 μg/ml) [71]. More in vitro, in vivo, and clinical studies are required to assess the toxicity of this weed, as it is critical to focus research on the plant’s safety and efficacy to use it adequately.
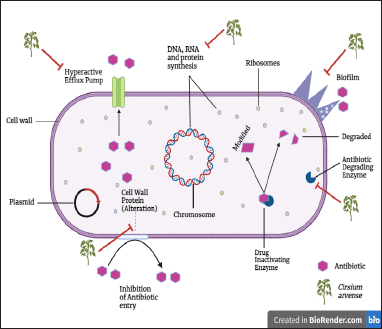 | Figure 4. Mechanistic basis of antibacterial action of C. arvense (Created using Biorender.com). [Click here to view] |
BIOPROSPECTING OF C. ARVENSE
Cirsium arvense is a widespread weed, but its potential for bioprospecting was not explicitly addressed. Despite being seen as an invasive plant in agricultural fields, C. arvense extracts have strong antioxidant properties, making them a viable source of antioxidants [67]. Its antimicrobial activity has also been investigated; tracin, hispidulin, and luteolin have antibacterial and antifungal effects [58]. Iranian C. arvense extracts displayed antibacterial efficacy against various bacterial strains [40]. Cirsium arvense was employed to generate silver nanoparticles with a high biological value and better E. coli inhibition activity [84]. Diverse phytoconstituents were responsible for the synthesis and biological activity of plant-mediated nanoparticles, as evidenced by several reports [85–89]. Therefore, C. arvense’s varied phytocomposition can be used in the future. However, in Tasmania, C. arvense root and foliage extracts prevented the germination and growth of several plant species, which may make it difficult for pasture and crop species to establish in C. arvense-infested environments [90]. Although its weeding potential may restrict its uses, but the biological potential of this opens up a new avenue for bioprospecting.
CONCLUSION AND FUTURE PERSPECTIVES
Cirsium arvense is a globally distributed weed that grows in various habitats. Ethnomedicinally, the plant is employed against gastrointestinal ailments, hypertension, bleeding, metrorrhagia, scabies, pyogenic infections, ulcers, and skin infections. Kaempferol-3-O-β-D-glucopyranoside, quercetin-3-O-β-D-glucopyranoside, hispidulin-7-O-β-D-glucopyranoside, luteolin-5-O-β-D-glucopyranoside, caffeic acid, chlorogenic acid, enicin, rutin, stigmasterol, and acacetin represent diverse phytocomposition of this weed. The current review study highlighted antioxidant, antibacterial, and antiproliferative activities. The major limitation of the antimicrobial studies is that researchers did not reported MIC, as IZD evaluation is only a preliminary study. Cirsium arvense extracts’ antiproliferative ability against HeLa, A43, and MCF7 cell lines was evaluated, but vast research is still necessary. Although C. arvense has been utilized in various ethnomedicines, its pharmacological potential has yet to be thoroughly investigated, especially its toxicity (LC50 51 μg/ml).
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
This research was supported by the Ministry of Ayush, Government of India, under Ayurswasthya Yojana.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Kumari A, Verma R, Sharma M, Chauhan P, Kumar A. Evaluation of phytochemical, antioxidant, antibacterial and anti-cancerous activity of Ficus auriculata Lour. and Osyris wightiana Wall. ex Wight. Bull Environ Pharmacol Life Sci. 2018;7:64–70.
2. Balkrishna A, Juyal R, Devi R, Kumar J, Prakash A, Pathak P, et al. Ethnomedicinal status and pharmacological profile of some important orchids of Uttarakhand (North Western Himalayas), India. J Orchid Soc India. 2020;34:137–47.
3. Sharma S, Kumari A, Dhatwalia J, Guleria I, Lal S, Upadhyay N, et al. Effect of solvents extraction on phytochemical profile and biological activities of two Ocimum species: a comparative study. J Appl Res Med Aromat Plants. 2021;25:100348. CrossRef
4. Dhatwalia J, Kumari A, Verma R, Upadhyay N, Guleria I, Lal S, et al. Phytochemistry, pharmacology, and nutraceutical profile of Carissa species: an updated review. Molecules. 2021;26(22):1–34. CrossRef
5. Sonam KA, Kumar V, Guleria I, Sharma M, Kumar A, Alruways MW, et al. Antimicrobial potential and chemical profiling of leaves essential oil of Mentha species growing under North-West Himalaya conditions. J Pure Appl Microbiol. 2021;15(4):2229–43. CrossRef
6. Sharma K, Verma R, Kumar D, Nepovimova E, Kuca K, Kumar A, et al. Ethnomedicinal plants used for the treatment of neurodegenerative diseases in Himachal Pradesh, India in Western Himalaya. J Ethnopharmacol. 2022;293:115318. CrossRef
7. Harborne JB, Turner BL. Plant chemosystematics. London, UK: Academic Press; 1984.
8. Plants of the World Online. 2023.
9. Sharma M, Sharma M, Bithel N, Sharma M. Ethnobotany, phytochemistry, pharmacology and nutritional potential of medicinal plants from Asteraceae family. J Mountain Res. 2022;17(2):67–83. CrossRef
10. Flora of China. 2023.
11. Bureš P, Wang YF, Horová L, Suda J. Genome size variation in central European species of Cirsium (Compositae) and their natural hybrids. Ann Bot. 2004;94:353–63. CrossRef
12. Kadota Y. Species diversification of genus Cirsium (Asteraceae) in Japan. Korean J Plant Taxon. 2007;37:335–49. CrossRef
13. Dang S. Plants profile for Cirsium vulgare USDA. Plant Database. 1984.
14. Lee WB, Kwon HC, Cho OR, Lee KC. Phytochemical constituents of Cirsium setidens Nakai and their cytotoxicity against human cancer cell lines. Arch Pharm Res. 2002;25(5):628–35. CrossRef
15. Won BL, Hak CK, Ock RC, Kang CL, Sang UC, Nam IB, et al. The constituents of Cirsium japonicum D.C. var. takaoense. Kitamura. Isolation of two new flavonoids cirsitakaoside (IV) and cirsitakaogenin (VI). Chem Pharm Bull. 1978;26:2036–9. CrossRef
16. Jolanta N, Jan G. Flavonoid compounds from the flowers of Cirsium rivulare (JACQ) all. Acta Pol Pharm. 2003;60(1):87–9.
17. Luo W, Wu B, Tang L, Li G, Chen H, Yin X. Recent research progress of Cirsium medicinal plants in China. J Ethnopharmacol. 2021;280:114475. CrossRef
18. Shahrajabian MH. Spear thistle (Cirsium vulgare L.) and ramsons (Allium ursinum L.), impressive health benefits and high-nutrient medicinal plants. Pharmacogn Commun. 2021;11:168–71. CrossRef
19. Flora of North America. 2023.
20. Tiley GE. Biological flora of the British Isles: Cirsium arvense (L.) scop. J Ecol. 2010;98(4):938–83. CrossRef
21. Khan A, Amin A, Khan MA, Ali I. In vitro screening of Circium arvense for potential antibacterial and antifungal activities. Pak J Pharm Sci. 2011;24(4):519–22.
22. Travlos I, Roussis IE, Karampasis N, Tabaxi I, Papadimitriou D, Katsenios N, et al. Thistles of Greece and their potential value as medicinal crops: study on their first growth. Bull UASVM Hortic. 2016;73:1–2. CrossRef
23. Moerman DE. Native American medicinal plants: an ethnobotanical dictionary. Portland, OR: Timber Press; 2009.
24. Vardhana R. Medicinal and the economic plants. New Delhi, India: Shree Publishers and Distributors, vol. 3; 2013.
25. Khan ZUH, Ali F, Khan SU, Ali I. Phytochemical study on the constituents from Cirsium arvense. Mediter J Chem. 2011;1(2):64–9. CrossRef
26. Orhan C, Sahin N, Akdemir F, Markiewicz-Zukowska R, Borawska MH, Isidorov VA, et al. The effect of Cirsium arvense extract on antioxidant status in Quail. Br Poultry Sci. 2013;54(5):620–6. CrossRef
27. Hossain ML, Monjur-Al-Hossain ASM, Sadhu SK. HPLC profiling and evaluation of in vitro antioxidant activity of Cirsium arvense L. (Family: Asteraceae). J Pharmacogn Phytochem. 2016;5(1):272–7.
28. Favrelière E, Ronceux A, Pernel J, Meynard JM. Nonchemical control of a perennial weed, Cirsium arvense, in arable cropping systems. A review. Agron Sustain Dev. 2020;40(4):1–17. CrossRef
29. Aggarwal G, Kaur G, Bhardwaj G, Mutreja V, Sohal HS, Nayik GA, et al. Traditional uses, phytochemical composition, pharmacological properties, and the biodiscovery potential of the genus Cirsium. Chemistry. 2022;4(4):1161–92. CrossRef
30. Mitra S, Mukherjee SK. Flora and ethnobotany of West Dinajpur District, West Bengal. Dehradun, India: Bishen Singh Mahendra Pal Singh; 2013.
31. Efloras: Flora of China. Cirsium arvense. 2023.
32. Chhikara A, Rohilla P, Singh L, Kumar D, Antil R, Dahiya P. Cirsium arvense: a multi-potent weed. Ann Biol. 2020;36(3):442–7.
33. Weiner MA. Earth medicine-earth food: plant remedies, drugs, and natural foods of the North American Indians. New York, NY: Ballantine Books; 1980.
34. Uddin SN, Rahman MM. Traditional uses of ethnomedicinal plants of Chittagong hill tracts. Dhaka, Bangladesh: Bangladesh National Herbarium; 2006.
35. Duke JA, Ayensu ES. Medicinal plants of China. Michigan, China: Reference Publication, vol. 1; 1985.
36. Li X, Wang W. Chinese materia medica: combinations & applications. Hertfordshire, UK: Donica Publishing; 2002.
37. Agarwal VS. Directory of Indian economic plants. Dehradun, India: Bishen Singh Mahendra Pal Singh; 2003.
38. Wu J. An illustrated Chinese material medica. New York, NY: Oxford University Press; 2005.
39. Liu Z, Liu L. Essentials of Chinese medicine. London, UK: Springer, vol. 2. 2009.
40. Dehjurian A, Lari J, Motavalizadehkakhky A. Anti-bacterial activity of extract and the chemical composition of essential oils in Cirsium arvense from Iran. J Essent Oil-Bear Plants. 2017;20(4):1162–6. CrossRef
41. David W. Herbalpedia: the ultimate herbal encyclopedia. Silver Spring, PA: Silver Springer; 2013.
42. Singh R, Upadhyay SK, Rani A, Kumar P, Sharma P, Sharma I, et al. Ethnobotanical study of weed flora at district Ambala, Haryana, India: comprehensive medicinal and pharmacological aspects of plant resources. Int J Pharm Res. 2020;12(1):1941–56. CrossRef
43. Coffey T. The history and folklore of North American wildflowers. Boston, NY: Houghton Mifflin; 1993.
44. Foster S, Duke JA. Peterson field guide to medicinal plants and herbs of Eastern/Central North America, 2nd ed. Boston, NY: Houghton Mifflin; 2000.
45. Gupta AK, Tandon N (Eds.). Reviews on Indian medicinal plants. New Delhi, India: Indian Council of Medical Research, vol. 6; 2004.
46. Sood SK, Chandel R, Sharma S, Kumar S. Unusual folk plants and drugs of India. Jaipur, India: Aavishkar Publishers; 2014.
47. Baral SR, Kurmi PP. A compendium of medicinal plants in Nepal. Kathmandu, Nepal: Mass Printing Press; 2006.
48. Sood SK, Kumari P, Thakur R, Bassi SK, Thakur A. Herbal medicine. Jaipur, India: Pointer Publishers; 2015.
49. Nuzzo V. Element stewardship abstract for Cirsium arvense. Arlington, VA: The Nature Conservancy; 1997.
50. Gupta VK. Traditional and folk herbal medicine: recent researches. New Delhi, India: Daya Publishing House; 2012.
51. Günbatan T, Gürbüz I, Özkan AMG. The current status of ethnopharmacobotanical knowledge in Çamlidere (Ankara, Turkey). Turk J Bot. 2016;40:241–9. CrossRef
52. Kaul MK. Medicinal plants of Kashmir & Ladakh, temperate & cold Arid Himalaya. New Delhi, India: Indus Publishing Company; 1997.
53. Rai Y. A color handbook of flowering & medicinal plants. New Delhi, India: Biotech Books; 2015.
54. Shah R. Edible plants of North West Himalaya (Uttarakhand). Dehradun, India: Bishen Singh Mahendra pal Singh; 2015.
55. Chauhan PP. Ethnobotanical studies of wild edible plants used by ethnic people in Pabbar valley, District Shimla, Himachal Pradesh. Worldwide Int J Multidiscip Res. 2022;8(04):5–10. CrossRef
56. Akhtar N, Mirza, B. Phytochemical analysis and comprehensive evaluation of antimicrobial and antioxidant properties of 61 medicinal plant species. Arabian J Chem. 2018;11:1223–35. CrossRef
57. Popova YV. The pharmacognostic study Cirsium arvense (L.) Scop. and C. vulgare (Savi) Ten. Flora of Ukraine [Doctoral Thesis]. Zaporizhia, Ukraine: Zaporizhzhia State Medical University; 2020.
58. Khan ZUH, Khan S, Chen Y, Wan P. In vitro antimicrobial activity of the chemical constituents of Cirsium arvense (L). Scop. J Med Plant Res. 2013;7(25):1894–8. CrossRef
59. Ashmita P, Singh L, Kumar D, Antil R, Dahiya P. Cirsium arvense: a multi-potent weed. Ann Biol. 2020;36:442–7.
60. Asolkar LV, Kakkar KK, Chakre O. Second supplement to, glossary of Indian medicinal plants with active principles (Part 1). New Delhi, India: National Institute of Science Communication (CSIR); 1992.
61. Nadkarni KM. Indian materia medica, 3rd ed. Mumbai, India: Popular Prakashan, vol. 1; 1996.
62. Li TSC. Chinese and related North American: herbs, phyto-pharmacology and therapeutic values, 2nd ed. Boca Raton, FL: CRC Press; 2009.
63. Ferdosi MF, Khan IH, Javaid A, Fardosi MF. GC-MS examination of methanolic extract of Cirsium arvense flower. Pak J Weed Sci Res. 2021;27(2):173–80.
64. Banaras S, Javaid A, Shoaib A, Ahmed E. Antifungal activity of Cirsium arvense extracts against phytopathogenic fungus Macrophomina phaseolina. Planta Daninha. 2017;35:1–10. CrossRef
65. Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30(1):11–26. CrossRef
66. Gulcin I. Antioxidants and antioxidant methods: an updated overview. Arch Toxicol. 2020;94(3):651–715. CrossRef
67. Demirtas I, Tufekci AR, Yaglioglu AS, Elmastas M. Studies on the antioxidant and antiproliferative potentials of Cirsium arvense subsp. vestitum. J Food Biochem. 2017;41(1):e12299. CrossRef
68. Nazaruk J, Czechowska SK, Markiewicz R, Borawska MH. Polyphenolic compounds and in vitro antimicrobial and antioxidant activity of aqueous extracts from leaves of some Cirsium species. Nat Prod Res. 2008;22(18):1583–8. CrossRef
69. Nazaruk J. Antioxidant activity and total phenolic content in Cirsium five species from North-East region of Poland. Fitoterapia. 2008;79(3):194–6. CrossRef
70. Csupor-Löffler B, Hajdú Z, Réthy B, Zupkó I, Máthé I, Rédei T, et al. Antiproliferative activity of Hungarian Asteraceae species against human cancer cell lines. Part II. Phytother Res. 2009;23(8):109–15. CrossRef
71. Hossain ML, Monjur-Al-Hossain ASM, Saha S, Sadhu SK. Assessment of biological activity on Cirsium arvense L. Algerian J Nat Prod. 2017;5:417–26. CrossRef
72. WHO. Antimicrobial resistance. Geneva, Switzerland: WHO; 2023.
73. Balkrishna A, Rohela A, Kumar A, Kumar A, Arya V, Thakur P, et al. Mechanistic insight into antimicrobial and antioxidant potential of Jasminum species: a herbal approach for disease management. Plants. 2021;10(6):1–25. CrossRef
74. Sekyere JO, Asante J. Emerging mechanisms of antimicrobial resistance in bacteria and fungi: advances in the era of genomics. Future Microbiol. 2018;13(2):241–62. CrossRef
75. Walsh C. Molecular mechanisms that confer antibacterial drug resistance. Nature. 2000;406(6797):775–81. CrossRef
76. Tenover FC. Mechanisms of antimicrobial resistance in bacteria. Am J Infect Control. 2006;119(6):S3–10. CrossRef
77. Khameneh B, Diab R, Ghazvini K, Bazzaz, BSF. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb Pathog. 2016;95:32–42. CrossRef
78. Kongkham B, Prabakaran D, Puttaswamy H. Opportunities and challenges in managing antibiotic resistance in bacteria using plant secondary metabolites. Fitoterapia. 2020;147:104762. CrossRef
79. Levy SB. The challenge of antibiotic resistance. Sci Am. 1998;278(3):46–53. CrossRef
80. Eldin AB, Ezzat M, Afifi M, Sabry O, Caprioli G. Herbal medicine: the magic way crouching microbial resistance. Nat Prod Res. 2023; 37: 1–10. CrossRef
81. Nayaka HB, Londonkar RL, Umesh MK, Tukappa A. Antibacterial attributes of apigenin, isolated from Portulaca oleracea L. Int J Bacteriol. 2014;2014:175851. CrossRef
82. Lopez-Romero JC, González-Ríos H, Borges A, Simões M. Antibacterial effects and mode of action of selected essential oils components against Escherichia coli and Staphylococcus aureus. Evid Based Complement Alternat Med. 2015;2015:795435. CrossRef
83. Sadiq MB, Tarning J, Aye Cho TZ, Anal AK. Antibacterial activities and possible modes of action of Acacia nilotica (L.) Del. against multidrug-resistant Escherichia coli and Salmonella. Molecules. 2017;22(1):47. CrossRef
84. Barbinta-Patrascu ME, Ungureanu C, Besliu D, Lazea-Stoyanova A, Iosif, L. Bio-active nanomaterials phyto-generated from weed herb Cirsium arvense. Optoelectron Adv Mater Rapid Commun. 2020;14(9-10):459–65.
85. Balkrishna A, Kumar A, Arya V, Rohela A, Verma R, Nepovimova E, et al. Phytoantioxidant functionalized nanoparticles: a green approach to combat nanoparticle-induced oxidative stress. Oxid Med Cell Longev. 2021;2021:1–20. CrossRef
86. Khatana C, Kumar A, Alruways MW, Khan N, Thakur N, Kumar D, et al. Antibacterial potential of zinc oxide nanoparticles synthesized using Aloe vera (L.) Burm. f.: a green approach to combat drug resistance. J Pure Appl Microbiol. 2021;15(4):1907–14. CrossRef
87. Dhatwalia J, Kumari A, Chauhan A, Mansi K, Thakur S, Saini RV, et al. Rubus ellipticus sm. Fruit extract mediated zinc oxide nanoparticles: a green approach for dye degradation and biomedical applications. Materials. 2022;15(10):3470. CrossRef
88. Thakur N, Thakur N, Chauhan P, Kumar K, Jeet K, Kumar A, et al. Futuristic role of nanoparticles for treatment of COVID-19. Biomater Polym Horiz. 2022;1(2):1–22. CrossRef
89. Thakur N, Thakur N, Kumar K, Kumar A. Tinospora cordifolia mediated eco-friendly synthesis of cobalt doped TiO2 NPs for degradation of organic methylene blue dye. Mater Today: Proc. 2023. CrossRef
90. All GMB. The allelopathic activity of californian thistle (Cirsium arvense (L.) Scop.) in Tasmania. Weed Res. 1975;15(2):77–81. CrossRef