INTRODUCTION
Protein drugs have several advantages over chemical drug molecules as they have high bioactivity, specificity, good solubility, high safety profile, and lower side effects. They majorly treat autoimmune disorders, cancer, and cardiovascular disorders. However, they are used mainly by a parenteral route, limiting their therapeutic application to a certain extent [1–4]. Many noninvasive routes were developed; among them, the oral route is preferred due to its ease of administration and high patient compliance [5]. However, oral administration of peptides is very challenging due to their poor permeability and rapid degradation by gastrointestinal (GI) peptidases [6]. They have short circulating half-lives as they undergo rapid systemic clearance and are also rapidly degraded by proteases and peptidases, poorly absorbed by intestinal mucosal membranes on oral administration [7]. Enzymes and the proteases in blood plasma can break down peptides into inactive metabolites, leading to shorter half-lives of 2–30 minutes [8]. Also, if the peptides are highly hydrophilic and size below 60 kDa, they will be filtered by the kidneys within minutes from the circulation [9]. It has been observed that nanotechnology can be used as a comprehensive tool for the delivery of peptide molecules [2,10–12]. Several studies have reported that herbal compounds, such as piperine, quercetin, ginger, naringin, genistein, glycyrrhizin, and sinomenin, can improve the bioavailability of drug molecules with nanocarriers. Among them, the bioavailability enhancement by piperine was well established [13,14].
It has been reported that piperine has multiple activities. Research recently explained the binding behavior of piperine with myoglobin (a protein that carries oxygen in muscle cells) using computational approaches. The study observed that the myoglobin–piperine complex’s antioxidant property depended on piperine concentration. With an increase in piperine concentration, there was an increase in free iron release from myoglobin. The study could explain the structural changes in a protein molecule after binding with piperine, leading to changes in its antioxidant activity. Piperine acted as a ligand, and the protein myoglobin was a receptor. Piperine–myoglobin complex exerted more antioxidant activity when compared to free piperine [15]. Nowadays, molecular dynamics (MD) simulations are widely used as they help to understand the interactions between proteins and other compounds. MD simulations can predict changes in protein secondary structure, stability, and binding energy due to the complex dynamic processes in protein systems. The technique can be used in drug design to simulate various experimental conditions, such as pH, temperature, and solvent changes. Applying heat, high pressure, and physical and chemical treatments during formulation can lead to protein denaturation. MD simulation can predict the effect of behavior and structural changes of protein during processing and their effect on protein functional characteristics [16]. Proteins can also be used as carriers as they are biodegradable and sustainable. Soybean protein isolate and soybean soluble polysaccharide were used as vehicles for hyperoside administration. Hyperoside achieved a high encapsulation efficiency of 85.56% by retaining its antioxidant activity. Soybean protein isolate tends to aggregate in acidic conditions, which was prevented by adding anionic polysaccharide-soybean soluble polysaccharide. Incorporating soluble soybean polysaccharides inhibited the aggregation of soybean protein isolate in acidic conditions [17]. This concept is beneficial for delivering life-saving drugs, such as insulin orally, encapsulated in nanocarriers and coated with an amino polysaccharide such as chitosan.
Docking studies are not only useful to understand the binding of piperine with peptides, but also can be used for certain disease diagnosis. Molecular docking analysis of piperine with cell cycle proteins, such as CDK2, CDK4, Cyclin D, and Cyclin T, suggested the optimal binding of piperine with these proteins [18]. Another study reported the interaction of apoptotic signaling proteins such as Bax, Caspase 3, Cox2, and Caspase 9 with piperine. These proteins are associated with colo rectal cancer; this interaction will be useful in considering piperine as a potential candidate in colon cancer research [19].
Piperine was first isolated from the fruits of Piper nigrum from black and white pepper grains by Hans Christian Orsted in 1819 [20–22]. It is considered the world’s first bioenhancer, which can enhance the bioavailability of drugs by 30%–200% [23,24]. Bose first reported an enhanced anti-asthmatic effect of AdhatodaVasicaon coadministration with piperine in the 1920s [25]. Piperine is an alkaloid belonging to the family Piperaceae present in Piper longum and P. nigrum with many medicinal values [26]. Piperine is hypothesized to exert its bioenhancing activity through various mechanisms such as DNA receptor binding, regulating cell signal transduction, inhibiting drug efflux pump, stimulating gut amino acid transporters, and drug absorption. Piperine also inhibits the production of glucuronic acid, human P-glycoprotein (P-gp), and cytochrome P450 3A4(CYP450 3A4), thereby preventing the first-pass elimination of many drugs. CYP1A1, CYP1B1, CYP1B2, CYP2E1, and CYP3A4 are some drug-metabolizing enzymes inhibited by piperine. Other suggested mechanisms for the bioenhancing action of piperine are making drug target receptors more responsive, acting as receptors for drug molecules, increasing drug absorption by increased vasodilation, and increasing drug transport by modulating cell membranes [21,27–32]. 10% concentration of piperine can act as a bioenhancer, but its dose varies with different drugs [33]. Risorine is a fixed-dose combination developed by the Indian Institute of Integrative Medicine (Jammu) in 2009 with Cadila Pharmaceuticals. Risorine contains 200 mg rifampicin, 300 mg isoniazid, and 10 mg piperine. Piperine reduces the dose of rifampicin from 450 to 200 mg, enhancing its bioavailability by 60% [34]. A combination of black pepper, long pepper, and ginger, collectively known as “Trikatu,” was used in most Ayurvedic formulations. “Trikatu” in Sanskrit means three acrids; the presence of piperine made “Trikatu” more effective in treating diseases [35–40].
MECHANISMS INVOLVED IN THE ORAL ABSORPTION OF PROTEIN DRUGS
After oral administration, peptide drug molecules must cross numerous enzymatic, mucosal, and epithelial barriers to reach the systemic circulation. Lipid-based nanocarriers are one of the most promising drug delivery systems for oral peptide administration. They can interact with cell surfaces through endocytosis, transcytosis, fusion with the cell membrane, and overcoming the epithelial barrier [41]. Combining nanotechnology with bioenhancers can improve solubility, prevent drug degradation in different physiological pH conditions, and improve bioavailability. The combination can also result in sustained drug release with drug targeting and reduced dosage and toxicity.
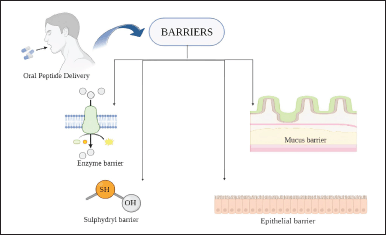
Barriers to the delivery of oral protein and peptide drugs
Enzymatic barrier
Various substrate-specific enzymes in the digestive cavity can create a vast enzymatic barrier hindering the absorption of protein and peptide drugs. Pepsin is the major enzyme in the stomach, causing enzymatic cleavage of proteins and peptides. Endopeptidases, for example, trypsin, chymotrypsin, elastase, and exopeptidases carboxypeptidase A and B, can rapidly degrade protein drugs. For example, insulin was completely degraded by trypsin, α chymotrypsin, and elastase within 1 hour. Other enzymes that can degrade peptides are membrane-bound enzymes in the brush border membrane. Cytosolic enterocyte enzymes, such as lysosomes, also cause protein degradation [42–44].
Sulfhydryl barrier
Inactive conjugates will be formed by peptide drugs possessing thiol or disulfide substructures in the GI tract by thiol/disulfide exchange reactions. Endogenous thiols, e.g., glutathione, dietary proteins, and mucus glycoproteins, will be barriers. Casein peptones degraded lanreotide in 2 hours, and desmopressin formed three different disulfide conjugates with glutathione under physiological conditions [41,45–47].
Mucus barrier
The mucus, secreted by goblet cells, composed of mucin, enzymes, electrolytes, and water, protects the intestine by acting as a lubricant. Mucus can prevent pathogens and toxins and restrict drug diffusion and absorption, resulting in low bioavailability. The thickness of the mucus layer in GI epithelia varies in different regions. The thickest mucus layers are in the stomach (800 μm) and colon (110–160 μm), forming a 3-D network of mucus glycoproteins. Ionic interactions, hydrogen bonding, and hydrophobic interactions will limit the permeation of peptide molecules through the mucus layer. The high viscosity of the mucus layer can reduce the peptide diffusivity and residence time. The mucus in Peyer’s patches allows nanoparticle permeation to blood circulation, which is the main absorption route for oral nanoparticles. Peptides with a molecular weight greater than 6.5 kDa can permeate the mucus gel layer to a certain extent, whereas polypeptides with a molecular mass greater than 12.4 kDa are restricted. Moreover, the continuous secretion and replacement of mucus make it extensively challenging for the peptide molecules to pass this unstirred layer to enter the intestinal epithelium [2,5,48,49].
Epithelial barrier
Peptide drugs must cross a monolayer of intestinal epithelial barrier to enter the systemic circulation, which is challenging. Intestinal epithelial cells and Microfold cells (M cells) are the primary transmembrane transport cells in the GI tract. The drug will be absorbed through the cross-cell channel and undertake passive diffusion, carrier-mediated, or vesicle transport. The permeability of drugs decreases when the size is greater than 1 kDa and, hence, is size-dependent. As peptides are hydrophilic, they can not easily pass through the lipid bilayers of the epithelium. Systemic uptake is possible only after the opening of tight junctions between the cells, which is possible with the help of specific permeation enhancers. Permeation enhancers can improve drug transport by transcellular (fluidizing the cell membranes) or paracellular (tight junction rearrangement) pathways. The effect of phenyl piperazine (PPZ) and sodium deoxycholate (SDC) in enhancing the intestinal permeation of different molecular weight dextrans (4, 10, 40, and 70 kDa) was investigated in mice and Caco-2 monolayers [50]. Both effectively improved dextran’s permeability depending on its molecular weight, i.e., they could improve the permeation of dextrans based on their size. PPZ and SDC could enhance the permeation of 4 and 10 kDa dextrans in in vitro studies, whereas in in vivo studies, SDC improved the permeation of all four dextrans, and PPZ showed the same effect [50–53] (Fig. 1). Chitosan microparticles were formulated to deliver the macromolecular compound fluorescein isothiocyanate dextran 4,400 with piperine as a bioenhancer by intranasal route. Microparticles with piperine showed a 1.2-fold increase in macromolecular delivery across nasal epithelia compared to microparticles without piperine [54]. This approach can be a promising alternative for noninvasive macromolecular delivery across nasal epithelia.
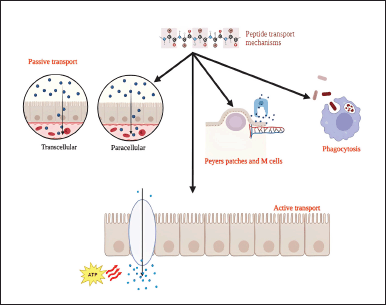
DRUG TRANSPORT MECHANISMS
Commonly observed drug transport mechanisms for peptide drugs are active and passive transport. Protein and peptide drug molecules in nanoparticles are postulated to pass through the GI tract in four ways—transmembrane transport, receptor-mediated transport, carrier-mediated transport, and M-cell transport (Fig. 2).
Active transport involves Adenosine triphosphate (ATP) usage, where drug molecules move from lower to higher concentrations against the gradient by transmembrane proteins. It is also known as facilitated diffusion or carrier-mediated transport. This mechanism was mainly involved in the intestinal absorption of di and tri peptides with the help of a carrier. Newey and Smyth [55], in 1959, first reported the presence of a peptide transport system in the mammalian gut. They also absorbed amino β lactam antibiotics, renin, and angiotensin-converting enzyme inhibitors [38].
Passive transport in which Fick’s law of diffusion governs the drug transfer rate. Here, the drug molecules diffuse in the same direction of concentration gradient without energy expenditure. Passive diffusion is a combination of two processes: paracellular and transcellular transport. Paracellular transport uses water-filled pores or channels for the transport of drug molecules. This route is preferred for transporting low molecular weight (size lower than 500 Da), hydrophilic, small peptide fragments. Diffusion through the paracellular route depends on molecular dimension, overall ionic charge, and physicochemical properties and, hence, is limited. Most therapeutic peptides possess molecular weight beyond 700 Da and cannot cross the tight epithelial junctions, resulting in low bioavailability. Modification of either the drug molecule or the tight junction associated with the paracellular pathway may increase the permeation of macromolecules [56–59]. Transcellular transport is good for lipophilic drugs with a relatively higher affinity for the lipophilic lipid membrane. Drug molecules will permeate through apical and basolateral membranes, and hence, lipophilicity is the key component to a certain extent. It is also observed that the number of polar groups and the energy required to break water-peptide hydrogen bonds will improve drug transport through this route [60].
 | Figure 1. Barriers in oral peptide delivery. [Click here to view] |
 | Figure 2. Different drug transport mechanisms. [Click here to view] |
It is also reported that peptide drugs can be absorbed through gut-associated lymphoid tissue (GALT). GALT consists of Peyer’s patches with specialized epithelial cells known as M cells that can improve the efficacy of orally administered peptides. M cells are a part of the mucosal immune system with the potential to transport bacteria, viruses, and antigens. The follicle-associated epithelium of Peyer’s patches has a limited number of goblet cells and very little mucus, potentiating particle uptake by M cells. M cells permit the transcytose of particles without passing through lysosomes, thereby protecting them from degradation. Even though less than 1% of the intestine is occupied by M cells, specific M cell targeting can help overcome the limitations of its low availability for efficient oral drug delivery [49,61].
There is much literature supporting the uptake of nanoparticulate formulations by the GI tract. Nanocarriers play a unique role in delivering protein and peptide drugs as they can permeate mucosal barriers by protecting the encapsulated drug [62,63]. Once the nanoparticles are taken up by M cells, the drug will be transported to capillary vessels, then to portal circulation and reach the liver, as such or as a free drug. Before reaching the systemic circulation, the drug undergoes first-pass hepatic metabolism by various liver enzymes. This pattern of drug transport was observed for insulin-loaded nanoparticles after oral administration, behaving similarly to physiologic insulin. Another transport mechanism followed was nanoparticles carried to blood vessels by the mesenteric lymph nodes and finally entering the systemic circulation by the thoracic duct. This transport mechanism will show actions similar to subcutaneously injected insulin [64–66].
Phagocytic mechanism and other mechanisms
Intestinal epithelial cells and M cells are the essential transmembrane transporters in the GI tract. However, the nanoparticle uptake is significantly higher from M cells than from intestinal epithelial cells. Peptide drug molecules selectively adhere to corresponding glycoproteins and get absorbed by M cells. Lymphocytes, dendritic cells, and phagocytes are gathered on the lateral basal surface of the cell membrane, which will shorten the distance for the drug particles to reach the systemic circulation [67–69].
Receptor or carrier-mediated pathways have the benefits of high efficiency and selectivity. Receptor-mediated endocytosis is focused on the receptor type with no emphasis on drug size, where the drug binds to corresponding ligands through receptors on the membrane and may undergo phagocytosis. The high efficiency of receptor binding arises due to the ability of cytokines to bind strongly to specific receptors on target cells. Receptor selectivity is the extent to which a receptor can bind with a specific drug than other molecules. Carrier-mediated transport is mainly observed in the case of small molecules such as oligopeptides, monosaccharides, angiotensin-converting enzymes, and amino acids. Drug permeability can be improved by ligand modification in the polypeptide drug structure and binding with receptors in intestinal cell membranes [2,70].
BINDING OF PROTEIN DRUGS TO BLOOD COMPONENTS
Protein and peptide drugs are highly susceptible to serum and tissue proteases, resulting in short half-lives due to rapid clearance and low bioavailability. Plasma protein binding can be considered as an approach to improve the pharmacokinetic properties of drug molecules. It was identified that a series of peptides with core sequence DICLPRWGCLW would bind with serum albumin with high affinity. Albumin is the major plasma protein with a half-life of 19 days in humans. Therefore, a combination of rapidly cleared molecules with albumin can prolong their actions in the body. When linked with albumin by acylation with fatty acids, insulin was found to have a prolonged effect in rabbits when injected subcutaneously [71]. Zorzi et al. [72] developed a “piggyback” strategy in which a ligand bound peptides with albumin. They have used an easily synthesized albumin-binding ligand with a high affinity for human albumin based on a peptide-fatty acid chimera. The approach increased the elimination half-life of cyclic peptides over 7 hours. In anti-thrombotic therapy, conjugation to peptide factor XII inhibitor prolonged the half-life of 5 hours from 13 minutes.
HEPATIC/RENAL METABOLISM OF PEPTIDE DRUGS
Most peptide drugs undergo first-pass metabolism in the liver before reaching the systemic circulation, resulting in low systemic bioavailability. Peptide drugs have short half-lives and will be easily excreted and, hence, are preferred to be administered by parenteral routes or by chemical modifications [73]. The CYP450 mixed function oxidase reaction catalyzing hydroxylation is a common drug metabolism reaction in the liver and kidney. Renal metabolism and elimination of protein and peptide drugs involve glomerular filtration, hydrolysis, conjugation, reabsorption, and peritubular extraction [74]. Peptides undergo high first-pass extraction with low absorption mainly due to enzymatic and pH-mediated hydrolysis in the liver and Gastro intestinal tract (GIT) [75].
In rats, the first-pass hepatic metabolism of peptide drugs after oral administration was investigated using metkephamid and thyrotropin-releasing hormone (TRH) as the model peptides. A single liver perfusion technique was used to mimic in vivo liver metabolism. It was observed that adding bovine serum albumin (BSA) 3% w/v to rat liver perfusate could prevent the hepatic metabolism of metkephamid. After intestinal absorption, free metkephamid was rapidly hydrolyzed by hepatocytes. Only 40% of metkephamid was recovered in protein-free conditions and enhanced to 70%–75% in the presence of BSA. In the case of TRH, it was not found bound to BSA. During a single pass liver perfusion, TRH was not significantly degraded. Therefore, the liver is not the primary site for TRH degradation, and first-pass metabolism does not affect TRH like metkephamid. The fraction of TRH metabolized by the liver was less than 10%, which indicates 90% of TRH remained as such [76]. Leuprorelin, cyclosporin, cetrorelix, and desmopressin were incubated in human liver-derived incubation systems: liver S9 fraction, human primary hepatocytes, and upcyte hepatocytes. S9 fraction formed the highest metabolites for leuprorelin and cetrorelix; primary hepatocytes and liver S9 produced similar metabolite profiles for desmopressin and cyclosporin. Metabolism of cyclosporin, leuprorelin, and cetrorelix was CYP (NADPH) dependent in liver S9 [77].
EFFECT OF PIPERINE ON PROTEIN DRUG ABSORPTION AND METABOLIC PATHWAYS
Effect of piperine in GI permeation of drugs
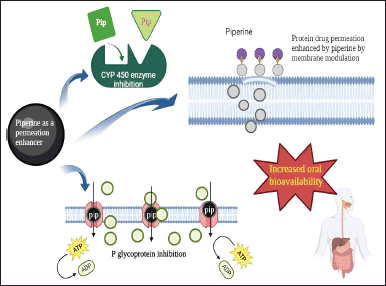
Piperine is a well-established bioenhancer for many drugs. It is hypothesized that piperine can increase drug absorption by altering the lipid membrane dynamics. Due to the apolar nature of piperine, it can easily partition the lipid core and assist the permeation of the molecules attached to it. Khajuria et al. [78] studied the effect of piperine on the membrane fluidity of the intestinal brush border membrane. Membrane fluidity studies were performed using an apolar fluorescence probe, pyrene. Pyrene measured the fluid properties of the hydrocarbon core and confirmed an increase in intestinal brush border membrane fluidity. The excimer/monomer ratio of pyrene evaluated membrane fluidity changes. The study also observed that piperine altered enzyme kinetics by stimulating leucine amino peptidase and glycyl-glycine dipeptidase. Piperine enhanced the fluidity of the brush border membrane at 5, 10, and 20 mg/kg body weight after 5–15 minutes from administration by reducing the ability of membrane lipids to act as stearic constraints and modifying the enzyme conformation. Thus, we can conclude that piperine altered membrane dynamics and permeation characteristics, induced protein synthesis, and enhanced intestinal absorptive surface. Membrane fluidity enhancement by piperine can improve the permeability characteristics by increasing microvilli length and the absorptive surface of the small intestine without damaging it [73]. Piperine was a permeation enhancer for capsaicin delivery across nasal epithelial models. It was observed that piperine opened the tight junctions, resulting in a higher modulatory effect on paracellular permeation across the nasal epithelium [79]. In another study, cosmoperine, a piperine derivative, was compared with piperine in enhancing the permeation of lidocaine by the transdermal route. 1.5% w/w of both enhancers showed similar permeation effects for lidocaine, but 3% w/w cosmoperine exerted better permeation activity than piperine. This difference is possibly due to the alterations in their partitioning behavior and not due to differences in their diffusion coefficient [80].
Permeation studies using cell lines and intestinal mucosa have significantly proved piperine’s role in improving drug permeability across these barriers. In the presence of piperine, an increase in permeation flux and permeability coefficient of curcumin was observed in ex vivo permeation studies using sheep intestinal mucosa and Caco-2 cell monolayer [81]. Permeation across these barriers was increased by the ability of piperine to modulate the tight cellular junctions.
When combined with vesicular structures, piperine can prolong the rate and extent of drug absorption by altering the pharmacokinetic parameters. It has already been reported that nanocarriers can reversibly open tight cellular junctions [61]. Hence, combining nanotechnology with a natural permeation enhancer such as piperine can improve drug absorption without any significant side effects. An increased absorption with decreased metabolism of omeprazole was observed when given along with piperine as gastro retentive microspheres. Piperine enhanced the area under plasma drug concentration (AUC) and Cmax and delayed the Tmax of omeprazole by altering the membrane cytoskeleton with increased fluidity [82]. Tenofovir was encapsulated in Solid lipid nanoparticles (SLNs) with piperine and chitosan as permeation enhancers to improve oral absorption. Absorption of tenofovir was limited by the tight junctions of the intestinal epithelium, which was reversed by piperine and chitosan [83]. All these studies confirmed piperine’s ability to modulate tight cellular junctions.
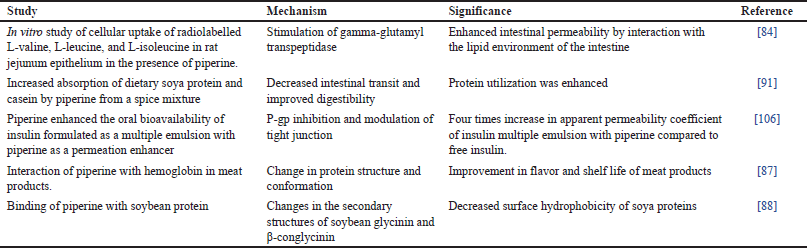
Although studies are few, it is postulated that piperine could enhance the absorption of protein drugs by similar mechanisms. Piperine interacted with γ-glutamyl transpeptidase and improved the radiolabelled amino acid uptake with increased lipid peroxidation in isolated epithelial cells of rat jejunum [84]. It was hypothesized that the ability of piperine to interact with the lipid environment led to increased permeability of intestinal epithelium.
Piperine is also known to increase the bioefficacy of proteins by influencing their metabolism. Oral administration of a 10 mg dose in male albino rats produced a significant rise in serum gonadotropins while reducing intratesticular testosterone levels [85].
Piperine is capable of binding with mammalian proteins such as bovine β-lactoglobulin (BLG), a major whey protein in milk [86]. Molecular docking studies revealed the ability of piperine to be accommodated within the central cavity of BLG and modify its absorption properties.
Alteration of the properties of dietary proteins by piperine was also reported. The conformation and structure of hemoglobin in meat proteins were altered by piperine, improving the nutritional quality and flavor of the former [87]. In a similar study [88], the binding of piperine with soybean proteins produced changes in the secondary structures of soybean glycinin and β-conglycinin. Molecular docking studies revealed the possible formation of microstructured complexes by electrostatic, hydrogen bonding, and hydrophobic interactions with decreased surface hydrophobicity.
Effect of piperine on digestive enzymes
When taken with food, spices such as piperine stimulate salivary amylase, pancreatic lipase, amylase, proteases, and intestinal digestive enzymes. However, it is already reported that piperine can inhibit gastric acid secretion in a dose-dependent manner. With increased concentration, piperine could also reduce the volume of gastric juice, gastric acidity, and pepsin activity. There is evidence that piperine could protect against gastric ulcers in rats and mice by inhibiting pepsin [89]. Piperine can alter enzyme kinetics by stimulating amino peptidase and glycyl-glycine dipeptidase activity [90]. It is reported that dietary intake of spices, such as piperine, ginger, ajowan, and cumin, enhanced fat and protein digestion and their absorption in rats. Rats were fed with casein and soy protein as the protein source, and each spice’s effect was investigated. Piperine significantly reduced the food transit time for both proteins and increased the apparent protein digestibility [91].
Effect of piperine on gut wall enzymes and transport proteins
Several studies reported that piperine increased the oral bioavailability of drugs by the former’s effect on gut wall enzymes. Piperine inhibited P-gp and enhanced the cellular uptake of rapamycin loaded in Poly(lactic-co-glycolic)acid (PLGA) nanoparticles [92] (Table 1). Piperine analog enhanced the oral bioavailability of etoposide by inhibiting P-gp and CYP3A4, enhancing its mucosal uptake. Analog reduced intestinal exsorption rate and total plasma clearance of etoposide [93]. Piperine improved the bioavailability of nisoldipine by inhibiting CYP4503A4 and reducing its first-pass hepatic metabolism [94]. Studies show piperine’s role in inhibiting the P-gp and CYP3A4 enzymes, which had significant roles in the first-pass metabolism of many drugs. Piperine inhibited P-gp substrates of digoxin and cyclosporin A, and CYP3A4 catalyzed the formation of verapamil metabolites [95]. When coadministered with warfarin, piperine decreased the warfarin metabolite concentration by inhibiting the CYP450 enzyme, CYP2C. Piperine prevented warfarin metabolism by inhibiting CYP450 and organic anion-transporting polypeptides, reducing hepatic uptake of warfarin and thereby preventing its metabolism [96]. The effect of piperine in improving the oral bioavailability of amoxicillin was evaluated by in situ single-pass intestinal perfusion method. It was observed that piperine inhibited the drug efflux pump, and the P-gp efflux transporter reduced the efflux and enhanced the bioavailability [97]. Piperine significantly enhanced the oral bioavailability of linarin by inhibiting P-gp-mediated efflux, improved intestinal absorption, increased AUC by 381%, and delayed gastric emptying [98]. Piperine inhibited the efflux of gentamicin and enhanced its antibacterial activity by increasing its intracellular concentration [99].
Piperine can prevent the metabolism of anticancer drugs by inhibiting CYP and P-gp, which will be a significant breakthrough. A synergistic antitumor activity was observed when piperine was combined with paclitaxel in the Michigan Cancer Foundation-7 (MCF-7) breast cancer cell line. The bioavailability of paclitaxel was enhanced by the inhibition of CYP3A4 and P-gp protein efflux transporters [100]. A combination of piperine with celecoxib inhibited colon adenocarcinoma with a synergistic effect. Piperine enhanced the bioavailability of celecoxib by 129% by inhibiting CYP3A4. The approach may benefit by using celecoxib at lower and safer concentrations and a novel way of treating colon cancer [101].
Piperine at a high dose can improve carbamazepine concentrations by inhibiting metabolic enzyme activities. This inhibition can decrease microsomal activity and rCYP3A2 mRNA and protein expression levels [102]. Piperine improved the oral bioavailability of protopanaxadiol by inhibiting CYP3A4, enhancing its absorption [103]. The oral bioavailability of domperidone was increased to 79.5% by piperine as a P-gp inhibitor. AUC and Cmax of domperidone were significantly improved by piperine inhibiting efflux transporter, P-gp [104]. Docetaxel conjugated in multiwalled carbon nanotube and coadministered with piperine as a permeation enhancer. Carbon nanotubes delayed the clearance, and piperine inhibited the CYP3A4 metabolism of docetaxel with increased AUC [105]. All these studies suggested that piperine significantly prevents drug metabolism by inhibiting major drug-metabolizing enzymes such as CYP and P-gp.
While considering oral absorption of proteins, gut wall enzymes and transport proteins play a significant role. It was found that piperine can inhibit gut wall P-gp, CYP3A4, and other drug-metabolizing enzymes. Even if few studies reported on piperine as a permeation enhancer in improving the oral bioavailability of peptide drugs, enough literature is available in which the role of piperine as a bioenhancer was proved. Recently, literature reported that piperine acted as a permeation enhancer to improve insulin permeation across Caco-2 cell lines. The study reported a fourfold increase in the apparent permeability coefficient of insulin from multiple emulsions incorporated with piperine compared with free insulin. They observed that piperine exerted bioenhancing activity and showed a significant hypoglycemic effect in rats by inhibiting P-gp [106].
 | Table 1. Role of piperine in enhancing the bioavailability of proteins. [Click here to view] |
Effect of piperine on drug blood components and protein binding
Piperine can bind significantly with human plasma as it is lipophilic. Piperine can improve the bioavailability of drugs by displacing them from major blood proteins such as albumin and alpha acid glycoprotein. It may enhance drug transport across biological barriers and help improve the drugs’ pharmacokinetics and pharmacodynamics [107]. The effect of piperine on the plasma protein binding of various drugs, such as diazepam, warfarin, salicylic acid, propanalol, and lidocaine, was investigated. Piperine manipulated the binding of both acidic and basic drugs in a concentration-dependent manner. Studies suggest piperine has a significant effect in displacing albumin-bound drugs. Piperine also increased the uptake of warfarin and propranolol by brain microvascular endothelial cells and, hence, can be considered adequate for modulating the impact of clinically significant molecules [108].
Effect of piperine on hepatic metabolism
The effect of black pepper on hepatic metabolism was reported by many researchers [109]. Piperine improves the oral bioavailability of silymarin by hepatic and intestinal glucuronidation inhibition, which can prevent the phase II metabolism of silymarin. It was evaluated that the drug absorption increased with an increase in piperine concentration and reduced the biotransformation rate of the drug in the intestine [110]. Piperine enhanced the bioavailability of silybin by 146%–181% by inhibiting efflux transporters BCRP and MRP2 in Caco-2 and transfected MDCKII cell lines. Piperine prevented the biliary excretion of silybin and its metabolites without affecting its phase 2 metabolism [111].
The oral bioavailability of curcumin is just 1%, as it cleared out quickly due to extensive hepatic metabolism. There are many studies in which piperine improved the oral bioavailability of curcumin by preventing its hepatic metabolism. Curcumin, when combined with piperine, could inhibit the glucuronidation of curcumin in vitro assay of rat intestinal microsomes. Piperine also enhanced curcumin permeability with a significant increase in AUC [112]. In another study by Rinwa et al. [113] piperine enhanced the neuroprotective action of curcumin in rats. Piperine prevented the intestinal degradation of curcumin by inhibiting hepatic and intestinal glucuronidation and restored the behavioral, biochemical, mitochondrial, and molecular alterations in depression-induced rats. Piperine improved the antigenotoxic effects of curcumin in overcoming benzopyrene-induced DNA damage. Piperine inhibited curcumin’s metabolism, enhancing its absorption by increasing the residence time [114]. An excipient-free curcumin solid dispersion was formulated with piperine to evaluate the effect of piperine as a permeation enhancer. When combined with curcumin in the coamorphous formulation, piperine inhibited the glucuronidation of curcumin and improved its GI membrane permeability [112].
Several other works proved piperine’s permeation enhancement activity by improving the drugs’ pharmacokinetic properties. 10 mg/kg of piperine enhanced the antihyperlipidemic potential of gemfibrozil by inhibiting its glucuronidation. Piperine enhanced the solubility and, thereby, the absorption of gemfibrozil, elevating its plasma levels within 1 hour [115]. The hepatoprotective activity of Aeglemarmelos was enhanced by piperine, inhibiting its metabolism and increasing its absorption [116]. Coadministration of emodin with piperine increased the oral bioavailability of emodin by inhibiting glucuronidation. Piperine significantly increased the AUC by 221%, Cmax by 258%, and delayed the clearance of emodin by 238% [117]. To overcome the extensive first-pass metabolisms and poor solubility of cannabinoids, they were incorporated in pro nanolipospheres with piperine as a permeation enhancer. Piperine inhibited the phase 1 and 2 metabolisms of cannabinoids with a three-fold increase in Cmax and a 1.5-fold increase in AUC [118]. Concurrent administration of resveratrol with piperine improved the anti-inflammatory activity of resveratrol in arthritic rats by the glucuronidation inhibition ability of piperine. They have observed that the bioavailability of resveratrol was improved by delaying its elimination by piperine at a dose of 10–20 mg/kg [119]. Anticancer and antioxidant potentials of epigallocatechin gallate were improved using piperine, which prevented its glucuronidation and increased its absorption with an increase in residence time in the GIT [120]. Piperine pro nanolipospheres improved the oral bioavailability of raloxifene by inhibiting phase II metabolism via glucuronidation. Piperine inhibited uridine diphosphate (UDP)-glucuronosyltransferases (UGT) enzymes involved in raloxifene’s metabolism and delayed its clearance [121]. All these studies establish that piperine plays a significant role in inhibiting the hepatic metabolism of drugs (Fig. 3).
 | Figure 3. Effects of piperine as a permeation enhancer. [Click here to view] |
Effect of piperine on protein stability
A significant concern with proteins is their denaturation, which several factors can cause. Proteins can be denatured by chemicals, heat, agitation, and pH changes, unfolding their polypeptide chains and making them inactive and toxic. Storing at lower temperatures can prevent protein denaturation to a certain extent [122]. It was reported in the literature that the “LVEALYL” segment in the insulin B chain is responsible for fibril formation. Piperine prevented insulin aggregation by attaching it to thermostable gold particles. Piperine could retain the native structures of insulin by interacting with its aggregation-prone residues. It was hypothesized that piperine interacted with the valine residue of “LVEALYL” of the insulin B chain and restricted its aggregation. The concept can be utilized to develop nanoparticle formulations to overcome the medical problems related to insulin aggregation [123].
CONCLUSION
Oral delivery of protein and peptide drugs is an evergreen research concern. Several strategies, such as absorption enhancers, chemical modifications, mucoadhesives, and enzyme inhibitors, have been investigated to improve the oral bioavailability of peptide drugs. However, all these approaches are associated with several drawbacks, such as damaging the epithelium, irritation, inflammation, and unwanted entry of pathogens. Piperine has significant effects on drug metabolizing enzymes and intestinal drug absorption. Piperine can also modulate the membrane dynamics and improve drug permeation by opening tight junctions. By inhibiting CYP3A4, P-gp and the first pass hepatic metabolism can delay the drug clearance and enhance oral bioavailability. Although there are not many studies that have established the role of piperine in improving the permeation of peptide drugs, there are clear indicators that such bioenhancers could contribute significantly to improving oral bioavailability of protein drugs. Thus, the use of herbal bioenhancers in promoting protein absorption is still a vastly unexplored area, even though there are several studies that report their success in enhancing the bioavailability of poorly available nonprotein drugs. One of the significant research concerns in delivering a protein drug by oral route is maintaining its stability till it reaches the target site. Piperine was reported to prevent protein aggregation and maintain protein stability. Data showed that lifestyle disorders like diabetes are on the rise. Hence, developing a viable oral alternative to the subcutaneous administration of insulin would make a significant difference to anitidiabetic therapy. Although there is plenty of research going on in developing an oral form of insulin, a successful clinically tested formulation is still far off. Using natural permeation enhancers such as piperine to improve the permeation of a macromolecular drug such as insulin can be a milestone. The concept can significantly reduce the side effects of insulin injections, reduce treatment costs, and improve patient compliance. Future studies on natural permeaton enhancers such as piperine must include the establishment of safe and effective doses in humans since much of the data are based on investigations in animals. The efficacy of other bioenhancers, such as zingiberine and quercetin, in improving the oral bioavailability of protein drugs can also be explored.
LIST OF ABBREVIATIONS
AUC, Area under the plot of plasma drug concentration with time; BLG, β-lactoglobulin; BSA, bovine serum albumin; Cmax, maximum concentration; CYP 450, Cytochrome P 450; GALT, gut-associated lymphoid tissue; GI, gastrointestinal; kDA, kilo Dalton; M cells, Microfold cells; MD, molecular dynamics; P-gp: P-glycoprotein, PPZ, phenyl piperazine; SDC, sodium deoxycholate; TRH, Thyrotropin-releasing hormone.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
FINANCIAL SUPPORT
There is no funding to report.
CONFLICTS OF INTEREST
The authors report no financial or any other conflicts of interest in this work.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Bruno BJ, Miller GD, Lim CS. Basics and recent advances in peptide and protein drug delivery. Ther Deliv. 2013 Nov;4(11):1443–67. CrossRef
2. Cao SJ, Xu S, Wang HM, Ling Y, Dong J, Xia RD, et al. Nanoparticles: oral delivery for protein and peptide drugs. AAPS PharmSciTech. 2019 Jul 1;20(5):1–11. CrossRef
3. Patel A, Cholkar K, Mitra AK. Recent developments in protein and peptide parenteral delivery approaches. Ther Deliv. 2014;5(3):337–65. CrossRef
4. He Y, Wang M, Zhang H, Zhang Y, Gao Y, Wang S. Protective properties of mesocellular silica foams against aggregation and enzymatic hydrolysis of loaded proteins for oral protein delivery. J Colloid Interface Sci. 2020;560:690–700. CrossRef
5. Zhu Q, Chen Z, Paul PK, Lu Y, Wu W, Qi J. Oral delivery of proteins and peptides: challenges, status quo and future perspectives. Acta Pharm Sin B. 2021 Aug 1;11(8):2416–48. CrossRef
6. Wang F, Sangfuang N, McCoubrey LE, Yadav V, Elbadawi M, Orlu M, et al. Advancing oral delivery of biologics: machine learning predicts peptide stability in the gastrointestinal tract. Int J Pharm. 2023;634:122643. CrossRef
7. Ibeanu N, Egbu R, Onyekuru L, Javaheri H, Khaw PT, Williams GR, et al. Injectables and depots to prolong drug action of proteins and peptides. Pharmaceutics. 2020;12(10):1–42. CrossRef
8. Cao SJ, Lv ZQ, Guo S, Jiang GP, Liu HL. An update—prolonging the action of protein and peptide drugs. J Drug Deliv Sci Technol. 2021;61(August):102124. CrossRef
9. Lamers C. Overcoming the shortcomings of peptide-based therapeutics. Futur Drug Discov. 2022;4(2):FDD75. CrossRef
10. Renukuntla J, Vadlapudi AD, Patel A, Boddu SHS, Mitra AK. Approaches for enhancing oral bioavailability of peptides and proteins. Int J Pharm. 2013;447(1–2):75–93. CrossRef
11. Bashyal S, Seo JE, Choi YW, Lee S. Bile acid transporter-mediated oral absorption of insulin via hydrophobic ion-pairing approach. J Control Release. 2021;338(March):644–61. CrossRef
12. Asfour MH. Advanced trends in protein and peptide drug delivery: a special emphasis on aquasomes and microneedles techniques. Drug Deliv Transl Res. 2021;11(1):1–23. CrossRef
13. Tatiraju DV, Bagade VB, Karambelkar PJ, Jadhav VM, Kadam V. Natural bioenhancers: an overview. J Pharmacogn Phytochem. 2013;2(3):55–60.
14. Majumdar SH, Kulkarni AS, Kumbhar SM. Yogvahi (Bioenhancer): an ayurvedic concept used in modern medicines. Int Res J Pharm Med Sci. 2018;1(1):20–5.
15. Wu D, Hu X, Cai Z, Zhang J, Geng F, Li H. Binding behavior and antioxidant study of spice extract piperine with meat myoglobin. Food Funct. 2023;14:6422–31. CrossRef
16. Hu X, Zeng Z, Zhang J, Wu D, Li H, Geng F. Molecular dynamics simulation of the interaction of food proteins with small molecules. Food Chem. 2023;405(November 2022). CrossRef
17. Wu D, Tang L, Zeng Z, Zhang J, Hu X, Pan Q, et al. Delivery of hyperoside by using a soybean protein isolated-soy soluble polysaccharide nanocomplex: fabrication, characterization, and in vitro release properties. Food Chem. 2022;386(March):132837. CrossRef
18. Selvaraj J. Molecular docking analysis of piperine with CDK2, CDK4, cyclin D and cyclin T proteins. Bioinformation. 2020;16(5):359–62. CrossRef
19. Kirubhanand C, Selvaraj J, Rekha UV, Vishnupriya V, Nalini D, Mohan SK, et al. Molecular docking data of piperine with Bax, Caspase 3, Cox 2 and Caspase 9. Bioinformation. 2020;16(6):458–61. CrossRef
20. Sindhoora D, Bhattacharjee A, Shabaraya AR. Bioenhancers: a comprehensive review. Int J Pharm Sci Rev Res. 2020;60(1):126–31.
21. Jain G, Patil UK. Strategies for enhancement of bioavailability of medicinal agents with natural products. Int J Pharm Sci Res. 2015;6(12):5315.
22. Mhaske DB, Sreedharan S, Mahadik KR. Role of piperine as an effective bioenhancer in drug absorption. Pharm Anal Acta. 2018;09(07):1–4. CrossRef
23. Atal N, Bedi KL. Bioenhancers: revolutionary concept to market. J Ayurveda Integr Med. 2010;1(2):96–9. CrossRef
24. Yurdakok-Dikmen B, Turgut Y, Filazi A. Herbal bioenhancers in veterinary phytomedicine. Front Vet Sci. 2018;5(OCT):1–8. CrossRef
25. Bose KG. Pharmacopoeia India. Calcutta, India: Bose Laboratories; 1929.
26. Gorgani L, Mohammadi M, Najafpour GD, Nikzad M. Piperine—the bioactive compound of black pepper: from isolation to medicinal formulations. Compr Rev Food Sci Food Saf. 2017;16(1):124–40. CrossRef
27. Kesarwani K, Gupta R. Bioavailability enhancers of herbal origin: an overview. Asian Pac J Trop Biomed. 2013 Apr 1;3(4):253–66. CrossRef
28. Tiwari A, Mahadik KR, Gabhe SY. Piperine: a comprehensive review of methods of isolation, purification, and biological properties. Med Drug Discov. 2020;7:100027. CrossRef
29. Peterson B, Weyers M, Steenekamp JH, Steyn JD, Gouws C, Hamman JH. Drug bioavailability enhancing agents of natural origin (bioenhancers) that modulate drug membrane permeation and pre-systemic metabolism. Pharmaceutics. 2019;11(1):33. CrossRef
30. Alexander A, Qureshi A, Kumari L, Vaishnav P, Sharma M, Saraf S, et al. Role of herbal bioactives as a potential bioavailability enhancer for active pharmaceutical ingredients. Fitoterapia. 2014;97:1–14. CrossRef
31. Singh S. Piperine: an effective bioenhancer for drug absorption. Pharm Drug Regul Aff J. 2021;4(1):1–3. CrossRef
32. Raghunath I, Koland M, Vadakkepushpakath AN, Kumar L, Shenoy SCS. Herbal bioenhancers with nanocarriers: a promising approach for oral peptide delivery. Int J Pharm Investig. 2022;13(1):07–13. CrossRef
33. Singh A, Deep A. Piperine?: a bioenhancer. Int J Pharm Res Technol. 2019;1(1):1–5. CrossRef
34. Atal N, Bedi KL. Bioenhancers: revolutionary concept to market. J Ayurveda Integr Med. 2010;1(2):96–9. CrossRef
35. Stojanovi?-Radi? Z, Pej?i? M, Dimitrijevi? M, Aleksi? A, Anil Kumar NV, Salehi B, et al. Piperine-A major principle of black pepper: a review of its bioactivity and studies. Appl Sci. 2019;9(20):1–29. CrossRef
36. Chivte VK, Tiwari SV, Nikalge APG. Bioenhancers?: a brief review bioenhancers. Adv J Pharm Life Sci Res. 2019;5(2):1–18.
37. Jhanwar B, Gupta S. Biopotentiation using herbs: novel technique for poor bioavailable drugs. Int J PharmTech Res. 2014;6(2):443–54.
38. Bai JPF, Amidon GL. Structural specificity of mucosal-cell transport and metabolism of peptide drugs: implication for oral peptide drug delivery. Pharm Res An Off J Am Assoc Pharm Sci. 1992;9(8):969–78.
39. Javed S, Ahsan W, Kohli K. The concept of bioenhancers in bioavailability enhancement of drugs—a patent review. J Sci Lett. 2016;1(3):143–65.
40. Jin ZH, Qiu W, Liu H, Jiang XH, Wang L. Enhancement of oral bioavailability and immune response of Ginsenoside Rh2 by co-administration with piperine. Chin J Nat Med. 2018;16(2):143–9. CrossRef
41. Haddadzadegan S, Dorkoosh F, Bernkop-Schnürch A. Oral delivery of therapeutic peptides and proteins: technology landscape of lipid-based nanocarriers. Adv Drug Deliv Rev. 2022;182:114097. CrossRef
42. Bernkop-Schnürch A, Kast CE, Guggi D. Permeation enhancing polymers in oral delivery of hydrophilic macromolecules: thiomer/GSH systems. J Control Release. 2003;93(2):95–103. CrossRef
43. Buckley ST, Bækdal TA, Vegge A, Maarbjerg SJ, Pyke C, Ahnfelt-Rønne J, et al. Transcellular stomach absorption of a derivatized glucagon-like peptide-1 receptor agonist. Sci Transl Med. 2018;10(468):eaar7047. CrossRef
44. Marschütz MK, Bernkop-Schnürch A. Oral peptide drug delivery: polymer-inhibitor conjugates protecting insulin from enzymatic degradation in vitro. Biomaterials. 2000;21(14):1499–507. CrossRef
45. Chakraborty S, Shukla D, Mishra B, Singh S. Lipid—an emerging platform for oral delivery of drugs with poor bioavailability. Eur J Pharm Biopharm. 2009;73(1):1–15. CrossRef
46. Schmitz T, Huck CW, Bernkop-Schnürch A. Characterisation of the thiol-disulphide chemistry of desmopressin by LC, μ-LC, LC-ESI-MS and Maldi-Tof. Amino Acids. 2006;30(1):35–42. CrossRef
47. Ijaz M, Bonengel S, Zupan?i? O, Yaqoob M, Hartl M, Hussain S, et al. Development of oral self nano-emulsifying delivery system(s) of lanreotide with improved stability against presystemic thiol-disulfide exchange reactions. Expert Opin Drug Deliv. 2016;13(7):923–9. CrossRef
48. Bernkop-Schnurch A, Fragner R. Investigations into the diffusion behaviour of polypeptides in native intestinal mucus with regard to their peroral administration. Pharm Sci. 1996;2(8):361–3.
49. Xu Y, Shrestha N, Préat V, Beloqui A. Overcoming the intestinal barrier: a look into targeting approaches for improved oral drug delivery systems. J Control Release. 2020;322(April):486–508. CrossRef
50. Fein KC, Gleeson JP, Newby AN, Whitehead KA. Intestinal permeation enhancers enable oral delivery of macromolecules up to 70 kDa in size. Eur J Pharm Biopharm. 2022;170:70–6. CrossRef
51. Brayden DJ, Hill TA, Fairlie DP, Maher S, Mrsny RJ. Systemic delivery of peptides by the oral route: formulation and medicinal chemistry approaches. Adv Drug Deliv Rev. 2020;157:2–36. CrossRef
52. Smart AL, Gaisford S, Basit AW. Oral peptide and protein delivery: intestinal obstacles and commercial prospects. Expert Opin Drug Deliv. 2014;11(8):1323–35. CrossRef
53. Donovan MD, Flynn GL, Amidon GL. Absorption of polyethylene glycols 600 through 2000: the molecular weight dependence of gastrointestinal and nasal absorption. Pharm Res. 1990;7:863–8. CrossRef
54. Weyers M, Peterson B, Hamman JH, Steenekamp JH. Formulation of chitosan microparticles for enhanced intranasal macromolecular compound delivery: factors that influence particle size during ionic gelation. Gels. 2022;8(11):686. CrossRef
55. Newey H, Smyth DH. The intestinal absorption of some dipeptides. J Physiol. 1959;145(1):48–56. CrossRef
56. Hirlekar RS, Patil EJ, Bhairy SR. Oral insulin delivery: novel strategies. Asian J Pharm. 2017;11(3):S434–43.
57. Salamat-Miller N, Johnston TP. Current strategies used to enhance the paracellular transport of therapeutic polypeptides across the intestinal epithelium. Int J Pharm. 2005;294(1–2):201–16. CrossRef
58. Antosova Z, Mackova M, Kral V, Macek T. Therapeutic application of peptides and proteins: parenteral forever? Trends Biotechnol. 2009;27(11):628–35. CrossRef
59. Anilkumar P, Badarinath AV, Naveen N, Prasad K, Reddy BR, Hyndhavi M, et al. A rationalized description on study of intestinal barrier, drug permeability and permeation enhancer. J Glob Trends Pharm Sci. 2011;2(4):431–49. CrossRef
60. Muheem A, Shakeel F, Jahangir MA, Anwar M, Mallick N, Jain GK, et al. A review on the strategies for oral delivery of proteins and peptides and their clinical perspectives. Saudi Pharm J. 2016;24(4):413–28. CrossRef
61. Lopes MA, Abrahim BA, Cabral LM, Rodrigues CR, Seiça RMF, de Baptista Veiga FJ, et al. Intestinal absorption of insulin nanoparticles: contribution of M cells. Nanomed Nanotechnol Biol Med. 2014;10(6):1139–51. CrossRef
62. Date AA, Hanes J, Ensign LM. Nanoparticles for oral delivery: design, evaluation and state-of-the-art. J Control Release. 2016;240:504–26. CrossRef
63. Batista P, Castro PM, Madureira AR, Sarmento B, Pintado M. Recent insights in the use of nanocarriers for the oral delivery of bioactive proteins and peptides. Peptides. 2018;101(December 2017):112–23. CrossRef
64. Reis CP, Damgé C. Nanotechnology as a promising strategy for alternative routes of insulin delivery. Methods Enzymol. 2012;508:271–94. CrossRef
65. Pabst O, Mowat AM. Oral tolerance to food protein. Mucosal Immunol. 2012;5(3):232–9. CrossRef
66. Lundquist P, Artursson P. Oral absorption of peptides and nanoparticles across the human intestine: opportunities, limitations and studies in human tissues. Adv Drug Deliv Rev. 2016;106:256–76. CrossRef
67. Liu C, Kou Y, Zhang X, Cheng H, Chen X, Mao S. Strategies and industrial perspectives to improve oral absorption of biological macromolecules. Expert Opin Drug Deliv. 2018 Mar 4;15(3):223–33. CrossRef
68. Al-Hilal TA, Alam F, Byun Y. Oral drug delivery systems using chemical conjugates or physical complexes. Adv Drug Deliv Rev. 2013;65(6):845–64. CrossRef
69. Toorisaka E, Watanabe K, Ono H, Hirata M, Kamiya N, Goto M. Intestinal patches with an immobilized solid-in-oil formulation for oral protein delivery. Acta Biomater. 2012;8(2):653–8. CrossRef
70. Amet N, Wang W, Shen WC. Human growth hormone-transferrin fusion protein for oral delivery in hypophysectomized rats. J Control Release. 2010;141(2):177–82. CrossRef
71. Dennis MS, Zhang M, Gloria Meng Y, Kadkhodayan M, Kirchhofer D, Combs D, et al. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. J Biol Chem. 2002;277(38):35035–43. CrossRef
72. Zorzi A, Middendorp SJ, Wilbs J, Deyle K, Heinis C. Acylated heptapeptide binds albumin with high affinity and application as tag furnishes long-acting peptides. Nat Commun. 2017;8(May):1–9. CrossRef
73. Han HK. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert Opin Drug Metab Toxicol. 2011;7(6):721–9. CrossRef
74. Weitzel KM. Bond-dissociation energies of cations—pushing the limits to quantum state resolution. Mass Spectrom Rev. 2011 Mar-Apr;30(2):221–35. CrossRef
75. Di L. Strategic approaches to optimizing peptide ADME properties. AAPS J. 2015;17(1):134–43. CrossRef
76. Taki Y, Sakane T, Nadai T, Sezaki H, Amidon GL, Langguth P, et al. First-pass metabolism of peptide drugs in rat perfused liver. J Pharm Pharmacol. 1998;50(9):1013–8. CrossRef
77. Jyrkäs J, Tolonen A. Hepatic in vitro metabolism of peptides; comparison of human liver S9, hepatocytes and upcyte hepatocytes with cyclosporine A, leuprorelin, desmopressin and cetrorelix as model compounds. J Pharm Biomed Anal. 2021;196:113921. CrossRef
78. Khajuria A, Thusu N, Zutshi U. Piperine modulates permeability characteristics of intestine by inducing alterations in membrane dynamics: influence on brush border membrane fluidity, ultrastructure and enzyme kinetics. Phytomedicine. 2002;9(3):224–31. CrossRef
79. Gerber W, Steyn D, Kotzé A, Svitina H, Weldon C, Hamman J. Capsaicin and piperine as functional excipients for improved drug delivery across nasal epithelial models. Planta Med. 2019;85(13):1114–23. CrossRef
80. Influence of Piperine and Cosmoperine® as Transdermal Drug Delivery. n.d. https://discovery.ucl.ac.uk/id/eprint/1351458/1/Murdan_piperine.docMurdan.pdf (accessed November 3, 2023).
81. Thomas G, Koland M. Composition of piperine with enteric-coated chitosan microspheres enhances the transepithelial permeation of curcumin in sheep intestinal mucosa and Caco-2 cells. J Heal Allied Sci NU. 2022;12(03):312–21. CrossRef
82. Boddupalli BM, Anisetti RN, Ramani R, Malothu N. Enhanced pharmacokinetics of omeprazole when formulated as gastroretentive microspheres along with piperine. Asian Pacific J Trop Dis. 2014;4(S1):129–33. CrossRef
83. Babu MR, Prakash PR, Devanna N. Exploring the oral absorption enhancing effect of piperine and chitosan on tenofovir loaded solid lipid nanoparticles. Int Res J Pharm. 2019;10(3):221–6. CrossRef
84. Johri RK, Thusu N, Khajuria A, Zutshi U. Piperine-mediated changes in the permeability of rat intestinal epithelial cells. The status of γ-glutamyl transpeptidase activity, uptake of amino acids and lipid peroxidation. Biochem Pharmacol. 1992;43(7):1401–7. CrossRef
85. Malini T, Manimaran RR, Arunakaran J, Aruldhas MM, Govindarajulu P. Effects of piperine on testis of albino rats. J Ethnopharmacol. 1999;64(3):219–25. CrossRef
86. Zsila F, Hazai E, Sawyer L. Binding of the pepper alkaloid piperine to bovine β-lactoglobulin: circular dichroism spectroscopy and molecular modeling study. J Agric Food Chem. 2005;53(26):10179–85. CrossRef
87. Hu X, Wu D, Tang L, Zhang J, Zeng Z, Geng F, et al. Binding mechanism and antioxidant activity of piperine to hemoglobin. Food Chem. 2022 Nov 15;394:133558. CrossRef
88. Zhang C, Niu Z, He Z, Ding Y, Wu G, Wu H, et al. Molecular interaction of soybean protein and piperine by computational docking analyses. Food Hydrocoll. 2023 Aug 31;146:109249. CrossRef
89. Platel K, Srinivasan K. Digestive stimulant action of spices: a myth or reality? Indian J Med Res. 2004;119(5):167–79.
90. Meghwal M, Goswami TK. Piper nigrum and piperine: an update. Phyther Res. 2013;27(8):1121–30. CrossRef
91. Prakash UNS, Srinivasan K. Influence of dietary spices on protein digestibility and absorption in experimental rats. Food Dig. 2013;4(2–3):69–75. CrossRef
92. Katiyar SS, Muntimadugu E, Rafeeqi TA, Domb AJ, Khan W. Co-delivery of rapamycin—and piperine-loaded polymeric nanoparticles for breast cancer treatment. Drug Deliv. 2016;23(7):2608–16. CrossRef
93. Najar IA, Sharma SC, Singh GD, Koul S, Gupta PN, Javed S, et al. Involvement of P-glycoprotein and CYP 3A4 in the enhancement of etoposide bioavailability by a piperine analogue. Chem Biol Interact. 2011;190(2–3):84–90. CrossRef
94. Rathee P, Kamboj A, Sidhu S. Enhanced oral bioavailability of nisoldipine-piperine-loaded poly-lactic-co-glycolic acid nanoparticles. Nanotechnol Rev. 2017;6(6):517–26. CrossRef
95. Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;302(2):645–50. CrossRef
96. Zayed A, Babaresh WM, Darweesh RS, El-Elimat T, Hawamdeh SS. Piperine alters the pharmacokinetics and anticoagulation of warfarin in rats. J Exp Pharmacol. 2020;12:169–79. CrossRef
97. Barve K, Ruparel K. Effect of bioenhancers on amoxicillin bioavailability. ADMET DMPK. 2015;3(1):45–50. CrossRef
98. Feng X, Liu Y, Wang X, Di X. Effects of piperine on the intestinal permeability and pharmacokinetics of linarin in rats. Molecules. 2014;19(5):5624–33. CrossRef
99. Khameneh B, Iranshahy M, Ghandadi M, Ghoochi Atashbeyk D, Fazly Bazzaz BS, Iranshahi M. Investigation of the antibacterial activity and efflux pump inhibitory effect of co-loaded piperine and gentamicin nanoliposomes in methicillin-resistant Staphylococcus aureus. Drug Dev Ind Pharm. 2015;41(6):989–94. CrossRef
100. Motiwala MN, Rangari VD. Combined effect of paclitaxel and piperine on a MCF-7 breast cancer cell line in vitro: evidence of a synergistic interaction. Synergy. 2015;2(1):1–6. CrossRef
101. Srivastava S, Dewangan J, Mishra S, Divakar A, Chaturvedi S, Wahajuddin M, et al. Piperine and celecoxib synergistically inhibit colon cancer cell proliferation via modulating Wnt/β-catenin signaling pathway. Phytomedicine. 2021;84(January):153484. CrossRef
102. Ren T, Yang M, Xiao M, Zhu J, Xie W, Zuo Z. Time-dependent inhibition of carbamazepine metabolism by piperine in anti-epileptic treatment. Life Sci. 2019;218(December 2018):314–23. CrossRef
103. Jin X, Zhang ZH, Sun E, Tan XB, Li SL, Cheng XD, et al. Enhanced oral absorption of 20(S)-protopanaxadiol by self-assembled liquid crystalline nanoparticles containing piperine: in vitro and in vivo studies. Int J Nanomedicine. 2013;8:641–52. CrossRef
104. Islam N, Irfan M, Hussain T, Mushtaq M, Khan IU, Yousaf AM, et al. Piperine phytosomes for bioavailability enhancement of domperidone. J Liposome Res. 2022;32(2):172–80. CrossRef
105. Raza K, Kumar D, Kiran C, Kumar M, Guru SK, Kumar P, et al. Conjugation of docetaxel with multiwalled carbon nanotubes and codelivery with piperine: implications on pharmacokinetic profile and anticancer activity. Mol Pharm. 2016;13(7):2423–32. CrossRef
106. Kaur I, Nallamothu B, Kuche K, Katiyar SS, Chaudhari D, Jain S. Exploring protein stabilized multiple emulsion with permeation enhancer for oral delivery of insulin. Int J Biol Macromol. 2021 Jan 15;167:491–501. CrossRef
107. Kobori T, Iwamoto S, Takeyasu K, Ohtani T. Biopolymers volume 85/number 4295. Biopolymers. 2007;85(4):392–406. CrossRef
108. Dubey RK, Leeners B, Imthurn B, Merki-Feld GS, Rosselli M. Piperine decreases binding of drugs to human plasma and increases uptake by brain microvascular endothelial cells. Phyther Res. 2017;31(12):1868–74. CrossRef
109. Srinivasan K. Black pepper and its pungent principle-piperine: a review of diverse physiological effects. Crit Rev Food Sci Nutr. 2007;47(8):735–48. CrossRef
110. Javed S, Kohli K, Ahsan W. Enhanced intestinal permeability of silymarin by natural products as bioenhancers—assessment by ex-vivo NonEverted rat Gut Sac study. Bioequivalence Bioavailab Int J. 2018;2(1):1–10. CrossRef
111. Bi X, Yuan Z, Qu B, Zhou H, Liu Z, Xie Y. Piperine enhances the bioavailability of silybin via inhibition of efflux transporters BCRP and MRP2. Phytomedicine. 2019;54:98–108. CrossRef
112. Wang R, Han J, Jiang A, Huang R, Fu T, Wang L, et al. Involvement of metabolism-permeability in enhancing the oral bioavailability of curcumin in excipient-free solid dispersions co-formed with piperine. Int J Pharm. 2019;561(february):9–18. CrossRef
113. Rinwa P, Kumar A, Garg S. Suppression of neuroinflammatory and apoptotic signaling cascade by curcumin alone and in combination with piperine in rat model of olfactory bulbectomy induced depression. PLoS One. 2013;8(4):2–12. CrossRef
114. Sehgal A, Kumar M, Jain M, Dhawan DK. Combined effects of curcumin and piperine in ameliorating benzo(a)pyrene induced DNA damage. Food Chem Toxicol. 2011;49(11):3002–6. CrossRef
115. Mohanalakshmi S, Bhatt S, Ashok Kumar CK. Enhanced antihyperlipidemic potential of gemfibrozil under co-administration with piperine. Curr Res Pharmacol Drug Discov. 2021;2(December 2020):100021. CrossRef
116. Rathee D, Kamboj A, Sachdev RK, Sidhu S. Hepatoprotective effect of Aegle marmelos augmented with piperine co-administration in paracetamol model. Rev Bras Farmacogn. 2018;28(1):65–72. CrossRef
117. Di X, Wang X, Liu Y. Effect of piperine on the bioavailability and pharmacokinetics of emodin in rats. J Pharm Biomed Anal. 2015;115:144–9. CrossRef
118. Cherniakov I, Izgelov D, Barasch D, Davidson E, Domb AJ, Hoffman A. Piperine-pro-nanolipospheres as a novel oral delivery system of cannabinoids: pharmacokinetic evaluation in healthy volunteers in comparison to buccal spray administration. J Control Release. 2017;266(August):1–7. CrossRef
119. El-Ghazaly MA, Fadel NA, Abdel-Naby DH, Abd El-Rehim HA, Zaki HF, Kenawy SA. Potential anti-inflammatory action of resveratrol and piperine in adjuvant-induced arthritis: effect on pro-inflammatory cytokines and oxidative stress biomarkers. Egypt Rheumatol. 2020;42(1):71–7. CrossRef
120. Dahiya S, Rani R, Dhingra D, Kumar S, Dilbaghi N. Conjugation of epigallocatechin gallate and piperine into a zein nanocarrier: implication on antioxidant and anticancer potential. Adv Nat Sci Nanosci Nanotechnol. 2018;9(3):035011. CrossRef
121. Izgelov D, Cherniakov I, Aldouby Bier G, Domb AJ, Hoffman A. The effect of piperine pro-nano lipospheres on direct intestinal phase II metabolism: the raloxifene paradigm of enhanced oral bioavailability. Mol Pharm. 2018;15(4):1548–55. CrossRef
122. Zapadka KL, Becher FJ, Gomes dos Santos AL, Jackson SE. Factors affecting the physical stability (aggregation) of peptide therapeutics. Interface Focus. 2017;7(6):20170030. CrossRef
123. Anand BG, Shekhawat DS, Dubey K, Kar K. Uniform, polycrystalline, and thermostable piperine-coated gold nanoparticles to target insulin fibril assembly. ACS Biomater Sci Eng. 2017;3(6):1136–45. CrossRef