INTRODUCTION
The use of medicinal herbs has skyrocketed in the last decades owing to their availability, low cost, agreement with patients’ values, and perceived safety and efficacy in preventing, managing, and treating several chronic diseases whose burden is increasing worldwide. According to recent reports from the World Health Organization, medicinal herbs are used in an estimated 88% of all countries. Besides, they contribute to the trillion-dollar industries of health, pharmaceutical markets, and beauty, which are growing continuously, as more than 40% of pharmaceutical products are manufactured from natural products [1].
Humans have undoubtedly used medicinal herbs since ancient times, and this has resulted in increasing people’s awareness of their properties. The presence of various physiologically active agents, namely, phytochemicals or secondary metabolites, constituting plants is the basis for their medicinal use. The constituents are saponins, terpenoids, glycosides, and alkaloids, which can act singly or synergistically. In addition, medicinal herbs are considered safe because they have been used for many centuries, and knowledge about them has accumulated over time. All these factors have led to their use in many therapeutic plans for patients with chronic diseases, either as an adjunct to conventional therapies or as an alternative [2].
Among many brutal chronic illnesses, chronic kidney disease (CKD) has been found to create a great surge in demand for medicinal herbs. This is because of the heavy load it adds on patients’ health, contributing to high rates of morbidity and mortality, which are reaching alarming proportions worldwide. The prevalence of CKD was reported in 2017 to range from 8.5% to 9.8%, leading to 1.2 million cases of death [3]. Also, it is estimated that more than 1.4 million patients with kidney disease have end-stage renal disease (ESRD), thus receiving renal replacement therapy worldwide [4]. According to the most recent annual reports from the United States Renal Data System, the prevalence of ESRD increased dramatically from 6% to 13.5% in many countries between 2006 and 2012, placing a greater burden on health insurance systems in these countries [5].
There are many risk factors for the development of kidney disease, which, according to recent epidemiological studies, can be divided into initiating and perpetuating risk factors. The former contributes to cycle initiation, leading to loss of nephrons, such as advanced age, male sex, and diabetes. On the other hand, perpetuating factors are the main drivers of disease progression. Examples include hypertension, diabetes mellitus, dyslipidemia, hyperuricemia, and proteinuria [6].
Pharmaceutical agents have been used to counteract the progression of kidney disease. Nevertheless, medicinal herbs are gaining an increasing interest in this context due to many factors, including socioeconomic status, in rural areas and developing countries; patients have considered herbal remedies as their resort in controlling and combating CKD, as these remedies are cheaper and reported to have fewer side effects compared to drugs. However, this “health claim” has resulted in less strict regulation over their use. In addition, many drugs can interact with other agents, and herbs are no exception, which warrants close monitoring, especially when using drugs with a narrow therapeutic index and an herbal drug that interacts with them [7]. Another rationale for that action to hold a place is the fact that the screening libraries for the structure and activity of drugs are not matched with those of traditional medicine [8]. In fact, pharmacological data are scarce on the presence of toxic phytochemicals and elements in plants, which are not consistent in concentration at all times and can be influenced by many determinants such as harvesting time, storage conditions, drying, and quality problems (contamination and temperature fluctuation). Furthermore, many countries are not mandating the conduction of toxicological research before introducing herbal products into the market, and they have no effective approaches for regulating them. The only available regulations for assessing the adverse drug reactions of herbs are the WHO International Drug Monitoring Programme in 2000, 2001, EU—The Directive on Traditional Herbal Medicinal Products, UK—Yellow Card Scheme for monitoring of ADRs, India—Ayurveda, Yoga & Naturopathy, Unani, Siddha, Sowa Rigpa and Homoeopathy, The National Medicinal Plants Board (2000) of the Indian government, USA FDA—The Dietary Supplement Health and Education Act, 1994, and Russia—Federal Service for Surveillance in Healthcare under the Ministry of Health [9]. This shows that regulatory practices for herbal medicines have been established in a few countries, mainly in developed countries, highlighting the need for further action on a broader scale. Therefore, a better understanding of medicinal herbs safety, especially in CKD, could optimize the prevention and management of disease onset, progression, and complications and push for further regulatory practices to be established. Furthermore, since there are many risk factors of complex and heterogeneous nature for kidney disease progression, it is important to determine the effects of herbal remedies on the control of these factors. Therefore, this review was undertaken to examine the literature in this context, evaluate the herbal mechanisms attributed to their influence on the progression of CKD, provide a better understanding and guidance on the use of herbal remedies in the treatment and management of CKD, and serve as a building block for future studies based on the recommendations to be made.
METHODS
This systematic review was undertaken through a thorough literature search via scientific health databases, including Google Scholar, PubMed, Ebscohost Research Databases, and Wileyplus. Specific keywords that are relevant to the influence of herbal remedies on the progression of CKD were utilized. The search terms (MeSH terms) were validated by all authors and were “chronic kidney disease,” “CKD,” “herbal remedies,” “herbs,” “progression,” “blood pressure,” “blood glucose,” “diabetic kidney disease,” “diabetic nephropathy,” “lipid profile,” “proteinuria,” “uric acid,” “hyperuricemia,” and “toxicity.” Consequently, 300–400 articles were found. Therefore, the limitation of the search was applied to include articles (1) written in English, (2) published in professional journals from the year (3) 2000 onward, and (4) categorized as research-based literature studies that (5) focused on assessing the safety and efficacy of herbal remedies in CKD patients in the aspect of disease progression or those under the setting of CKD progression risk conditions. On the contrary, (1) unpublished studies and (2) nonresearch studies were excluded. For criteria verification purposes, titles and abstracts were reviewed by all authors (four authors) independently, alleviating the risk of selection bias. Then, those meeting the inclusion requirements were fully read and reviewed by them. Also, data was collected from every study by all the article authors. Besides, the snowball method was employed for literature searches for references existing in the manuscripts. As a result of applying the inclusion and exclusion criteria, 49 articles were selected for this article. The whole search process (summarized in Fig. 1) was done manually by the authors for 2 weeks to ensure the credibility and reliability of the methodology adopted. EndNote, version X7 (Clarivate Analytics, Philadelphia, PA), was used to import the retrieved articles, and any identical journals were removed.
A framework with eight categories was defined, including (1) reduction of blood pressure, (2) reduction of blood sugar levels, (3) enhancement of lipid profiles, (4) reduction of proteinuria, (5) reduction of serum uric acid levels, (6) elevation of blood pressure, (7) aggravation of diabetes mellitus outcomes, and (8) elevation of serum uric acid levels. Further elaboration of the review was undertaken by separately addressing each category and scrutinizing every relevant article for the corresponding category.
This systematic review ensured the credibility of the studies included by the assessment of their risk of bias with the use of the second version of the Cochrane risk-of-bias tool (Supplementary Materials) for randomized trials (RoB 2), which is structured into a set of bias domains, of which each is focussed upon certain aspects of the study methodology.
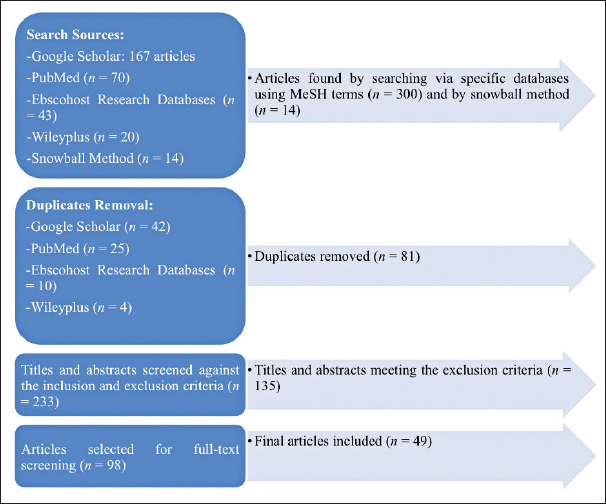 | Figure 1. A flowchart of the systematic review search strategy. [Click here to view] |
RESULTS AND DISCUSSION
Beneficial properties of herbal remedies in controlling CKD progression risk factors
Reduction of blood pressure
Many plant extracts have potential therapeutic value for the treatment of hypertension and other cardiovascular diseases, so their valuable effects could also make them useful for the control of the progression of CKD. For example, several herbs can suppress the renin-angiotensin system, which plays an important role in protecting the kidney. For instance, ergone in Polyporus umbellatus and pachymic acid B in Poria cocos showed the same effect in the cultured human kidney 2 and podocytes with tubulointerstitial fibrosis and glomerulosclerosis [10].
Antioxidant and anti-inflammatory properties are another strategy to combat high blood pressure in CKD. In a study, the effect of pomegranate juice from Punica granatum [Lythraceae] was investigated in 101 patients with chronic hemodialysis. Patients were randomly assigned to either a pomegranate juice group or a placebo group. The study found that pomegranate juice significantly reduced systolic blood pressure compared to placebo by mitigating oxidative damage [11]. Another recent study conducted on 41 hemodialysis patients yielded similar results in addition to a reduction in triglyceride (TG) levels [12].
Hypertension can stimulate the synthesis of endothelial cells and the release of vasoactive substances such as nitric oxide (NO), so such an effect can help reduce blood pressure, thus delaying CKD progression. For instance, in a randomized double-blinded controlled trial, the effects of capsules of Allium sativum [Alliaceae or Liliaceae] were assessed in trained male runners with coronary heart disease (CHD). Twenty-seven subjects were selected, and every individual received either the capsules or placebo for 16 weeks. The study found that garlic exerted hypotensive effects by inducing vasodilation and smooth muscle relaxation via the increase in the production of NO. Besides, those receiving it had their total antioxidant status and low-density lipoprotein (LDL) reduced, suggesting that garlic can decrease the risk of CHD effectively [13].
In a double-blinded randomized crossover study of 70 hemodialysis patients, garlic showed both antioxidant and antihypertensive effects by reducing the levels of homocysteine and oxidized LDL [14]. Potential side effects of it include gastrointestinal tract (GIT) disturbance, irritation, and nausea [15]. Theobroma cacao [Malvaceae], which is also mainly enriched with flavonoid constituents [16,17], has been shown to lower blood pressure similarly.
In addition, the combination of extracts from Astragalus membranaceus and Salvia miltiorrhiza can lead to an increase in endothelial NO synthase levels in patients with renal calculi [18]. However, the side effects of A. membranaceus were only seen when it was taken in excessive amounts, including allergy, agrypnia, headache, and GIT upset [19]. To add up, a single cross-over study investigated the effect of beet (Beta vulgaris) juice on hypertensive CKD patients. Seventeen CKD patients were recruited and randomly assigned to either a group receiving beet juice with a nitrate loading of 300 mg or a placebo group in which patients received water. Beet juice ingestion significantly increased plasma nitrate concentration and a remarkable decrease in peripheral systolic blood pressure, diastolic blood pressure, and mean arterial blood pressure compared to the control group [20].
Reduction of blood sugar levels
Serious complications of diabetes are common; in general, about one in three patients with diabetes eventually develop kidney disease, leading to diabetic nephropathy (DN), which is associated with significant morbidity and mortality rates, explaining the traditional use of herbal remedies to combat them [21]. In one study, 45 patients with early-stage diabetes DN were recruited and randomly assigned to receive either Zishentongluo (a decoction of extracts of Curcuma zedoaria, Astragalus, Cuscuta, Angelica, Radix Rehmanniae, Carthamus tinctorius, Perenniporia, Cornus, Schisandra, Epimedium, and earthworm) or benazepril for 3 months. It was found that hemoglobin A1C, fasting blood glucose (FBG), total cholesterol (TC), TGs, urine albumin excretion rate (UAER), serum creatinine, atrial natriuretic peptide, endothelin 1, and vascular endothelial growth factor were effectively improved in the zishentongluo group compared with the benazepril group [22].
The herbal formula Yiqi Huaju Qingli was studied in combination with Western medicine and in comparison with placebo with Western medicine in patients with metabolic syndrome and microalbuminuria. The results showed that treatment with herbal medicines significantly reduced body mass index, waist circumference, and waist-to-hip ratio. In addition, there was a significant reduction in FBG, 2-hour postprandial plasma glucose, homeostasis model assessment for insulin resistance, microalbuminuria, and UACR in both groups, with the herbal group showing a better effect. Both groups also showed a significant decrease in TC, LDL cholesterol (LDL-C), and TGs, with TGs being reduced more in the herbal group. Blood pressure was reduced in both treatments, with no significant differences noted [23].
Enhancement of lipid profiles
Dyslipidemia is one of the factors affecting the progression of kidney disease. CKD patients will also eventually develop this disease because they have significant proatherogenic lipid abnormalities that can be treated, and herbal remedies seem to be one of the options. The main mechanisms are lowering lipid levels, increasing lipid resistance, and oxidizing lipids by certain cofactors such as Cu (2+), as is the case with basil or Ocimum basilicum [Lamiaceae] [24].
In a study conducted on 90 CKD patients with dyslipidemia, Xuezhitong capsules (an extract of Allium macrostemon Bunge) were evaluated for their efficacy in this population. Patients were randomly divided into three groups. The first group received Xuezhitong capsules, while the second group received Xuezhitong capsules in combination with atorvastatin. The third group received atorvastatin only. After 3 months of therapy, the estimated glomerular filtration rate (eGFR) and UACR were significantly decreased in all groups, and the levels of TC, TG, and LDL-C decreased significantly in all three groups. The lowest levels were found in the second group, while the levels of high-density lipoprotein (HDL) increased and were also highest in the second group, indicating the usefulness of this herb in correcting dyslipidemia in CKD [25].
In a case report, a patient suffering from hypertension, dyslipidemia, type 2 diabetes mellitus, CKD, and hyperuricemia was administered Kangen Karyu extract daily for 6 months, resulting in a decrease in serum levels of TC, LDL-C, and TGs, and a decrease in blood pressure. Also, eGFR, uric acid, creatinine, creatine phosphokinase, aspartate transaminase, alanine aminotransferase, and γ-glutamyl transpeptidase were improved, indicating the effective role of this extract against metabolic syndrome [26].
Ginsenoside Rb1 (GS-Rb1), a ginseng-derived antioxidant, was studied in 197 patients with stage 2 or 3 CKD (early CKD) randomized to receive either GS-Rb1 or placebo for six consecutive months. As a result, the GS-Rb1 group (consisting of 91 patients) showed a significant relief of renal function impairment compared to the placebo group, and GS-Rb1 also reduced oxidative stress and inflammation and improved the lipid profile of recipients [27].
Reduction of proteinuria
Most progressive kidney diseases are associated with proteinuria, and many researchers have investigated using herbal remedies to correct these conditions. For example, the effect of Astragalus on proteinuria was studied by examining and recording cases of kidney disease. After patients received an Astragalus injection of 40 g per day for 3 weeks, proteinuria decreased significantly from 2,328 ± 3,157 to 1,017 ± 765 mg/day [28]. A more recent study showed similar results in 35 CKD patients, of which 15 had stage 4 CKD, while the rest had stage 5 CKD [29]. The combination of this herb with Angelica resulted in a remarkable decrease in proteinuria in 79 glomerulonephritis patients with stage 2 CKD in 1 randomized study comparing this intervention effect with that of losartan in another group of 79 patients [30].
Besides, 180 patients with diabetic kidney disease were enrolled in a randomized, double-blinded clinical trial, in which 122 of them were randomly assigned to receive the herbal granule formula Tangshen (consisting of A. membranaceus, Euonymus alatus, Rehmannia glutinosa, Citrus aurantium L., Cornus officinalis sieb, Rheum palmatum L., and Panax notoginseng). The remaining patients received a placebo (angiotensin-converting enzyme inhibitors/ angiotensin 2 receptor blockers) for 6 months. The results showed that UAER was not statistically different between the two groups in patients with microalbuminuria, whereas the group receiving the herbal remedy showed a statistically significant reduction in 24-hour urinary protein in patients with macroalbuminuria. eGFR also improved in patients with microalbuminuria and macroalbuminuria [31]. Adverse effects of this formula are subarachnoid hemorrhage, elevation in liver enzymes, urinary tract infections, and mild anemia [31], while those of P. notoginseng are mild in nature, and they include dryness, insomnia, restlessness, and dizziness [32].
In 2019, 600 patients with type 2 diabetes mellitus with or without DN in 10 hospitals were randomized to receive either Liuwei dihuang tablets (a Chinese formula of Rehmannia glutinosa, C. officinalis, Paeonia suffruticosa, Dioscorea opposita, P. cocos, and Alisma plantago-aquatica) and Ginkgo biloba tablets or placebos for two years in a randomized controlled trial. In patients with UACR of 30–299 mg/g at baseline, UACR decreased more significantly between baseline and follow-up in the group receiving herbal medicines than in the other group, indicating the efficacy of herbal medicines for DN [33]. Nevertheless, GIT upset was the main reported side effect of Liuwei dihuang pills in inhibiting small intestine activity and gastric emptying [33]. In contrast, the main reported side effects of G. biloba include stomach upset, constipation, headache, and dizziness [34].
Reduction of serum uric acid levels
Hyperuricemia is another factor that accelerates the decline of renal function. In recent decades, more attention has been paid to natural products as alternative agents for treating hyperuricemia. They either inhibit xanthine oxidase (XOD) (uricostatic), increase uric acid excretion (uricosuric), or exert both actions. Few articles in the literature examine the effects of herbs on hyperuricemia in CKD, so we evaluated those articles that were not conducted in this context (i.e., CKD) instead.
Dioscorea collettii [Dioscoreaceae] can lower serum uric acid levels by inhibiting XOD activity, downregulating URAT1, and upregulating OAT1 and OAT3 [35].
In one study, the effectiveness of curcumin from Curcuma longa was evaluated in 87 subjects with nonalcoholic fatty liver disease, who were randomly allocated to a group receiving curcumin (1,000 or a control one for 8 weeks). After the end of the follow-up period, it was reported that the consumption of curcumin led to a significant drop in serum TC, LDL-C, TGs, nonHDL-C, and uric acid levels [36]. Although this plant is rarely associated with side effects when taken in the short term, it can induce GIT disorders, deficiency in iron, and complications like diabetes mellitus when consumed for an extended period of time [37].
The herbal remedy of Plantago psyllium seeds, produced from Plantago ovata [Plantaginaceae], has been assessed for its effects on serum uric acid. A case report of a 50-year-old diabetic woman who took 100 mg of allopurinol followed by 5 g of psyllium seeds daily for 2 weeks revealed that the patient’s serum uric acid, urea, creatinine, TC, and TG levels normalized. This suggests that this herb can have a promising role as an adjunct to conventional therapies in managing such conditions [38]. Similarly, in another case report of a patient aged 51 years old with hyperuricemia, psyllium seeds were consumed for 40 days with a dose of 83.3 mg/kg. The 40-day treatment duration was subdivided into two courses; each was 20 days. In the first course, the remedy was consumed daily, while in the second course, the seeds were received every other day. The patient witnessed a reduction in serum uric acid to 8.1 mg/dl at the end of the former course in comparison to a drop to 6.8 mg/dl after 40 days without suffering from adverse effects, indicating that such a remedy can serve as an effective and safe option for managing hyperuricemia [39].
Harmful properties of herbal remedies in worsening CKD progression risk factors
Elevation of blood pressure
Studies addressing the nephrotoxic effects of herbal remedies in hypertensive CKD patients were scarce in the literature. Therefore, medicinal plants that increase blood pressure were studied assuming that they might have nephrotoxic effects in CKD patients.
Citrus auranticum [Rutaceae], also known as bitter orange, consists of two adrenergic agents: synephrine and octopamine, which can increase heart rate by more than 10 bpm in healthy recipients and increase systolic and diastolic blood pressure in healthy subjects by 10 and 9 mmHg, respectively, when combined with caffeine [40]. However, the presence of many phytochemicals in this plant can help in the management of anxiety, cancers of the lung and the prostate, GIT problems, and obesity [41]. Licorice or Glycyrrhiza glabra [Fabaceae] root extract can also lead to mineralocorticoid excess, causing hypertension, sodium retention, and hypokalemia [42]. It has many pharmacological benefits as an antioxidant, sedative, skin healing, anti-inflammatory, antiulcerative, antitussive, and expectorant agent [43]. In addition, the relationship between St. John’s wort or Hypericum perforatum [Hypericaceae] and hypertension is poorly understood. This plant can inhibit the reuptake of serotonin, leading to serotonin syndrome and consequently hypertension. Furthermore, a cheese reaction occurred because this plant contains hypericin, which acts as a monoamine oxidase inhibitor in vitro, resulting in a hypertensive crisis [44]. This plant possesses neuroprotective activities and has been used for many years for minor burns, depression, and anxiety [45].
Moreover, various parts of Arnica montana [Asteraceae] have been shown to increase blood pressure [46]. Despite the exertion of this adverse effect, they can increase blood perfusion, promote healing, and alleviate arthritic pains [47]. Furthermore, Ephedra distachya L. [Ephedraceae] contains many chemical compounds, such as ephedrine, pseudoephedrine, norpseudoephedrine, nor-ephedrine, and methylephedrine that can increase blood pressure [48]. These ingredients have been utilized to treat asthma, nasal congestion, and bronchitis [49].
Paullinia cupana [Sapindaceae] seeds contain 2.5%–7% caffeine (200 mg/dose), which can cause an increase in blood pressure and stimulate the release of catecholamines [50]. The extracts of this plant can exert a psychoanaleptic effect and serve as stimulants due to the caffeine content, as well as have a weight-reducing effect by altering fat metabolism. The high content of phenols also makes this plant a powerful antioxidant and antimicrobial agent [51].
Aggravation of diabetes mellitus outcomes
A thorough review of the literature revealed studies on the nephrotoxic effects of herbs on diabetes and renal function but not in the context of CKD. Therefore, they were examined in this article under the assumption that they might have various drawbacks in CKD patients.
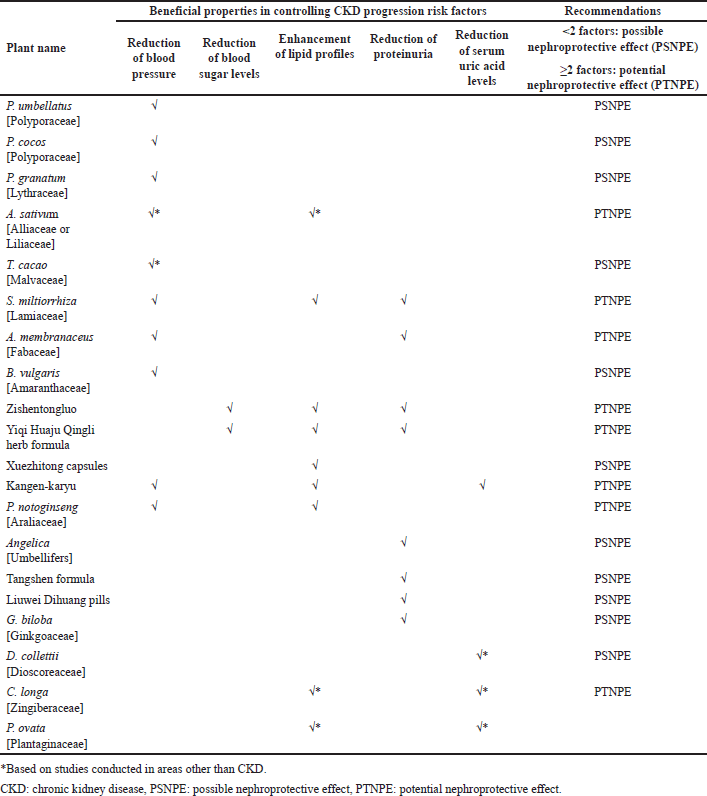 | Table 1. Pharmacological effects of plants and herbal preparations with recommendations on whether they are possibly or potentially nephroprotective. [Click here to view] |
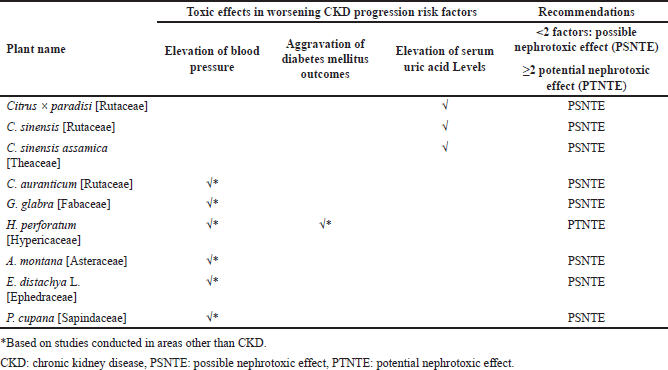 | Table 2. Pharmacological effects of plants and herbal preparations with recommendations on whether they are possibly or potentially nephrotoxic. [Click here to view] |
Extracts of St. John’s wort leaves and flowers led to insulin resistance and attenuation of insulin-stimulated glucose uptake [52]. In one case report, this herbal remedy led to acute kidney injury in a patient who consumed it as tea, leading to hemodialysis due to acute renal failure [53]. This is the first case in which such an adverse effect of St. John’s wort has been reported. Nevertheless, St. John’s wort has been shown in many studies to be a good option for treating hyperglycemia and improving insulin secretion [54].
Elevation of serum uric acid levels
As for the nephrotoxicity of herbal medicines in the context of hyperuricemia, few data were found on this topic. In one study, purple grape juice (Citrus × paradisi) and black tea (Camellia sinensis assamica) displayed enhancement in XOD activity at low doses. In contrast, pink grapefruit juice and orange juice (Citrus sinensis) promoted XOD activity at the doses measured [55]. Purple grape juice has been found to possess beneficial effects on managing diabetes, cardiovascular diseases, cancer, and neurodegenerative disorders [56]. In contrast, as constituents of black tea, flavonoids are responsible for the plant blood pressure beneficial properties, enhancement of blood circulation, and amelioration of endothelial dysfunction [57]. Orange juice has been used to treat many ailments such as constipation, tuberculosis, stress, cramps, hypertension, and menstrual problems [58].
Given the fact that some of the studies reviewed in this article were not conducted in the setting of CKD, we cannot conclude whether a certain plant is nephroprotective or not. Instead, we can assume whether it has a possible or potential nephroprotectivity or nephrotoxicity based on its net effects in controlling/ aggravating CKD progression risk factors.
Tables 1 and 2 summarize the above plants and herbal preparations with recommendations on whether they are possibly or potentially nephroprotective or nephrotoxic, respectively. In addition, a more comprehensive overview of each plant’s benefits/indications and reported side/adverse effects is illustrated in Table 3.
Quality assessment and risk of bias
This article reviewed different types of studies, including randomized trials, nonrandomized studies, and case reports. Therefore, although the RoB 2 tool is specialized for randomized trials, it was utilized in this article by every author independently to assess every article selected to minimize the potential bias of this systematic review. A summary of the assessment conducted is presented in Table 4 for every study by taking the average of the authors’ appraisal, while Figure 2 demonstrates the percentage of studies possessing a certain degree of bias (red: high risk of bias; yellow: unclear risk of bias; and green: low risk of bias). Articles displaying an unclear risk of bias in all domains did not conduct an actual action, sparing them from the assessment employed in this tool.
Limitations
There are some limitations to this literature review. First, the literature lacks articles that evaluate the effects of herbal medicines on CKD patients, especially from the aspect of harmful effects. This is disappointing because decisions on using such agents in CKD need to be based on solid research conducted in such a context. Second, some studies investigated the influence of herbal formulas in CKD without specifying the concentration or strength of each plant as a constituent, limiting the ability to indicate whether that plant extract was responsible for the effect obtained in the study, as was the case in other studies that investigated the same constituent as a single entity rather than as part of a formula. Such studies focused more on the description of the participants and the methods of analysis but paid little attention to the herbal formula administered.
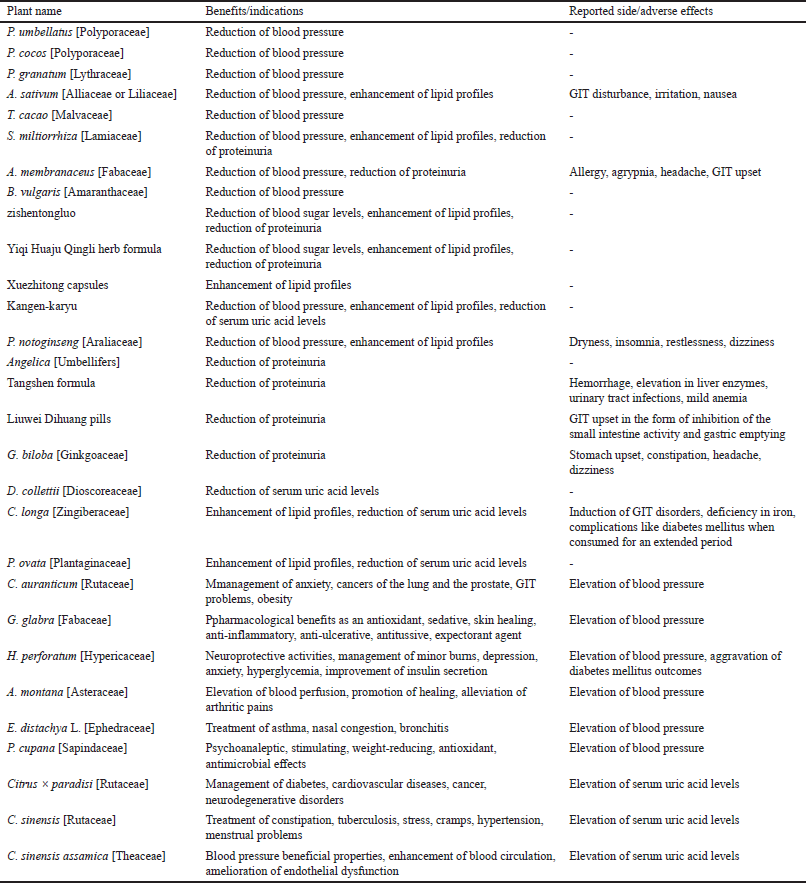 | Table 3. Pharmacological benefits/indications and reported side/adverse effects of plants and herbal preparations. [Click here to view] |
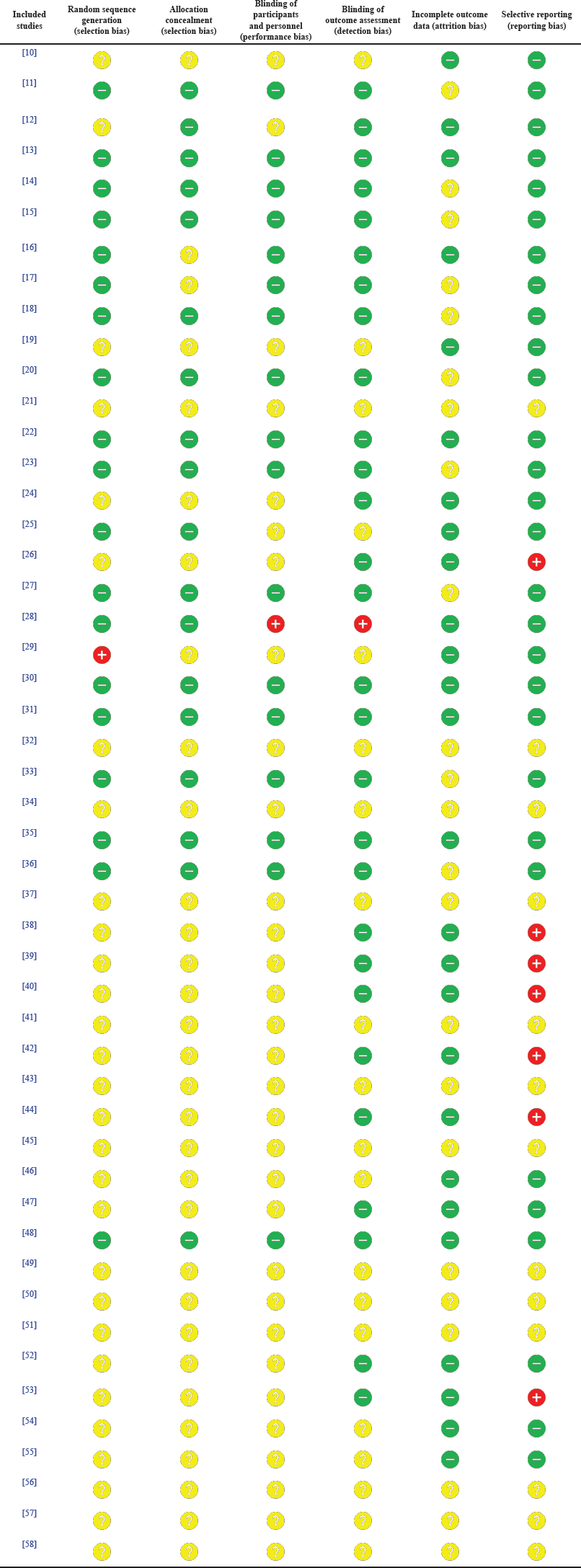 | Table 4. A summary of the RoB assessment of the included studies. [Click here to view] |
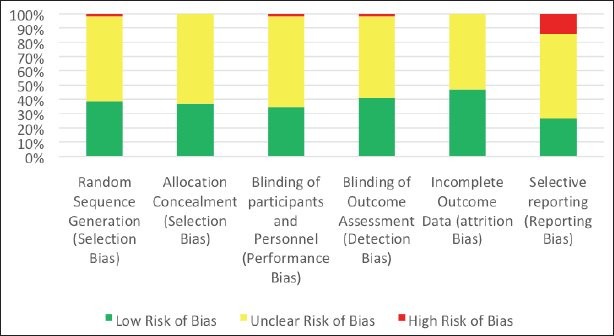 | Figure 2. A summary of the RoB assessment of the included studies. [Click here to view] |
CONCLUSION AND FUTURE DIRECTIONS
Owing to its perceived benefits and safety, the world has been witnessing a soaring increase in the use of herbal medicine for the prevention, management, and treatment of chronic diseases like CKD, which is associated with alarming rates of morbidity and mortality globally. Although pharmaceutical and chemical agents are available to control the risk factors for the progression of CKD, most of them are associated with side effects and adverse reactions, making them unpleasant for many patients with vulnerable kidneys. Therefore, herbal remedies are sought due to their natural ingredients and safety. However, they cannot be used in all cases because some are nephrotoxic, which can aggravate kidney damage and inflammation. Therefore, selecting an appropriate remedy with sufficient safety and efficacy for patients with CKD is crucial.
Agents that are nephrotoxic, such as C. auranticum, G. glabra, H. perforatum (has a potential nephrotoxic effect), A. montana, E. distachya L., P. cupana, Citrus × paradisi, and C. sinensis, must be avoided.
Plants with beneficial properties in delaying CKD progression may be used, especially those with potential nephroprotective effects, such as A. sativum, S. miltiorrhiza, A. membranaceus, zishentongluo, Yiqi Huaju Qingli herb formula, Kangen-karyu, P. notoginseng, C. longa, and P. ovata [Plantaginaceae].
Further research needs to be conducted to fill the gap in the literature created by the fact that several studies on the influence of herbal remedies have not been conducted in CKD, limiting the applicability and reliability of these studies. Therefore, it is suggested that future studies must be conducted in humans with CKD to obtain more conclusive results. In addition, based on the findings of this literature review, it is recommended that the safety and efficacy of a formulation of an herbal product with beneficial effects must be tested in all risk factors of CKD, considering the concentration of each ingredient used, e.g., a formulation containing extracts of P. umbellatus (blood pressure-lowering effect), zishentongluo (blood sugar-lowering effect), Kangen-karyu (lipid profile-improvement effect), Angelica (proteinuria-lowering effect), and C. longa (serum uric acid-lowering effect).
LIST OF ABBREVIATIONS
CKD: chronic kidney disease; ESRD: end-stage renal disease; TGs: triglycerides; NO: nitric oxide; CHD: coronary heart disease; LDL: low-density lipoprotein; DN: diabetic nephropathy; FBG: fasting blood glucose; TC: total cholesterol; UAER: urine albumin excretion rate; LDL-C: low-density lipoprotein cholesterol; eGFR: estimated glomerular filtration rate; GS-Rb1: ginsenoside Rb1; XOD: xanthine oxidase; NAFLD: nonalcoholic fatty liver disease; HDL-C: high-density lipoprotein cholesterol.
ACKNOWLEDGMENTS
The authors would like to thank Ajman University and Al Ain University for their cooperation and support in this study. Further, authors thank Ajman University for paying the article processing fee of this manuscript.
AUTHORS’ CONTRIBUTION
MMA: conceptualization; data curation; supervision; visualization; writing-original draft; writing-review and editing. YN: data curation; project administration; resources; writing—original draft. SP: methodology; formal analysis; investigation; validation; writing-review and editing. MJA: validation; visualization; writing—review and editing.
CONFLICTS OF INTEREST
The authors of this article declare no conflicts of interest.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. World Health Organization. WHO global centre for traditional medicine. World Health Organization. [cited 2022 Oct 08]. Available from: https://www.who.int/initiatives/who-global-centre-for-traditional-medicine#:~:text=88%25%20of%20all%20countries%20are,yoga%2C%20indigenous%20therapies%20and%20others
2. Mensah ML, Komlaga G, Forkuo AD, Firempong C, Anning AK, Dickson RA. Toxicity and safety implications of herbal medicines used in Africa. Herb Med. 2019;63:1992-0849. CrossRef
3. Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395(10225):709–33. CrossRef
4. Zoccali C, Bolignano D. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395:709–33. CrossRef
5. Saran R, Robinson B, Abbott KC, Agodoa LY, Albertus P, Ayanian J, et al. US renal data system 2016 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis. 2017;69(3):A7–8. CrossRef
6. Taal MW, Brenner BM. Predicting initiation and progression of chronic kidney disease: developing renal risk scores. Kidney Int. 2006;70(10):1694–705. CrossRef
7. Shaito A, Thuan DT, Phu HT, Nguyen TH, Hasan H, Halabi S, et al. Herbal medicine for cardiovascular diseases: efficacy, mechanisms, and safety. Front Pharmacol. 2020;11:422.
8. Davison EK, Brimble MA. Natural product derived privileged scaffolds in drug discovery. Curr Opin Chem Biol. 2019;52:1–8. CrossRef
9. Mandal SC, Chakraborty R, Sen S. Evidence based validation of traditional medicines: a comprehensive approach. Singapore: Springer; 2022.
10. Chen L, Chen DQ, Wang M, Liu D, Chen H, Dou F, et al. Role of RAS/Wnt/β-catenin axis activation in the pathogenesis of podocyte injury and tubulo-interstitial nephropathy. Chem Biol Interact. 2017;273:56–72. CrossRef
11. Shema-Didi L, Sela S, Ore L, Shapiro G, Geron R, Moshe G, et al. One year of pomegranate juice intake decreases oxidative stress, inflammation, and incidence of infections in hemodialysis patients: a randomized placebo-controlled trial. Free Radic Biol Med. 2012;53(2):297–304. CrossRef
12. Boldaji RB, Akhlaghi M, Sagheb MM, Esmaeilinezhad Z. Effects of pomegranate juice on cardiometabolic risk factors and biomarkers of oxidative stress and inflammation in hemodialysis patients, a crossover controlled trial. J Sci Food Agric. 2019;100:846–54. CrossRef
13. Zhang XH, Lowe D, Giles P, Fell S, Board AR, Baughan JA, et al. A randomized trial of the effects of garlic oil upon coronary heart disease risk factors in trained male runners. Blood Coagul Fibrinolysis. 2001;12(1):67–74.
14. Asgharpour M, Khavandegar A, Balaei P, Enayati N, Mardi P, Alirezaei A, et al. Efficacy of oral administration of Allium sativum powder “garlic extract” on lipid profile, inflammation, and cardiovascular indices among hemodialysis patients. Evid Based Complement Alternat Med. 2021;2021:6667453. CrossRef
15. Jafari F, Tabarrai M, Abbassian A, Jafari F, Ayati MH. Effect of garlic (Allium sativum) supplementation on premenstrual disorders: a randomized, double-blind, placebo-controlled trial. Evid Based Complement Alternat Med. 2021;2021:9965064.
16. Di Renzo GC, Brillo E, Romanelli M, Porcaro G, Capanna F, Kanninen TT, et al. Potential effects of chocolate on human pregnancy: a randomized controlled trial. J Matern Fetal Neonatal Med. 2012;25(10):1860–7.
17. Taubert D, Berkels R, Roesen R, Klaus W. Chocolate and blood pressure in elderly individuals with isolated systolic hypertension. JAMA. 2003;290(8):1029–30.
18. Sheng B, He D, Zhao J, Chen X, Nan X. The protective effects of the traditional Chinese herbs against renal damage induced by extracorporeal shock wave lithotripsy: a clinical study. Urol Res. 2011;39(2):89–97. CrossRef
19. Jin H, Luo Q, Zheng Y, Nurahmat M, Wu J, Li B, et al. CD4+ CD25+ Foxp3+ T cells contribute to the antiasthmatic effects of Astragalus membranaceus extract in a rat model of asthma. Int Immunopharmacol. 2013;15(1):42–9.
20. Kemmner S, Lorenz G, Wobst J, Kessler T, Wen M, Günthner R, et al. Dietary nitrate load lowers blood pressure and renal resistive index in patients with chronic kidney disease: a pilot study. Nitr Oxide. 2017;64:7–15. CrossRef
21. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease. Clin J Am Soc Nephrol. 2017;12(12):2032–45. CrossRef
22. Ma J, Xu L, Dong J, Wei H, Zhi Y, Ma X, et al. Effects of zishentongluo in patients with early-stage diabetic nephropathy. Am J Chin Med. 2013;41(02):333–40. CrossRef
23. Wang TZ, Chen Y, He YM, Fu XD, Wang Y, Xu YQ, et al. Effects of Chinese herbal medicine Yiqi Huaju Qingli Formula in metabolic syndrome patients with microalbuminuria: a randomized placebo-controlled trial. Eur J Integr Med. 2013;11(3):175–83. CrossRef
24. Bravo E, Amrani S, Aziz M, Harnafi H, Napolitano M. Ocimum basilicum ethanolic extract decreases cholesterol synthesis and lipid accumulation in human macrophages. Fitoterapia. 2008;79(7–8):515–23. CrossRef
25. Feng Y, Liang W, Liang W, Xiao X, Zhang Y. Effects of XueZhiTong capsules on chronic kidney disease patients with dyslipidemia. Trop J Pharm Res. 2022;21(1):177–83. CrossRef
26. Park CH, Kitazawa T, Futamura A, Hirana H, Shoji M, Osanai M, et al. Therapeutic potential of Chinese prescription Kangen-karyu for patient with lifestyle-induced metabolic syndrome. Drug Discov Ther. 2020;14(5):252–5. CrossRef
27. Xu X, Lu Q, Wu J, Li Y, Sun J. Impact of extended ginsenoside Rb1 on early chronic kidney disease: a randomized, placebo-controlled study. Inflammopharmacology. 2017;25(1):33–40. CrossRef
28. Junfeng S, Hanwei Z, Chong Z. Therapeutic efficacy of Astragalus in patients with chronic glomerulonephritis. Acta Univ Med Secondae Shanghai. 2002;22(3):245–7.
29. Okuda M, Horikoshi S, Matsumoto M, Tanimoto M, Yasui H, Tomino Y. Beneficial effect of Astragalus membranaceus on estimated glomerular filtration rate in patients with progressive chronic kidney disease. Hong Kong J Nephrol. 2012;14(1):17–23. CrossRef
30. Peicheng S, Xuejun Y, Liqun H. Effect of Astragali and Angelica particle on proteinuria in Chinese patients with primary glomerulonephritis. J Tradit Chin Med. 2016;36(3):299–306. CrossRef
31. Li P, Chen Y, Liu J, Hong J, Deng Y, Yang F, et al. Efficacy and safety of tangshen formula on patients with type 2 diabetic kidney disease: a multicenter double-blinded randomized placebo-controlled trial. PLoS One. 2015;10(5):e0126027. CrossRef
32. Tan J, Zhang H, Zhang L, Xu H. Ginseng and “Shanghuo”(fireness): a comprehensive review from the viewpoints of TCM theory and modern science. Food Funct 2023;14: 3437–53.
33. Shi R, Wang Y, An X, Ma J, Wu T, Yu X, et al. Efficacy of co-administration of liuwei dihuang pills and Ginkgo biloba tablets on albuminuria in type 2 diabetes: a 24-month, multicenter, double-blind, placebo-controlled, randomized clinical trial. Front Endocrinol. 2019;10:100. CrossRef
34. Aritonang DY. The potency of Ginkgo biloba in treating tinnitus: a review. Eureka Herba Indonesia. 2022;3(2):145–50.
35. Guo Y, Lu H, Gan J, Li D, Gao J, Zhang C. Efficacy of Chinese herbal medicine Jiangniaosuan formula for treatment of hyperuricemia: study protocol for a double-blinded noninferiority randomized controlled clinical trial. Trials. 2022;23(1):1. CrossRef
36. Panahi Y, Kianpour P, Mohtashami R, Jafari R, Simental-Mendía LE, Sahebkar A. Curcumin lowers serum lipids and uric acid in subjects with nonalcoholic fatty liver disease: a randomized controlled trial. J Cardiovasc Pharmacol. 2016;68(3):223–9.
37. Ayman EL, Hassan SM, Mohamed AM, Mohammed HM. Tumeric or Curcuma longa Linn. In Nonvitamin and nonmineral nutritional supplements. Cambridge, MA: Academic Press; 2019. pp 447–53.
38. Ebadollahi-Natanzi A, Arab-Rahmatipour G. Psyllium together with allopurinol can efficiently reduce the increased serum level of uric acid, creatinine and urea: a case report. Iran J Toxicol. 2017;11(4):51–6.
39. Ebadollahi-Natanzi A, Arabrahmatipour G. Uric acid lowering effects of psyllium seeds on a hyperuricemic patient: a case report and review of literature. Asia Pac J Med Toxicol. 2020;9(1):21–4.
40. Jack S, Desjarlais-Renaud T, Pilon K. Bitter orange or synephrine: update on cardiovascular adverse reactions. CMAJ. 2007;176(8):1230.
41. Suntar I, Khan H, Patel S, Celano R, Rastrelli L. An overview on Citrus aurantium L.: its functions as food ingredient and therapeutic agent. Oxid Med Cell Longev. 2018;2018:7864269.
42. Murphy SC, Agger S, Rainey PM. Too much of a good thing: a woman with hypertension and hypokalemia. Clin Chem. 2009;55(12):2093–6. CrossRef
43. Pastorino G, Cornara L, Soares S, Rodrigues F, Oliveira MB. Liquorice (Glycyrrhiza glabra): a phytochemical and pharmacological review. Phytother Res. 2018;32(12):2323–39.
44. Madhavan P, Nallu R, Samat A. MON-429 malady or remedy? hypertensive crisis in a young woman with use of St. John’s Wort for depression. J Endocr Soc. 2019;3(Supplement_1):MON-429.
45. Oliveira AI, Pinho C, Sarmento B, Dias AC. Neuroprotective activity of Hypericum perforatum and its major components. Front Plant Sci. 2016;7:1004.
46. Judžentien? A, B?dien? J. Analysis of the chemical composition of flower essential oils from Arnica montana of Lithuanian origin. Chemija. 2009;20(3):190–4.
47. Röhrl J, Piqué-Borràs MR, Jaklin M, Werner M, Werz O, Josef H, et al. Anti-inflammatory activities of Arnica montana planta tota versus flower extracts: analytical, in vitro and in vivo mouse paw oedema model studies. Plants. 2023;12(6):1348.
48. Elnabtity AM, Selim MF. Norepinephrine versus ephedrine to maintain arterial blood pressure during spinal anesthesia for cesarean delivery: a prospective double-blinded trial. Anesth Essays Res. 2018;12(1):92–7. CrossRef
49. Ghavam M, Soleimaninejad Z. An overview of the various uses of Ephedra distachya L. from the past to the present. Avicenna J Pharm Res. 2020;1(2):82–6.
50. Jellin JM, Gregory PJ, Batz F, Hitchens K, Bonakdar R, Scott GN. Pharmacist’s letter/prescriber’s letter natural medicines comprehensive database. Stockton, CA: Therapeutic Research Faculty; 2000. pp 1–527.
51. de Carvalho FA, Lorenzo JM, Pateiro M, Bermúdez R, Purriños L, Trindade MA. Effect of guarana (Paullinia cupana) seed and pitanga (Eugenia uniflora L.) leaf extracts on lamb burgers with fat replacement by chia oil emulsion during shelf life storage at 2 C. Food Res Int. 2019;125:108554.
52. Richard AJ, Amini ZJ, Ribnicky DM, Stephens JM. St. John’s Wort inhibits insulin signaling in murine and human adipocytes. BBA Mol Basis Dis. 2012;1822(4):557–63. CrossRef
53. Adibelli Z, Karacay I, Demir M, Duran C. St. John’s Wort (Hypericum perforatum)-related acute kidney injury. Blood Purif. 2022;51(6):520-222. CrossRef
54. Liang C, Hao F, Yao X, Qiu Y, Liu L, Wang S, et al. Hypericin maintians PDX1 expression via the Erk pathway and protects islet β-cells against glucotoxicity and lipotoxicity. Int J Biol Sci. 2019;15(7):1472–87. CrossRef
55. Dew TP, Day AJ, Morgan MR. Xanthine oxidase activity in vitro: effects of food extracts and components. J Agric Food Chem. 2005;53(16):6510–5. CrossRef
56. Razavi BM, Hosseinzadeh H. A review of the effects of Citrus paradisi (grapefruit) and its flavonoids, naringin, and naringenin in metabolic syndrome. Bioactive food as dietary interventions for diabetes. Cambridge, MA: Academic Press; 2019. pp 515–43.
57. Naveed M, BiBi J, Kamboh AA, Suheryani I, Kakar I, Fazlani SA, et al. Pharmacological values and therapeutic properties of black tea (Camellia sinensis): a comprehensive overview. Biomed Pharmacother. 2018;100:521–31.
58. Favela-Hernández JM, González-Santiago O, Ramírez-Cabrera MA, Esquivel-Ferriño PC, Camacho-Corona MD. Chemistry and pharmacology of Citrus sinensis. Molecules. 2016;21(2):247.
SUPPLEMENTARY MATERIAL
Supplementary data can be downloaded from the journal’s website