INTRODUCTION
Cancer continues to be a major financial burden on healthcare systems worldwide, representing one of the costliest diseases. It also stands as the second leading cause of death globally, according to the World Health Organization. The disease caused 10 million deaths by 2020, with a high incidence in low- and low-middle-income countries [1]. However, recent studies in the United States have shown that cancer death rates from leukemia, melanoma, lung cancer, and kidney cancer are declining [2]. Furthermore, the projected number of new cancer cases and deaths in the United States by the year 2023 is estimated to be 1,958,310 and 609,820, respectively.
Cancer comprises a group of diseases characterized by display uncontrolled division (beyond the regular limits), invasion (direct extension and penetration of adjacent tissues), and sometimes metastasis (spread to other parts of the body through lymph or blood) [3]. Despite the diversity in the tissues where cancer can originate and the unique characteristics of each type, there is a fundamental process in cancer development that is shared among all forms of the disease. Among the various types of cancer, the most common in men include prostate, stomach, lung, colorectal, and liver cancers, while breast, lung, cervical, colorectal, and thyroid cancers are the most prevalent in women. [1].
Cancer treatments encompass a variety of approaches, including surgery, radiation therapy, and systemic treatments such as chemotherapy, targeted therapy, hormonal therapy, and immunotherapy. While these treatments have shown significant success in combating cancer, they often come with associated side effects. These adverse effects can vary depending on the treatment type and the individual patient, ranging from mild discomfort to more severe complications [4].
In the majority of malignancies, chemotherapy still stands as the foremost effective and commonly employed treatment. While current chemotherapy treatments, such as those based on 5-FU and oxaliplatin, demonstrate effectiveness in combating cancer, they are associated with a range of toxic effects, including nephrotoxicity, ototoxicity, neurotoxicity, cardiotoxicity, hematological toxicity, hepatotoxicity, gastrointestinal toxicity, and myelosuppression, among others. Such adverse effects often lead to dose limitations or discontinuation of treatment effects [5–7]. As cancer statistics continue to rise and the associated toxicity of conventional chemotherapy becomes a greater concern, intensive endeavors are underway to investigate and create novel pharmaceutical agents with potential chemopreventive properties for cancer. In this sense, several studies have been carried out to develop new synthetic alternative substances with high anticancer activity.
Dihydropyrimidinone (DHPM) is a heterocyclic compound that has been synthesized and studied for its potential as a drug candidate in various therapeutic areas. The DHPM is distinguished by its multifunctional scaffold, which displays a wide range of biological activities. [8–10] and especially anti-cancer activity. Monastrol (Fig. 1) is considered a DHPM derivative with promising anticancer activity, which was first described by Mayer et al. [11], and originally identified in a phenotype-based screening of a chemical library. DHPM and monastrol are both heterocyclic compounds; monastrol has a 4-methoxyphenyl group substituted at the 2-position of the heterocyclic ring, while DHPM has an acyclic substituent at this position. Monastrol triggers mitotic arrest at the G2/M phase by targeting the mitotic kinesin-5 motor protein Eg5 in mammalian cells, causing inhibition of the motility, therefore leading to cell apoptosis [12]. It has been demonstrated that this compound exhibits varying antiproliferative effects in breast adenocarcinoma MCF-7 cells and mammary epithelium HB4a cells [13]. The antiproliferative activity of this compound in MCF-7 cells is associated with cell cycle arrest in the M phase, displaying characteristics indicative of mitotic slippage without inducing neurotoxicity [14].
Despite its demonstrated activity in vitro, monastrol exhibits non-drug-like properties, such as poor solubility, low half-life, and limited bioavailability, which have hindered its further development as a therapeutic agent. To overcome these challenges, carrier-based nanoparticle drug delivery has emerged as a promising alternative approach. This approach aims to improve the effectiveness and patient compliance of drugs by enhancing their targeting capabilities. In this context, monastrol was successfully loaded onto mesoporous silica nanoparticles (MSNPs) and coated with hydrogels to achieve controlled drug delivery. Hanif et al. [15] tested monastrol-loaded MSNPs on human cervical epithelial malignant carcinoma (HeLa) cell lines and observed the successful delivery of monastrol into the cancer cells in vitro.
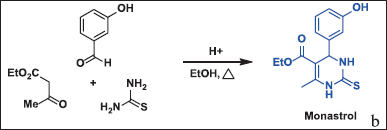 | Figure 1. Biginelli condensation in the monastrol synthesis. [Click here to view] |
Monastrol has been used in medicinal chemistry as a scaffold for new anticancer agents, such as molecular derivatives or hybrids [9]. These compounds have demonstrated enhanced antiproliferative activity against various cancer cell lines compared to monastrol itself [16,17]. Monastrol and DHPM are obtained using the Biginelli condensation (Fig. 1), consisting of a reaction of an aldehyde with thiourea and a β-ketoester catalyzed by a strong acid [18]. Recently, novel synthetic methodologies have been devised, leading to improved yields compared to the original procedures. These innovative approaches involve the combination of Lewis acids and/or transition metal salts [19].
Synthesis and anticancer activity assessment of monastrol derivatives and hybrids are currently active areas of research, with high scientific output of new compounds so do their potential antiproliferative effects on cancer cell lines. As a result, it becomes challenging to have a comprehensive view of all the studies on this topic due to the vast volume of information available in the scientific literature. The bibliometric analysis is a scientometric tool that helps researchers to have a holistic overview of the available information on a knowledge area. Furthermore, a bibliometric analysis of compounds based on monastrol is still lacking. Therefore, the aim of this work was to conduct a bibliometric analysis of monastrol, their derivatives, and hybrids to describe and analyze the research trend and the main outputs of this field.
METHODS
Database selection and search strategy
Data was acquired from the Scopus database on 28 February 2023 and retrieved the most relevant documents related to monastrol. The search equations were queried within the fields of the article title, abstract, and keywords, and were refined to articles and reviews as described elsewhere [20–22]. To cover the studies related to monastrol and their derivatives and hybrids, two search equations were designed as follows:
Search equation #1: TITLE-ABS-KEY (monastrol) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”)) AND (EXCLUDE (PUBYEAR, 2023)).
Timespan: 1999 to 2022.
Search equation #2: (TITLE-ABS-KEY (monastrol) AND TITLE-ABS-KEY (hybrids) OR TITLE-ABS-KEY (derivatives)) AND (LIMIT-TO (DOCTYPE, “ar”) OR LIMIT-TO (DOCTYPE, “re”)) AND (EXCLUDE (PUBYEAR, 2023)).
Timespan: 1999 to 2022.
Figure 2 shows a flowchart of the methodology used during the search.
Data export and bibliometric indicators
The information retrieved from the database were abstract, keywords, and both citation and bibliographical information. Data was downloaded from Scopus, and exported in Excel® format. Subsequently, VOSviewer 1.6.18 was used for visualization and data analysis [23]. The volume and growth of publications, subject areas, co-occurrence keywords network visualization, co-occurrence keywords overlay visualization, leading institutions and journals, and collaboration networks among the countries are the bibliometric indicators that were evaluated.
RESULTS
Evolution of publishing papers related to monastrol
A total of 409 records were retrieved from the Scopus database for the first search equation. 83.86% and 16.14% of them correspond to research articles and reviews, respectively. Figure 3 shows the growth of published studies related to monastrol from 1999 to 2022. In this timespan, an increase in the number of issued papers was observed between 1999 and 2006. Then, a slight decrease was perceived and the records oscillated around twenty documents. Furthermore, the main areas of knowledge of those records were: (i) biochemistry, genetics, and molecular biology, (ii) chemistry, and (iii) pharmacology, toxicology, and pharmaceutics, with 35.3, 17.6% and 17.4% of the records. In addition, the United States ranked first, followed by China, Germany, India, the United Kingdom, and Brazil as the leading countries in the number of publications (Fig. 4a).
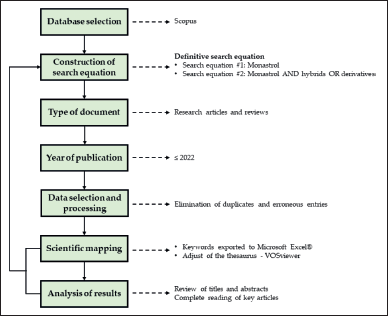 | Figure 2. Methodological flowchart for bibliometric analysis studies. [Click here to view] |
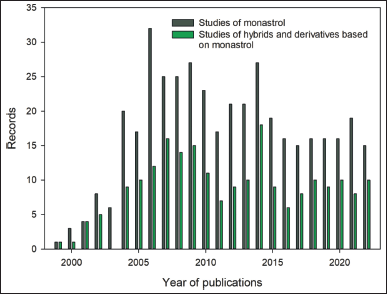 | Figure 3. Evolution of published studies related to monastrol and their hybrids and derivatives from 1999 to 2022. [Click here to view] |
Likewise, for the second search equation, 212 records were retrieved from the Scopus database. Here, the scientific production was not uniform but since 2004 the records range between 6 and 18 (Fig. 3). Concerning the main areas of knowledge, the top three were the same as for the first search equation, with biochemistry, genetics, and molecular biology keeping its first place, but chemistry, and pharmacology, toxicology, and pharmaceutics exchanged their positions, ranked third and second, respectively. For this search, the United States, China, and India, followed by Brazil and the United Kingdom, were the leading countries in terms of the number of publications (Fig. 4b).
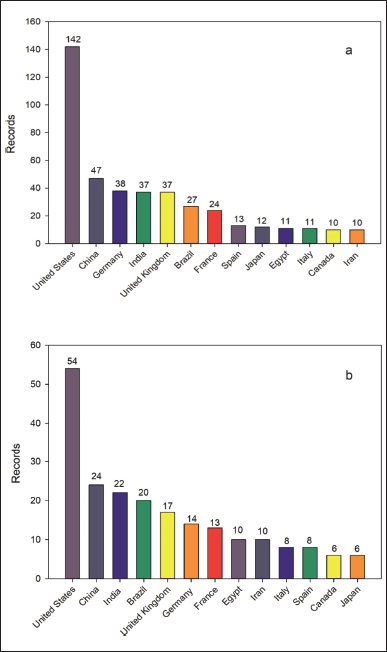 | Figure 4. Top 10 leading countries of studies related to monastrol between 1999 and 2022. a) Studies of monastrol. b) Studies of hybrids and derivatives based on monastrol. [Click here to view] |
Journals, institutions, and publications with the highest impact
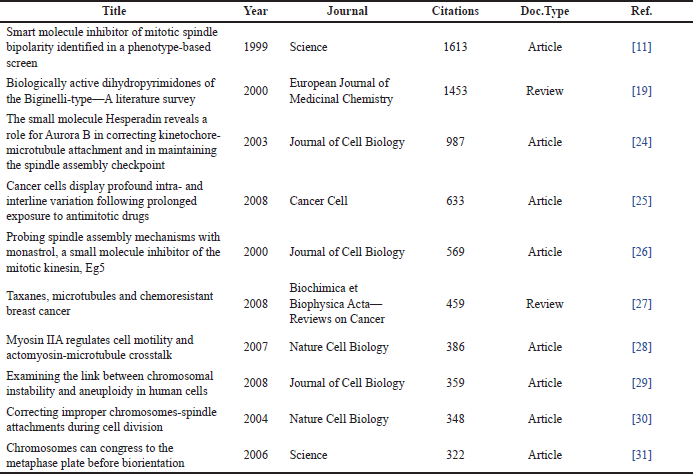
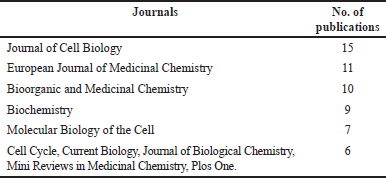
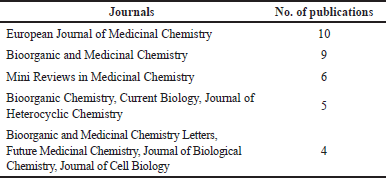
Table 1 shows the top 10 cited studies related to monastrol, where 8 and 2 are research papers and reviews, respectively. In addition, the top five leading institutions on this topic are displayed in Table 2. As expected, the leading institutions are located in the top countries previously mentioned. Tables 3 and 4 show the leading journals for the first and second search equations, respectively. As predictable, the searches share most of them.
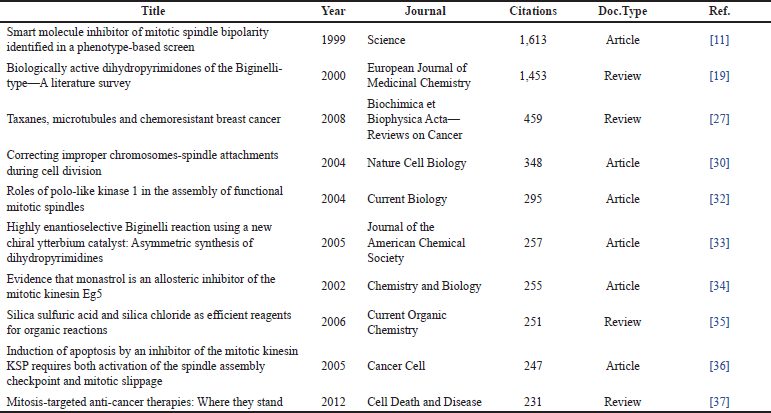
In addition, Table 5 shows the top 10 cited studies for the second search, where 6 are research articles and four are reviews.
Bibliometric networks of monastrol and their hybrids and derivatives
The co-occurrence of the author’s keywords network contains five clusters and 24 nodes (Fig. 5a). Among them, molecular docking studies, treatment of breast cancer (red, purple, and green clusters), anticancer activities and inhibitors (yellow and blue clusters) worth being highlighted. Figure 5b shows the topics the researchers focused on between 2008 and 2018. Clearly, studies of molecular docking, pharmacophore, and cytotoxicity based on monastrol were concentrated in 2018 (see yellow nodes in Fig. 5b).
 | Table 1. The top 10 most cited studies related to monastrol between 1999 and 2022. [Click here to view] |
 | Table 2. The top 5 institutions publishing studies related to monastrol between 1999 and 2022. [Click here to view] |
 | Table 3. The top 5 journals publishing studies related to monastrol between 1999 and 2022. [Click here to view] |
 | Table 4. The top 5 journals publishing studies related to hybrids and derivatives based on monastrol between 1999 and 2022. [Click here to view] |
 | Table 5. The top 10 most cited studies related to hybrids and derivatives based on monastrol between 1999 and 2022. [Click here to view] |
Concerning the co-occurrence of the author’s keywords network of studies based on hybrids and derivatives of monastrol, Figure 6a displayed 27 nodes within 6 clusters. Similar nodes are observed compared with 5a, highlighting molecular docking, breast cancer, DHPM, and monastrol analogs. Once again, molecular docking was one of those that concentrated the studies in 2018, along with works on cytotoxicity and cell cycle analysis (Fig. 6b). Interestingly, studies of hybrids based on monastrol also appeared as a hot topic between 2016 and 2018 (Fig. 6b) but with a small node, indicating that it is an emerging topic in this field. Furthermore, Figure 7 shows the countries’ collaboration networks. Clearly, the United States, China, India, Brazil, the United Kingdom, and Germany displayed the biggest nodes in this network. This is therefore consistent with Figure 4.
Since the bibliometric results of this work indicated that the studies related to monastrol have been focused on molecular docking, pharmacophore, and cytotoxicity of their hybrids and derivatives during the last years, the discussion was concentrated on these themes
DISCUSSION
Derivatives and hybrids based on monastrol as potential anticancer agents
In Figure 8, we show some examples of the vast collection of monastrol hybrids and derivatives, which have been active against different cancer lines. A range of DHPM derivatives incorporating different N-heterocyclic moieties was successfully synthesized and subjected to cytotoxicity testing. Notably, Compound A displayed remarkable activity against NCI-H460, SK-MEL-5, and HL-60 (TB) cell lines, inhibiting their growth by 88%, 86%, and 85%, respectively. Importantly, Compound A demonstrated increased safety on normal cells compared to doxorubicin, a standard chemotherapy agent. Further investigation of A549 cells treated with Compound A revealed cell cycle arrest at the G2/M phase and pro-apoptotic activity, verified by annexin V-FITC staining [18].
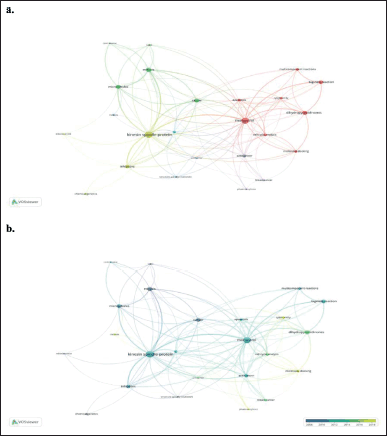 | Figure 5. Bibliometric network of studies related to monastrol between 1999 and 2022. a) Research-topic map. b) Research-topic map with time overlap. The nodes represent the author´s keywords while the size of the circle indicates their occurrences (i.e., the number of publications that have the corresponding term in their title or abstract). In addition, the width of the line linking them is proportional to the strength of the relationship between the author´s keywords, and the distance between them indicates the relatedness of the nodes. Note: minimum number of occurrences of a keyword is four. [Click here to view] |
Monastrol derivative B showed cytotoxicity activity with an MG-MID value of 43.16%. This compound displayed antiproliferative activity against many cell lines. Compound B exhibited notable effects on non-small-cell lung cancer NCI-H522 cells with a growth inhibition rate of −42.26% and MDA-MB-468 breast cancer cells with a rate of −1.10% at a single dose-assay concentration of 10−5 M. Furthermore, cell cycle analysis of NCI-H522 cells treated with compound B revealed cell cycle arrest at the G2/M phase [38]. Conjugates of monastrol C, containing proline, have demonstrated promising in vitro cytotoxic activity against leukemia. Notably, they exhibited good cytotoxic potency against the SR cell line of the leukemia sub-panel with an inhibitory effect of 53.3%. Furthermore, against the HL-60 (TB) and RPMI-8226 cell lines, the conjugates displayed inhibitory effects of 43.2% and 27.1%, respectively. Molecular docking studies have revealed that these conjugates (C) exhibit superior interactions at the kinesin Eg5 receptor compared to monastrol, suggesting their potential as potent anticancer agents. [39]. Monastrol-triazole hybrid D showed good cytotoxicity against cancer cell lines MCF-7 (breast), OVCAR-3 (ovarian), and SKOV-3 (ovarian) with IC50 of 22.0, 24.2, and 29.1 μM, respectively [40].
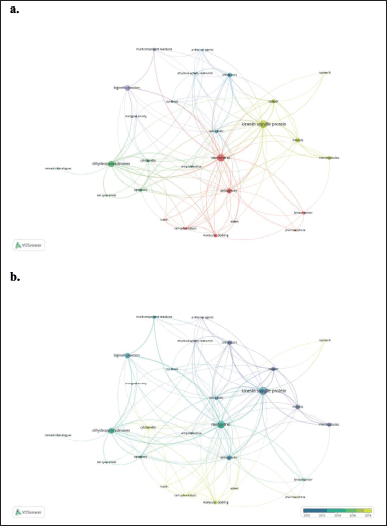 | Figure 6. Bibliometric network of studies related to hybrids and derivatives based on monastrol between 1999 and 2022. a) Research-topic map. b) Research-topic map with time overlap. The nodes represent the author´s keywords while the size of the circle indicates their occurrences (i.e. the number of publications that have the corresponding term in their title or abstract). In addition, the width of the line linking them is proportional to the strength of the relationship between the author´s keywords, and the distance between them indicates the relatedness of the nodes. Note: minimum number of occurrences of a keyword is three. [Click here to view] |
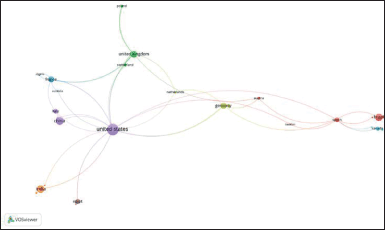 | Figure 7. Bibliometric map of global collaboration network among countries researching hybrids and derivatives based on monastrol between 1999 and 2022. The nodes represent the countries while the size of the circle denotes the number of publications. In addition, the width of the line linking them is proportional to the strength of the relationship between countries, and the distance between them indicates the relatedness of the nodes. The minimum number of documents in a country is one. [Click here to view] |
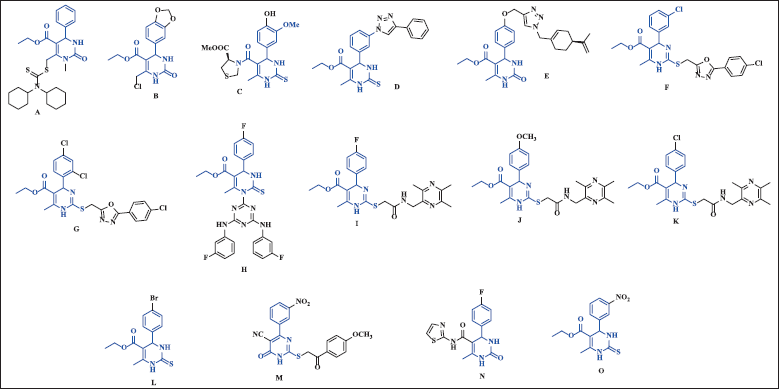 | Figure 8. Chemical structure of monastrol and their hybrids and derivatives. [Click here to view] |
Perillyl-DHPM hybrid E exhibited promising antiproliferative activity against OVCAR-3 (ovarian) with a TGI (concentration that produces total growth inhibition, or cytostatic effect) value of 6.90 μM. In addition, this hybrid also showed activity against cell lines U251 (glioma) and UACC-62 (melanoma) with IC50 of 12.43 μM and 19.18 μM, respectively [41].
Monastrol-oxadiazole hybrids F and G exhibited significant activity against leukemia HL-60 (TB) and MOLT-4 cells, with IC50 values of 56 nM and 80 nM, respectively. These hybrids demonstrated greater potency compared to monastrol, which had IC50 values of 147 nM and 215 nM for those cell lines. Cell cycle analysis of HL-60 (TB) cells treated with hybrid F and MOLT-4 cells treated with hybrid G indicated cell cycle arrest at the G2/M phase and pro-apoptotic activity, as evidenced by annexin V-FITC staining [36]. In addition, monastrol-1,3,5-triazine hybrid H was evaluated for its anticancer activity against HeLa (cervical cancer), MCF-7 (breast cancer), HL-60 (human promyelocytic leukemia), and HepG2 (hepatocellular carcinoma) cell lines, showing inhibitory activities of 39.7% ± 0.81%, 41.5% ± 0.31%, 23.1% ± 0.36%, and 31.2% ± 0.82%, respectively. Also, this compound was tested over MCF 12A (normal epithelial breast cell line), which was non-toxic [43].
Monastrol analogs with ligustrazine moiety (I-K) demonstrated significant antiproliferative activity against three major types of human tumor cell lines: breast MCF-7, colon HCT-116, and liver HepG-2. The IC50 values for these analogs ranged between 11.3 μM and 47.6 μM, indicating their potential as effective agents against these cancer cell lines. These compounds were most potent than monastrol (IC50 118-142 μM) [44]. The tetrahydropyrimidine-5-carboxylate derivative (L) demonstrated a notable cytotoxic activity against three human cancer cell lines, namely MCF-7, HepG-2, and AGS, with IC50 values of 59.97 ± 1.84 μM, 62.72 ± 1.11 μM, and 55.39 ± 0.98 μM, respectively [45]. On the other hand, the pyrimidinone derivative (M) displayed cytotoxic activity against four cancer cell lines, including HCT-116, HeLa, HepG-2, and MCF-7, with IC50 values ranging from 3.75 μM to 5.13 μM. Interestingly, it demonstrated safety on the normal human cell line WI-38, with a selectivity index value of 13.7 on HCT-116 cells, suggesting its potential as a promising candidate in cancer treatment. This compound showed arrested at the G2/M phase on HCT-116 cells with considerable apoptotic effect [46]. A 2-aminothiazolyl/benzothiazolyl derivative of 3,4-DHPMs (N) was active against AGS cell lines (IC50 7.06 μg/ml), being more cytotoxic than cis-platin (IC50 11.49 ± 1.35) [47].
Compound O, also known as LaSOM 65, exhibited a remarkable two-fold increase in activity against rat (C6) and human (U138-MG) glioma cell lines compared to monastrol. Furthermore, this compound demonstrated potent antitumoral activity against the Sarcoma 180 murine model when administered daily through intraperitoneal administration at a dose of 90 mg/kg for 7 days, resulting in a remarkable over 70% reduction in tumor growth [48]. Compound O showed good pharmacokinetic and tissue distribution and did not show signs of acute toxicity in an investigation in Wistar rats [49].
Molecular docking studies of monastrol derivatives and hybrids as a tool for anticancer drugs prospection
Molecular docking is a computational technique that predicts the binding mode and affinity of small molecules to a target protein. It is widely used in drug discovery and design to screen large databases of compounds and identify potential lead candidates that can modulate a protein of interest. Specifically, several recent studies have used molecular docking to identify potential monastrol analogs with improved Eg5 inhibitory activity. Eg5 is a member of the kinesin-5 family, playing a crucial role in cancer development by ensuring the proper assembly and maintenance of the bipolar spindle during the mitosis process. This essential function makes Eg5 an attractive target for potential anticancer therapies, particularly in specific types of cancer [50].
Compound B showed a kinesin enzyme inhibitory activity with an IC50 = 1.47 μM. This structure was subjected to a molecular docking study using the crystal structure of the kinesin Eg5 enzyme to elucidate the observed outcomes. This study unveiled several notable attractive forces, primarily through hydrogen bonding, with distinct amino acid residues (such as Glu116 and Glu118) in the active site. These findings suggest that the enhanced binding interactions of compound B with the Eg5 enzyme could be responsible for its superior activity compared to monastrol [38]. For monastrol analogs bearing ligustrazine moiety (I-K), molecular docking simulations were carried out on the most potent cytotoxic targets within the active site of the kinesin Eg5 protein. These compounds exhibited significant H-bonding interactions with critical amino acids Glu116 and Glu118, resembling the native ligand monastrol. These findings highlight the essential role of the pyrimidine nucleus, which incorporates thio-N-methylacetamide at position 2 and ethyl carboxylate at position 5, in the binding characteristics. These specific modifications appear to be crucial for the favorable binding interactions and enhanced activity of those compounds compared to monastrol, emphasizing its potential as a promising candidate for further investigation in anticancer therapies targeting Eg5 [38].
Docking studies of the monastrol derivative (L) revealed a favorable binding affinity towards the Eg5 enzyme, facilitated by three hydrogen bonding interactions. These interactions involve the ligand’s hydroxyl group with the oxygen carbonyl group in Glu118, the NH of THPM ring with the oxygen carbonyl group in Glu116, and the oxygen of the ester moiety with the hydroxyl group of Tyr211 [45]. Subsequent tests for Eg5 inhibitory activity of the pyrimidinone derivative M demonstrated a significant inhibition with an IC50 value of 12.89 μM when compared to monastrol (IC50 = 14.89 μM). The docking results revealed interactions with various amino acid residues in the binding site, which contributed to increased stability in the ligand-receptor complex, resulting in improved Eg5 inhibitory activity. These findings suggest the potential of compound M as a promising Eg5 inhibitor with enhanced activity compared to monastrol [46]. Molecular dynamics and molecular docking simulations applied to 2-aminothiazolyl/benzothiazolyl derivatives of 3,4-DHPMs (N) were conducted for exploring the binding model on the allosteric site of Eg5. The presence of a 4-(3-bromo phenyl) substituent resulted in the most potent inhibitory effect on cancer cell migration. This effect could be attributed to the larger size and higher hydrophobicity of the substituent, enabling stable and balanced binding interactions with Eg5 [47].
CONCLUSION
Monastrol and its hybrids and derivatives have been a subject of increasing interest in the scientific community since it was first reported in 1999, given the promising results as potential anticancer compounds. A significant number of articles and reviews have been published, with a peak in production between 1999 and 2006, followed by a slight decrease and stabilization of around 20 articles per year. Monastrol hybrids and derivatives have displayed cell cycle arrest, pro-apoptotic activity, and good cytotoxic potency against various cancer cell lines, such as lung, breast, ovarian, glioma, and melanoma. In addition, the monastrol O derivative showed good results in a preclinical and pharmacokinetic study. The network analysis revealed the most frequent topics in the scientific literature related to monastrol, which included molecular docking studies, anticancer activities against cancer, and inhibitors.
ACKNOWLEDGMENTS
The authors acknowledge the University of Antioquia (grant CODI 2020-32831, Código BUPP ES84200120) for financial support.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ETHICAL APPROVAL
This article does not contain any studies with human participants or animals performed by any of the authors.
INFORMED CONSENT
For this type of study, formal consent is not required.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. World Health Organization. Cancer fact sheet [Internet]. 2022 [cited 2023 May 5]. Available from: https://www.who.int/news-room/fact-sheets/detail/cancer
2. Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin [Internet]. 2023 Jan 12;73(1):17–48. Available from: https://onlinelibrary.wiley.com/doi/10.3322/caac.21763
3. Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res [Internet]. 2008;25(9):2097–116. Available from: https://doi.org/10.1007/s11095-008-9661-9
4. Miller KD, Nogueira L, Mariotto AB, Rowland JH, Yabroff KR, Alfano CM, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin [Internet]. 2019 Sep 1;69(5):363–85. Available from: https://doi.org/10.3322/caac.21565
5. McQuade RM, Bornstein JC, Nurgali K. Anti-colorectal cancer chemotherapy-induced diarrhoea: current treatments and side-effects. Int J Clin Med. 2014;05(07):393–406.
6. Rejhová A, Opattová A, ?umová A, Slíva D, Vodi?ka P. Natural compounds and combination therapy in colorectal cancer treatment. Eur J Med Chem [Internet]. 2018;144:582–94. Available from: https://www.sciencedirect.com/science/article/pii/S0223523417310656
7. Aslam MS, Naveed S, Ahmed A, Abbas Z, Gull I, Athar MA. Side effects of chemotherapy in cancer patients and evaluation of patients opinion about starvation based differential chemotherapy. J Cancer Ther. 2014;05(08):817–22.
8. Ashok M, Holla BS, Kumari NS. Convenient one pot synthesis of some novel derivatives of thiazolo[2,3-b]dihydropyrimidinone possessing 4-methylthiophenyl moiety and evaluation of their antibacterial and antifungal activities. Eur J Med Chem [Internet]. 2007;42(3):380–5. Available from: https://www.sciencedirect.com/science/article/pii/S0223523406003217
9. Kaur R, Chaudhary S, Kumar K, Gupta MK, Rawal RK. Recent synthetic and medicinal perspectives of dihydropyrimidinones: a review. Eur J Med Chem [Internet]. 2017;132:108–34. Available from: https://www.sciencedirect.com/science/article/pii/S0223523417301812
10. Naidu BN, Sorenson ME, Patel M, Ueda Y, Banville J, Beaulieu F, et al. Synthesis and evaluation of C2-carbon-linked heterocyclic-5-hydroxy-6-oxo-dihydropyrimidine-4-carboxamides as HIV-1 integrase inhibitors. Bioorganic Med Chem Lett [Internet]. 2015;25(3):717–20. Available from: http://europepmc.org/abstract/MED/25529736
11. Mayer TU, Kapoor TM, Haggarty SJ, King RW, Schreiber SL, Mitchison TJ. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science (1979) [Internet]. 1999 Oct 29;286(5441):971–4. Available from: https://www.science.org/doi/10.1126/science.286.5441.971
12. Russowsky D, Canto RFS, Sanches SAA, D’Oca MGM, de Fátima Â, Pilli RA, et al. Synthesis and differential antiproliferative activity of biginelli compounds against cancer cell lines: monastrol, oxo-monastrol and oxygenated analogues. Bioorg Chem [Internet]. 2006;34(4):173–82. Available from: https://www.sciencedirect.com/science/article/pii/S0045206806000253
13. Marques LA, Semprebon SC, Niwa AM, D’Epiro GFR, Sartori D, de Fátima Â, et al. Antiproliferative activity of monastrol in human adenocarcinoma (MCF-7) and non-tumor (HB4a) breast cells. Naunyn Schmiedebergs Arch Pharmacol [Internet]. 2016;389(12):1279–88. Available from: https://doi.org/10.1007/s00210-016-1292-9
14. Yoon SY, Choi JE, Huh JW, Hwang O, Lee HS, Hong HN, et al. Monastrol, a selective inhibitor of the mitotic kinesin Eg5, induces a distinctive growth profile of dendrites and axons in primary cortical neuron cultures. Cell Motil [Internet]. 2005 Apr;60(4):181–90. Available from: https://doi.org/10.1002/cm.20057
15. Hanif H, Nazir S, Mazhar K, Waseem M, Bano S, Rashid U. Targeted delivery of mesoporous silica nanoparticles loaded monastrol into cancer cells: an in vitro study. Appl Nanosci. 2017 Nov;7(8):549–55.
16. de Fátima Â, Braga TC, Neto L da S, Terra BS, Oliveira BGF, da Silva DL, et al. A mini-review on biginelli adducts with notable pharmacological properties. J Adv Res [Internet]. 2015;6(3):363–73. Available from: https://www.sciencedirect.com/science/article/pii/S2090123214001295
17. Sharma R, Jadav SS, Yasmin S, Bhatia S, Khalilullah H, Ahsan MJ. Simple, efficient, and improved synthesis of biginelli-type compounds of curcumin as anticancer agents. Med Chem Res [Internet]. 2015;24(2):636–44. Available from: https://doi.org/10.1007/s00044-014-1146-2
18. Mostafa AS, Selim KB. Synthesis and anticancer activity of new dihydropyrimidinone derivatives. Eur J Med Chem [Internet]. 2018;156:304–15. Available from: https://www.sciencedirect.com/science/article/pii/S0223523418305609
19. Kappe CO. Recent advances in the biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc Chem Res [Internet]. 2000 Dec;33(12):879–88. Available from: https://doi.org/10.1021/ar000048h
20. Ramírez-Malule H, López-Agudelo VA, Gómez-Ríos D. Candida auris: a bibliometric analysis of the first ten years of research (2009–2018). J Appl Pharm Sci [Internet]. 2020 Mar;10(3):12–21. Available from: https://japsonline.com/abstract.php?article_id=3084&sts=2
21. Gómez-Ríos D, López-Agudelo VA, Ramírez-Malule H. Repurposing antivirals as potential treatments for SARS-CoV-2: from SARS to COVID-19. J Appl Pharm Sci [Internet]. 2020 May ;10(5):1–9. Available from: https://japsonline.com/abstract.php?article_id=3133&sts=2
22. Sanchez-Ledesma LM, Ramírez-Malule H, Rodríguez-Victoria JA. Volatile fatty acids production by acidogenic fermentation of wastewater: a bibliometric analysis. Sustainability. 2023;;15(3):2370.
23. van Eck NJ, Waltman L. Software survey: vOSviewer, a computer program for bibliometric mapping. Scientometrics. 2010;84(2):523–38.
24. Hauf S, Cole RW, LaTerra S, Zimmer C, Schnapp G, Walter R, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore-microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003 Apr 28;161(2):281–94.
25. Gascoigne KE, Taylor SS. Cancer cells display profound intra- and interline variation following prolonged exposure to antimitotic drugs. Cancer Cell. 2008 Aug 12;14(2):111–22.
26. Kapoor TM, Mayer TU, Coughlin ML, Mitchison TJ. probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5 7 [Internet]. J Cell Biol. 2000;150. Available from: http://www.jcb.org
27. McGrogan BT, Gilmartin B, Carney DN, McCann A. Taxanes, microtubules and chemoresistant breast cancer. Biochim Biophys Acta Rev Cancer. 2008;1785:96–132.
28. Even-Ram S, Doyle AD, Conti MA, Matsumoto K, Adelstein RS, Yamada KM. Myosin IIA regulates cell motility and actomyosin-microtubule crosstalk. Nat Cell Biol. 2007 Mar;9(3):299–309.
29. Thompson SL, Compton DA. Examining the link between chromosomal instability and aneuploidy in human cells. J Cell Biol. 2008 Feb 25;180(4):665–72.
30. Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM. Correcting improper chromosomes-spindle attachments during cell division. Nat Cell Biol. 2004;6(3):232–7.
31. Kapoor TM, Lampson MA, Hergert P, Cameron L, Cimini D, Salmon ED, et al. Chromosomes can congress to the metaphase plate before biorientation. Science (1979). 2006 Jan 20;311(5759):388–91.
32. Sumara I, Giménez-Abiá JF, Gerlich D, Hirota T, Kraft C, De La Torre C, et al. Roles of Polo-like Kinase 1 in the assembly of functional mitotic spindles. Curr Biol. 2004;14:1712–22. doi: https://doi.org/10.1016/j.cub.2004.09.049
33. Huarig Y, Yang F, Zhu C. Highly enantioselective Biginelli reaction using a new chiral ytterbium catalyst: asymmetric synthesis of dihydropyrimidines. J Am Chem Soc. 2005 Nov 30;127(47):16386–7. doi: https://doi.org/10.1021/ja056092f
34. Maliga Z, Kapoor TM, Mitchison TJ. Evidence that monastrol is an allosteric inhibitor of the mitotic kinesin Eg5. Chem Biol [Internet]. 2002 Sep;9(9):989–96. Available from: https://linkinghub.elsevier.com/retrieve/pii/S1074552102002120
35. Salehi P, Ali Zolfigol M, Shirini F, Baghbanzadeh M. Silica sulfuric acid and silica chloride as efficient reagents for organic reactions. Curr Org Chem. 2006 Nov 1;10(17):2171–89. doi: https://doi.org/10.2174/138527206778742650
36. Tao W, South VJ, Zhang Y, Davide JP, Farrell L, Kohl NE, et al. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell. 2005;8(1):49–59. doi: https://doi.org/10.1016/j.ccr.2005.06.003
37. Chan KS, Koh CG, Li HY. Mitosis-targeted anti-cancer therapies: where they stand. Cell Death Dis. 2012;3:e411.
38. El-Hamamsy MH, Sharafeldin NA, El-Moselhy TF, Tawfik HO. Design, synthesis, and molecular docking study of new monastrol analogues as kinesin spindle protein inhibitors. Arch Pharm (Weinheim) [Internet]. 2020 Aug ;353(8):2000060. Available from: https://doi.org/10.1002/ardp.202000060
39. Malik MS, Adil SF, Seddigi ZS, Morad M, Jassas RS, Thagafi II, et al. Molecular modelling assisted design of napthalimide-dihydropyrimidinone conjugates as potential cytotoxic agents. J Saudi Chem Soc [Internet]. 2021;25(5):101226. Available from: https://www.sciencedirect.com/science/article/pii/S1319610321000314
40. Hassan HMA, Denetiu I, Sakkaf K, Khan KA, Pushparaj PN, Gauthaman K. Synthesis and biological evaluation of triazolyl monastrol analogues using Cu-catalyzed click chemistry. Heterocycles. 2017;94(10):1856–69.
41. Vendrusculo V, de Souza VP, M. Fontoura LA, M. D’Oca MG, Banzato TP, Monteiro PA, et al. Synthesis of novel perillyl–dihydropyrimidinone hybrids designed for antiproliferative activity. Medchemcomm [Internet]. 2018;9(9):1553–64. Available from: http://xlink.rsc.org/?DOI=C8MD00270C
42. Ragab FAF, Abou-Seri SM, Abdel-Aziz SA, Alfayomy AM, Aboelmagd M. Design, synthesis and anticancer activity of new monastrol analogues bearing 1,3,4-oxadiazole moiety. Eur J Med Chem [Internet]. 2017;138:140–51. Available from: http://europepmc.org/abstract/MED/28667871
43. Srivastava JK, Pillai GG, Bhat HR, Verma A, Singh UP. Design and discovery of novel monastrol-1,3,5-triazines as potent anti-breast cancer agent via attenuating epidermal growth factor receptor tyrosine kinase. Sci Rep [Internet]. 2017;7(1):5851. Available from: https://doi.org/10.1038/s41598-017-05934-5
44. Mohamed AM, Elnaggar DH, Elsayed MA, Abdel-Hafez NA, Mostafa EA, Elasasy MEA, et al. Design, docking studies, and anticancer activity of newly synthesized monastrol analogues bearing ligustrazine moiety. Russ J Gen Chem [Internet]. 2022;92(11):2400–14. Available from: https://doi.org/10.1134/S1070363222110251
45. Asham H, Bohlooli S, Dostkamel D, Rezvanpoor S, Sepehri S. Design, synthesis, and biological screening for cytotoxic activity of monastrol analogues. Polycycl Aromat Compd [Internet]. 2022 Sep;42(8):4863–77. Available from: https://doi.org/10.1080/10406638.2021.1913424
46. Thabit MG, Mostafa AS, Selim KB, Elsayed MAA, Nasr MNA. Insights into modulating the monastrol scaffold: development of new pyrimidinones as Eg5 inhibitors with anticancer activity. Arch Pharm (Weinheim) [Internet]. 2022 Oct ;355(10):2200029. Available from: https://doi.org/10.1002/ardp.202200029
47. Sagha M, Mousaei F, Salahi M, Razzaghi-Asl N. Synthesis of new 2-aminothiazolyl/benzothiazolyl-based 3,4-dihydropyrimidinones and evaluation of their effects on adenocarcinoma gastric cell migration. Mol Divers [Internet]. 2022;26(2):1039–51. Available from: https://doi.org/10.1007/s11030-021-10229-z
48. Canto RFS, Bernardi A, Battastini AMO, Russowsky D, Eifler-Lima VL. Synthesis of dihydropyrimidin-2-one/thione library and cytotoxic activity against the human U138-MG and Rat C6 glioma cell lines. J Braz Chem Soc [Internet]. 2011 Jul;22(7):1379–88. Available from: http://www.scielo.br/scielo.php?script=sci_arttext&pid=S0103-50532011000700025&lng=en&nrm=iso&tlng=en
49. Torres BGS, Uchôa FDT, Pigatto MC, Azeredo FJ, Haas SE, Dallegrave E, et al. Pre-clinical pharmacokinetics and acute toxicological evaluation of a monastrol derivative anticancer candidate LaSOM 65 in rats. Xenobiotica [Internet]. 2014 Mar;44(3):254–63. Available from: https://doi.org/10.3109/00498254.2013.822131.
50. Lu M, Zhu H, Wang X, Zhang D, Xiong L, Xu L, et al. The prognostic role of Eg5 expression in laryngeal squamous cell carcinoma. Pathology. 2016 Apr 1;48(3):214–8.