INTRODUCTION
The urgent need for treatment alternatives to face the pandemic of novel coronavirus disease 2019 (COVID-19) has motivated the scientific community to test several available commercial compounds with therapeutic potential as a rapid response to the increasing number of critical cases and casualties. According to the World Health Organization (WHO), most of the people with COVID-19 experience mild-to-moderate respiratory illness and recover without requiring special treatment, but older adults may develop an associated kind of pneumonia when underlying other medical conditions such as cardiovascular diseases, diabetes, chronic respiratory disease, cancer, or HIV (World Health Organization, 2020). The COVID-19 is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which resembles other zoonotic respiratory coronavirus outbreaks: the SARS-CoV and the Middle East respiratory syndrome coronavirus (MERS-CoV) of 2002–2003 and 2012–2013, respectively.
There are 253 genome sequences of SARS-CoV-2 deposited in GenBank (GenBank, taxid: 2697049). The genome characterization and phylogenetic analysis of independent research groups have found that SARS-CoV-2 belongs to β-coronavirus (such as SARS-CoV and MERS-CoV), with high similarities (89%) to the genome of bat coronavirus (Chan et al., 2020; Lu et al., 2020). The SARS-CoV-2 genome is a single-stranded RNA containing 29-kb, 9,860 amino acids, with a 38% of guanine-citosine content, and is composed of a 5′-untranslated region, an open reading frame (orf) 1a/b codifying non-structural proteins for replication, structural proteins including spike surface glycoproteins (S), small envelope protein (E), membrane (M), and nucleocapsid proteins such as orf3, 6, 7a, 7b, 8, and 9b, and 3′-untranslated region (Chan et al., 2020).
S-glycoproteins include two subunits: the S1 and S2 subunits (Park et al., 2019b). The S1 subunit contains a signal peptide, followed by an N-terminal domain and a receptor-binding domain responsible for the binding of the host cell receptor, whereas the S2 subunit contains a conserved fusion peptide, heptad repeats 1 and 2, transmembrane domain, and cytoplasmic domain responsible for the fusion of the viral and cellular membranes (Chan et al., 2020; Simmons et al., 2004). A recent study has demonstrated that, similar to SARS-CoV, human angiotensin-converting enzyme 2 can mediate the entry into host cells through the binding to (S) glycoproteins from SARS-CoV-2, which could partially explain the efficient transmission of SARS-CoV-2 in humans (Walls et al., 2020).
COVID-19 treatment is still limited to symptomatology management and standard therapeutics for viral pneumonia. Some antiviral treatments are preliminary recommended, including interferon-α used an immune response enhancer with antiviral activity, lopinavir/ritonavir antiretrovirals used for HIV treatment, ribavirin used for parainfluenza and human respiratory syncytial virus, and chloroquine an antimalarial and amebicide (Chinese Centre for Disease Control and Prevention, 2020; Dong et al., 2020). Thus, drug repurposing is a practical alternative to respond effectively to novel diseases since developing, testing, and mass production of new drugs and vaccines could take a long time, costing thousands of human lives.
The objective of this study is to provide a bibliometric overview of the studies related to the main potential antiviral treatments for SARS-CoV-2 in connection with the previous outbreaks of SARS-CoV and MERS-CoV. In this regard, a bibliometric analysis constitutes a systematic tool for monitoring the research efforts on a specific field, offering the scientific community, a panorama regarding the study network and impact and evolution of the research during a specific timespan (Gómez-Ríos and Ramírez-Malule, 2019; Ramirez-Malule, 2018; Ramírez-Malule et al., 2020).
MATERIALS AND METHODS
For this study, data search and collection were performed in the Scopus database. The search strategy included the terms in the article title, abstract, and keywords. The “document type” was limited only to articles, and software VOS viewer 1.6.10 was used for visualization and data analysis (van Eck and Waltman, 2010).
Search 1 (April 4, 2020): COVID-19 or 2019-ncov or SARS-CoV-1 or SARS-CoV-2 or SARS or MERS-CoV and chloroquine. Timespan: 1994–2020. 127 articles.
Search 2 (April 4, 2020): COVID-19 or 2019-ncov or SARS-CoV-1 or SARS-CoV-2 or SARS or MERS-CoV and hydroxychloroquine. Timespan: 2006–2020. 4 articles.
Search 3 (April 4, 2020): COVID-19 or 2019-ncov or SARS-CoV-1 or SARS-CoV-2 or SARS or MERS-CoV and lopinavir. Timespan: 2003–2020. 66 articles.
Search 4 (April 4, 2020): COVID-19 or 2019-ncov or SARS-CoV-1 or SARS-CoV-2 or SARS or MERS-CoV and ritonavir. Timespan: 1999–2020. 68 articles.
Search 5 (April 4, 2020): COVID-19 or 2019-nCoV or SARS-CoV-1 or SARS-CoV-2 or SARS or MERS-CoV and remdesivir. Timespan: 2017–2020. 13 articles.
Search 6 (April 4, 2020): COVID-19 or 2019-nCoV or SARS-CoV-1 or SARS-CoV-2 or SARS or MERS-CoV and ribavirin. Timespan: 1996–2020. 326 articles.
Search 7 (April 4, 2020): COVID-19 or 2019-nCoV or SARS-CoV-1 or SARS-CoV-2 or SARS or MERS-CoV and interferon. Timespan: 1988–2020. 593 articles.
RESULTS AND DISCUSSION
Evolution of research in antiviral options for the treatment of SARS-CoV & SARS-CoV-2 (COVID-19)
Chloroquine/hydroxychloroquine, lopinavir/ritonavir, ribavirin, and interferon have been tested in vitro as alternatives for the treatment of pneumonia associated with both SARS-CoV and MERS-CoV. Some of those drugs have reached the clinical phase, emerging as the first options for facing the COVID-19 pandemics, considering availability, cost, and production capacity (Al-Tawfiq and Memish, 2017; Stockman et al., 2006). The dynamic evolution of the published studies involving commercial drugs with confirmed antiviral activity – chloroquine/hydroxychloroquine, lopinavir/ritonavir, remdesivir, ribavirin, and interferon – related to COVID-19, 2019-ncov, SARS-Cov-1, SARS-CoV-2, and MERS-CoV, respectively, are shown in Figure 1. A total of 127, 4, 66, 68, 13, 326, and 593 records were retrieved from Scopus for the previously mentioned antivirals, respectively. However, after eliminating duplicates, a total of 989 articles were obtained, and 55 of them were published in 2020.
An increasing tendency in the number of publications was observed after the first SARS outbreak in 2003. A significant number of studies related to the combination of HIV-1 protease inhibitors lopinavir/ritonavir on SARS-CoV replication appeared since 2003 (Cinatl et al., 2005). Several in vitro studies showed that interferons (IFN-α, IFN-β, and IFN-γ) could inhibit SARS-CoV replication (Chen et al., 2004; Cinatl et al., 2003; Sainz et al., 2004; Ströher et al., 2004; Tan et al., 2004). The tests with ribavirin on animal and human cell lines (FRhK-4, MA-104, PK-15, Caco2, CL-14, and human primary epithelial kidney cells) showed an effective inhibition of SARS-CoV replication (Koren et al., 2003; Morgenstern et al., 2005). A significant number of records is also observed for chloroquine/hydroxychloroquine between 2004 and 2010 since it was shown to effectively inhibit the spread of SARS-CoV in Vero E-6 cells (Keyaerts et al., 2004; Vincent et al., 2005). The number of published articles decreased during the period 2008–2011, but the emergence of the MERS-CoV outbreak of 2012 attracted attention again. In the case of MERS-CoV, the studies with interferon, ribavirin, ritonavir, and lopinavir also showed a variable activity in vitro (Al-Tawfiq and Memish, 2017). Notice that in 2019–2020, the number of records for chloroquine, ritonavir, and remdesivir started to increase as a consequence of very active research with these compounds as potential interventions for patients infected with SARS-CoV-2.
Figure 2A shows the bibliometric network of studies of different antivirals related to coronavirus outbreaks. A big group of topics related to SARS is identified, including connected major clusters for coronavirus, SARS, SARS-CoV, MERS-CoV, and pneumonia. The potential treatments, namely, interferon, lopinavir, ritonavir, ribavirin, corticosteroids, and monoclonal antibodies, appear as smaller clusters. Furthermore, 2019-nCov and COVID-19 appear as small connected clusters, but given the intense scientific activity about this topic, those clusters are expected to increase rapidly within the next months. Chloroquine is a connecting point between research works related to malaria and coronavirus, evidencing a significant number of studies addressing the antimalarial and antiviral activities of this compound. Therefore, the second group of topics is identified regarding the extended use of chloroquine and hydroxychloroquine as a treatment for malaria and its inhibition effect on the microorganism Plasmodium falciparum.
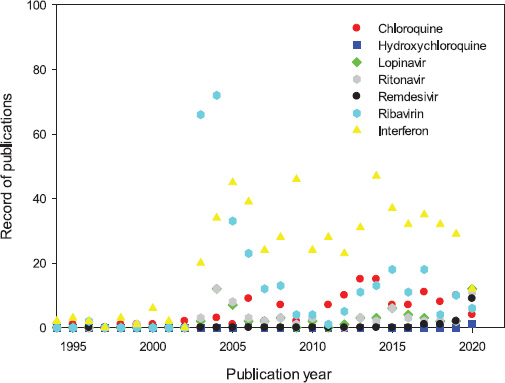 | Figure 1. Dynamic evolution of the studies of different antivirals related to COVID-19, 2019-ncov, SARS-Cov-1, SARS-CoV-2, and MERS-CoV. [Click here to view] |
Interestingly, Figure 2B shows that chloroquine and malaria have been intensively studied between 2010 and 2015, but it was also actively studied in SARS-CoV and MERS-CoV before 2015. The network shows that, now, chloroquine is extended to COVID-19, whereas lopinavir, ritonavir, and remdesivir were more explored for SARS-CoV. Aside, and not less important, Figure 2C shows the country collaboration network in the research of coronavirus outbreaks. The countries with an essential number of contagions appear as a part of the main contributors, namely, the U.S, China, Hong Kong, and Saudi Arabia, and to a lesser extent, Germany, Italy, Spain, France, the UK, Japan, South Korea, and Taiwan. Those countries engaged in collaboration with countries, where the number of cases was significant or needed to be controlled rapidly to avoid its rapid expansion (World Health Organization, 2003, 2017, 2020).
Repurposed antiviral treatments for COVID-19 based on clinical evidence
Figure 3 shows the antiviral treatment strategies for blocking the coronavirus replication cycle, adapted from Kupferschmidt and Cohen (2020). Replication of coronavirus begins with the entrance of virions to the host cells; it could occur by the attachment of spike glycoproteins to the angiotensin-converting enzyme 2 (ACE2) receptor (step 1a) or endocytosis (step 1b). Virions inject RNA into the cell and start an intracellular translation of polypeptides and proteins necessary for replication (step 2). Proteolysis (step 3) is necessary for producing functional proteins with catalytic activities such as the RNA-dependent RNA polymerases (RdRp). This enzyme is necessary for the replication of viruses, and here, genomic RNA is replicated, transcribed, and translated to form the structural proteins (step 4). Finally, viral proteins produced are processed through the endoplasmic reticulum and the Golgi apparatus (step 5), and viruses are assembled (step 7) and released via exocytosis (step 8). Antiviral treatments in red boxes represent the molecular process inhibited or induced during the coronavirus life cycle.
Interferon-α with corticosteroids or ribavirin
Cytokines known as type I interferons (IFNs) are secreted by infected cells activating intracellular antimicrobial programs in host cells and influencing the development of innate and adaptive immune responses (Ivashkiv and Donlin, 2014). In infected cells (Fig. 3, step 1a), the type I IFNs induce the transcription of IFN-stimulated genes, which codify proteins that suppress viruses by different mechanisms (McNab et al., 2015). It has been demonstrated that IFN-α, a type I interferon, inhibits the SARS-Co-V spread in vitro (Chen et al., 2004; Ströher et al., 2004; Tan et al., 2004). Some clinical studies explored the therapeutic effect of IFN-α on SARS-CoV and MERS-CoV patients. The outcomes have been not conclusive, but the synergistic effect of IFN-α with other drugs such as corticosteroids or antivirals results in more rapid resolution of pneumonia. Loutfy et al. (2003) performed a clinical trial on the therapeutic benefit and tolerability of combined corticosteroids-IFN-α treatment for SARS-CoV, showing a shorter resolution time (50%) compared with the control group treated with corticosteroids. The review of clinical findings in patients with SARS-CoV treated with four different protocols also showed that a treatment regime of IFN-α with high-dose corticosteroids avoided the need for mechanical ventilation in 100% of patients (Zhao et al., 2003).
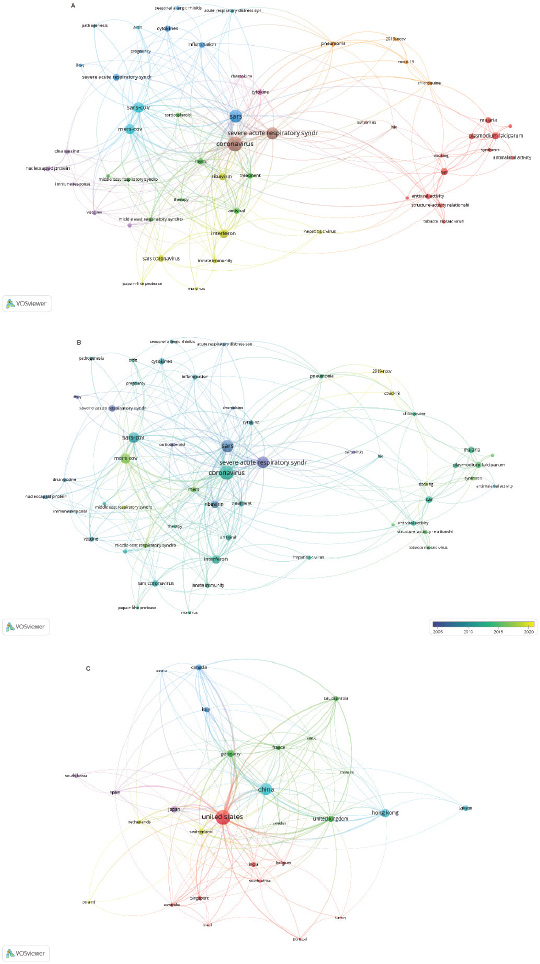 | Figure 2. Summary of antivirals studies related to COVID-19, 2019-ncov, SARS-Cov-1, SARS-CoV-2, and MERS-CoV. (A) Bibliometric network. (B) Research topic map with time overlay. (C) Visualization of the country collaboration network. Note: The minimum number of occurrences of a keyword is five. [Click here to view] |
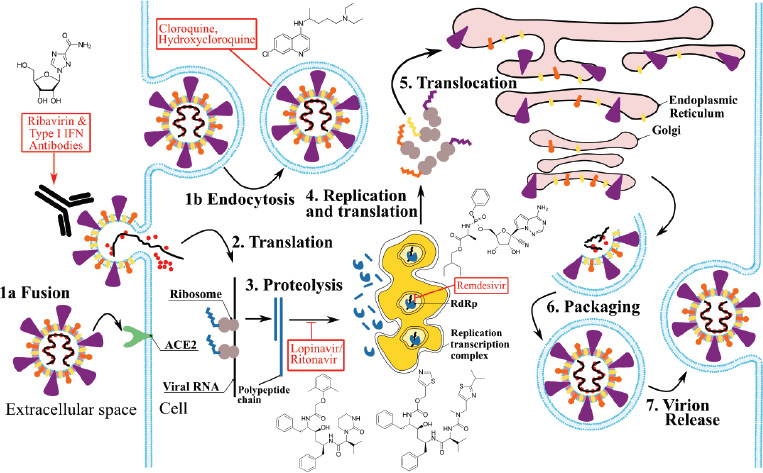 | Figure 3. Antiviral treatments strategies for blocking coronavirus replication cycle. Adapted from Kupferschmidt and Cohen, 2020. Replication of coronavirus begins with the entrance of virions to the host cells; it could occur by the attachment of spike glycoproteins to the ACE2 receptor (step 1a) or endocytosis (step 1b). Virions inject RNA into the cell and start an intracellular translation of polypeptides and proteins necessary for replication (step 2). Proteolysis (step 3) is necessary for producing functional proteins with catalytic activities such as the RdRp. This enzyme is necessary for replication of viruses, and here, genomic RNA is replicated, transcribed, and translated to form the structural proteins (step 4). Finally, viral proteins produced will be processed through the endoplasmic reticulum and golgi apparatus (step 5), and viruses are assembled (step 7) and released via exocytosis (step 8). Antiviral drugs (red boxes) are indicated in the process inhibited or induced in coronavirus life cycle; molecular drug structures are showed alongside. [Click here to view] |
Ribavirin itself did not show antiviral activity against SARS-CoV in vitro (Cinatl et al., 2003). Nevertheless, a positive synergistic effect was observed in vitro when used in combination with type I (α or β) IFNs (Chen et al., 2004; Morgenstern et al., 2005; Tan et al., 2004). Similar results were observed for MERS-CoV in Vero, Vero E6, and LLC-MK2 cell lines, suggesting that this combination could entail the benefits for MERS-CoV patients (Chan et al., 2013; Falzarano et al., 2013). Clinical trials and case series involving type I IFNs alone or in combination with ribavirin showed survival rates between 65% and 85%, but it is ineffective when used in the late stages of the disease (Al Ghamdi et al., 2016; Al-Tawfiq and Memish, 2017; Shalhoub et al., 2015).
In the case of SARS-CoV-2 infection, Lokugamage et al. (2020) found that pretreatment with recombinant interferon-α leads to a significant reduction in viral replication at 24- and 48-hour postinfection in Vero E6 cells. The treatment of critical care and noncritical care patients with a combination of interferon-α, methylprednisolone, moxifloxacin, and lopinavir showed preliminary positive results in 74% of critical patients, and at the same time, noncritical patients improved showing only mild symptoms (Qin et al., 2020).
Lopinavir/ritonavir
Viral proteases are endopeptidase enzymes codified in the viral RNA or DNA that catalyzes the cleavage of peptide bonds of viral polyproteins and host-cell proteins necessary for the viral maturation, life cycle, and infectivity (Konvalinka et al., 2015). Lopinavir/ritonavir is an inhibitor of HIV-1 protease that causes an antiviral effect on the maturation of virions (Fig. 3, step 3) and prevents the new waves of cellular infections (Chandwani and Shuter, 2008). Assays in fRhK-4 cell lines using lopinavir and lopinavir/ritonavir with ribavirin have shown some anti-SARS-CoV effects (Chen et al., 2004; Chu et al., 2004). A retrospective cohort study showed that SARS-CoV patients treated with lopinavir/ritonavir were associated with a reduction in the death rate (2.3%) compared with the cohort receiving the standard treatment (15.6%) and a lower requirement of corticosteroid therapy as well (Chan et al., 2003). Chu et al. (2004) performed a clinical trial with 41 patients infected with SARS-CoV and treated with a combination of lopinavir/ritonavir and ribavirin. The authors observed a significantly lower complication rate or death compared to the historical controls (2.4% vs. 28.8%) and a reduction in steroid usage and nosocomial infections as a consequence of decreasing in viral load and rising of peripheral lymphocyte count (Chu et al., 2004).
Based on the effectivity of the lopinavir/ritonavir and interferon combination in the clinical trials for SARS-CoV and primate animal model for MERS-CoV (Chan et al., 2015), two case reports suggested that lopinavir/ritonavir-based antiviral therapy with ribavirin and interferon possibly led to the disappearance of viral load after 3 and 6 days, respectively (Kim et al., 2016; Spanakis et al., 2014). The application of postexposure prophylaxis with lopinavir/ritonavir in healthcare workers was associated with a decrease (40%) in the infection risk without severe adverse effects (Park et al., 2019a). Thus, the addition of lopinavir/ritonavir to the standard treatment protocol treatment for SARS-CoV and MERS-CoV infections might be potentially associated with improved clinical outcomes.
In the case of COVID-19, the lopinavir/ritonavir combination has been used as an experimental therapy in several cases, and some clinical trials are under development, but the outcomes remain controversial. Some case reports suggested that treatment with lopinavir/ritonavir could help to significantly reduce the viral load (Lim et al., 2020; Young et al., 2020). Moreover, the use of lopinavir/ritonavir becomes more common as a therapeutic measure in hospital COVID-19 management protocols, being associated with benefits in small samples or individual cases (Bhatnagar et al., 2020; Huang et al., 2020; Liu et al., 2020a). Early antiviral treatment with lopinavir/ritonavir and arbidol (umifenovir) showed antiviral effects improving treatment efficacy in critical cases (Wang et al., 2020b; Xu et al., 2020). On the contrary, a retrospective study with 134 patients revealed no significant difference between lopinavir/ritonavir- and arbidol-treated and control groups in reducing the viral load (Yao et al., 2020a). A retrospective study suggests that lopinavir/ritonavir might improve the outcomes in COVID-19 although shortening of infection duration was not observed (Zhou et al., 2020). A randomized, controlled, open-label trial in adult patients with severe SARS-CoV-2 (199) revealed no benefits and similar mortality regarding the treatment with lopinavir/ritonavir compared with the standard care protocol (Cao et al., 2020). Nevertheless, given the severity condition of the patients of that study, the potential of lopinavir/ritonavir for early treatment is still a matter of analysis. A retrospective study with 120 patients showed that the median duration of viral load was shorter in the lopinavir/ritonavir-treated group (65%) than in the untreated group (35%), with a median duration of 22 days versus 28.5 days, respectively (Yan et al., 2020). According to the authors, only earlier, the administration of the treatment (≤10 days from symptom onset) was able to shorten the viral shedding. Currently, at least 15 clinical studies involving 2,606 patients are planned or being conducted to investigate the therapy based on the use of lopinavir/ritonavir (Belhadi et al., 2020).
Chloroquine/hydroxychloroquine
Chloroquine and hydroxychloroquine are weak bases that affect acid vesicles (endosomes, Golgi vesicles, and lysosomes), leading to the dysfunction of several enzymes. Weak bases tend to increase the pH of lysosomal and trans-Golgi network vesicles, to disrupt the enzymatic function, and to inhibit the post-translational modification of newly synthesized proteins (Savarino et al., 2003). The antiviral effect depends on the extent to which viruses use endosomes for entry into host cells (Fig. 3, step 1b) (Vincent et al., 2005). Furthermore, chloroquine would diminish the surface expression of the glycosylated forms of ACE2 receptor in Vero cells, affecting negatively the binding interactions between ACE2 and S glycoproteins (Fig. 3, step 1a) of SARS-CoV and possibly SARS-CoV-2 (Vincent et al., 2005). In 2001, Blau and Holmes (2001) investigated the effect of drugs (chloroquine and bafilomycin A), blocking the acidification of endosomes when infecting MRC-5 human lung epithelial cells with human coronavirus HCoV-229E. Keyaerts et al. (2004) reported chloroquine as an effective inhibitor of SARS-CoV in Vero E6 cells, showing a selectivity index of 30 and IC50 of 8.8 μM, approximately the same plasma concentration reached with the drug in malaria treatment. Similar results were also obtained by Vincent et al. (2005) in Vero E6 cells. Pretreatment with chloroquine of 0.1, 1, and 10 μM reduced the infectivity by 28%, 53%, and 100%, respectively, and a postinfection half-maximal inhibitory effect was estimated to occur at the concentrations of 4.4 μM. A screening test in Vero E6 cells with drugs approved by the U.S. Food and Drug Administration showed that chloroquine inhibits MERS-CoV replication at an early step with an EC50 of 3.0 μM and selectivity index of 19.4 (De Wilde et al., 2014).
Chloroquine has been reported as an in vitro inhibitor of SARS-CoV-2 at both entry and postentry stages in Vero E6 cells at an EC90 value of 6.90 μM, which can be clinically achievable in plasma patients receiving 500 mg (Wang et al., 2020a). In vitro tests with chloroquine and hydroxychloroquine in Vero cells showed that hydroxychloroquine was a better inhibitor of SARS-CoV-2 infection with an EC50 of 0.72 μM, whereas, for chloroquine, the EC50 was 5.47 μM (Yao et al., 2020b). On the contrary, Liu et al. (2020b) found the chloroquine to be a more effective inhibitor of SARS-CoV 2 in Vero E6 cells with an EC50 of 7.36 μM at the maximum multiplicity of infection (0.8) while an EC50 was observed for hydroxychloroquine. A loading dose of 800 mg/day of hydroxychloroquine followed by a maintenance dose of 400 mg/day for 4 days has been recommended for treating the SARS-CoV-2 infection (Yao et al., 2020). Early clinical trials in different Chinese hospitals with more than 100 patients demonstrated that chloroquine inhibited the exacerbation of pneumonia, improved the lung imaging findings, shortened disease, and reduced the viral load without adverse reactions (Gao et al., 2020). An open-label nonrandomized clinical trial in France using hydroxychloroquine and azithromycin showed a significant reduction of the viral load. After 6 days of treatment, 75% of the treated patients with 600 mg/day (20) were virologically cured (Gautret et al., 2020). According to Belhadi et al. (2020), 11 clinical trials are planned or in execution involving chloroquine and seven using hydroxychloroquine/chloroquine with a total of planned inclusions of 1,102 and 1,048, respectively.
Remdesivir
As shown in Figure 1, there are few studies regarding the effectivity of the antiviral remdesivir (GS-5734) in SARS-CoV and MERS-CoV. Remdesivir is a novel antiviral adenosine analog developed mainly as a treatment for Ebola virus, but it has also been found that remdesivir exhibits antiviral activity against other single-stranded RNA viruses including human zoonotic coronaviruses (Brown et al., 2019; Sheahan et al., 2017). As a nucleotide analog inhibitor, remdesivir interferes with RdRps as shown in Figure 3, step 4, specifically RdRps of MERS-CoV and Ebola virus (Agostini et al., 2018; Gordon et al., 2020; Tchesnokov et al., 2019). The antiviral activity of this compound has been assessed in primary human airway epithelial cells infected with both SARS-CoV and MERS-CoV, showing effectivity with the average EC50 values of 0.069 and 0.074 μM, respectively. Those concentrations are almost 100-fold lower than the potentially cytotoxic values. In vitro (Calu-3) and in vivo assays in mice and rhesus macaque animal models showed the effectivity of remdesivir in reducing the viral load and improving the respiratory function in MERS-CoV infection (de Wit et al., 2020; Sheahan et al., 2020). Remdesivir was also effective against SARS-CoV-2 postinfection in vitro in Vero E6 and human liver cancer Huh-7 cells, yielding an EC90 of 1.76 μM (Wang et al., 2020). The first case reported in the US of SARS-CoV-2 was treated with intravenous remdesivir in day 7 of hospitalization; no adverse events were observed, and only 1 day later, the clinical condition of the patient improved significantly up to the point of symptom resolution (Holshue et al., 2020). Since those results establish remdesivir as a promising drug for the treatment of COVID-19, at least five clinical trials are planned, enrolling 2,155 individuals to assess the effectiveness and safety of this therapy (Belhadi et al., 2020). However, remdesivir is still a prodrug in an advanced stage of development, and the regulatory issues must be first solved. Moreover, there is a limited possibility that remdesivir and other analogs reach the market in the short term in the required amounts to face the COVID-19 pandemic at accessible prices.
CONCLUSION
After 18 years since the first SARS outbreak, the studies in potential antiviral therapies for SARS-CoV and MERS-CoV have not progressed as fast as needed. This is reflected in the current uncertainties evidenced by the health personnel in the management of severe patients infected with the novel SARS-CoV-2. Although several potential drugs have been tested in vitro and in vivo for treating infections with SARS coronaviruses, the clinical evidence is still limited, and as a consequence, there is no proven antiviral therapy for any SARS disease. There is comparatively more evidence regarding the use of type I IFNs, ribavirin, and lopinavir/ritonavir for the treatment of SARS diseases, showing some benefits, but their application in late stages of infection is ineffective. Chloroquine, hydroxychloroquine, and remdesivir are the most promising alternatives for treating severe patients, even after several days of infection, but the associated clinical evidence is still minimal. Furthermore, from this group, the only licensed drugs are chloroquine and hydroxychloroquine, and therefore, they are readily available and cheap enough to be accessible for all healthcare personnel around the world.
ACKNOWLEDGMENT
Víctor A. López-Agudelo acknowledges the economic support of the Ministry of Science, Technology, and Innovation – MINCIENCIAS (National Ph.D. scholarship Conv. 727–2015).
CONFLICT OF INTEREST
None.
FUNDING
None.
REFERENCES
Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Smith EC, Case JB, Feng JY, Jordan R, Ray AS, Cihlar T, Siegel D, Mackman RL, Clarke MO, Baric RS, Denison MR. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio, 2018; 9:1–15. CrossRef
Al Ghamdi M, Alghamdi KM, Ghandoora Y, Alzahrani A, Salah F, Alsulami A, Bawayan MF, Vaidya D, Perl TM, Sood G. Treatment outcomes for patients with Middle Eastern Respiratory Syndrome Coronavirus (MERS CoV) infection at a coronavirus referral center in the Kingdom of Saudi Arabia. BMC Infect Dis, 2016; 16:1–7. CrossRef
Al-Tawfiq JA, Memish ZA. Update on therapeutic options for Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Expert Rev Anti Infect Ther, 2017; 15:269–75. CrossRef
Belhadi D, Peiffer-Smadja N, Lescure F-X, Yazdanpanah Y, Mentré F, Laouénan C. A brief review of antiviral drugs evaluated in registered clinical trials for COVID-19. medRxiv, 2020. CrossRef
Bhatnagar T, Murhekar M, Soneja M, Gupta N, Giri S, Wig N, Gangakhedkar R. Lopinavir/ritonavir combination therapy amongst symptomatic coronavirus disease 2019 patients in India: protocol for restricted public health emergency use. Indian J Med Res, 2020; 76:1532–9. CrossRef
Blau DM, Holmes KV. Human coronavirus HCoV-229E enters susceptible cells via the endocytic pathway. In: Advances in experimental medicine and biology. Springer, Berlin, Heidelberg, Germany, pp 193–8, 2001. CrossRef
Brown AJ, Won JJ, Graham RL, Dinnon KH, Sims AC, Feng JY, Cihlar T, Denison MR, Baric RS, Sheahan TP. Broad spectrum antiviral remdesivir inhibits human endemic and zoonotic deltacoronaviruses with a highly divergent RNA dependent RNA polymerase. Antiviral Res, 2019; 169:104541. CrossRef
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med, 2020;1–13. CrossRef
Chan JFW, Chan KH, Kao RYT, To KKW, Zheng BJ, Li CP, Li PT, Dai J, Mok FK, Chen H, Hayden FG, Yuen KY. Broad-spectrum antivirals for the emerging Middle East respiratory syndrome coronavirus. J Infect, 2013; 67:606–16. CrossRef
Chan JFW, Kok KH, Zhu Z, Chu H, To KKW, Yuan S, Yuen KY. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting Wuhan. Emerg Microbes Infect, 2020; 9:221–36. CrossRef
Chan JFW, Yao Y, Yeung ML, Deng W, Bao L, Jia L, Li F, Xiao C, Gao H, Yu P, Cai JP, Chu H, Zhou J, Chen H, Qin C, Yuen KY. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERSCoV infection in a nonhuman primate model of common marmoset. J Infect Dis, 2015; 212:1904–13. CrossRef
Chan KS, Lai ST, Chu CM, Tsui E, Tam CY, Wong MM, Tse MW, Que TL, Peiris JS, Sung J, Wong VC, Yuen KY. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med J, 2003; 9:399–406.
Chandwani A, Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: a review. Ther Clin Risk Manag, 2008; 4:1023–33. CrossRef
Chen F, Chan KH, Jiang Y, Kao RYT, Lu HT, Fan KW, Cheng VC, Tsui WH, Hung IF, Lee TS, Guan Y, Peiris JS, Yuen KY. In vitro susceptibility of 10 clinical isolates of SARS coronavirus to selected antiviral compounds. J Clin Virol, 2004; 31:69–75. CrossRef
Chinese Centre for Disease Control and Prevention. Diagnosis and treatment [Internet]. COVID-19 prevention and control. Beijing, 2020. Available via http://www.chinadaily.com.cn/specials/diagnosisandtreatment-Asia.pdf (Accessed 6 April 2020)
Chu CM, Cheng VCC, Hung IFN, Wong MML, Chan KH, Chan KS, Kao RY, Poon LL, Wong CL, Guan Y, Peiris JS, Yuen KY, HKU/UCH SARS Study Group. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax, 2004; 59:252–6. CrossRef
Cinatl J, Michaelis M, Hoever G, Preiser W, Doerr HW. Development of antiviral therapy for severe acute respiratory syndrome. Antiviral Res, 2005; 66:81–97. CrossRef
Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr H. Treatment of SARS with human interferons. Lancet, 2003; 362:293–4. CrossRef
De Wilde AH, Jochmans D, Posthuma CC, Zevenhoven-Dobbe JC, Van Nieuwkoop S, Bestebroer TM, van den Hoogen BG, Neyts J, Snijder EJ. Screening of an FDA-approved compound library identifies four small-molecule inhibitors of Middle East respiratory syndrome coronavirus replication in cell culture. Antimicrob Agents Chemother, 2014; 58:4875–84. CrossRef
de Wit E, Feldmann F, Cronin J, Jordan R, Okumura A, Thomas T, Scott D, Cihlar T, Feldmann H. Prophylactic and therapeutic remdesivir (GS-5734) treatment in the rhesus macaque model of MERS-CoV infection. Proc Natl Acad Sci, 2020;117:6771–6. CrossRef
Dong L, Hu S, Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19). Drug Discov Ther, 2020; 14:58–60. CrossRef
Falzarano D, De Wit E, Martellaro C, Callison J, Munster VJ, Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep, 2013; 3:1–6. CrossRef
Gao J, Tian Z, Yang X. Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends, 2020; 14:72–3. CrossRef
Gautret P, Lagier J-C, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, Courjon J, Giordanengo V, Vieira VE, Dupont HT, Honoré S, Colson P, Chabrière E, La Scola B, Rolain JM, Brouqui P, Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents, 2020; 105949. CrossRef
Gómez-Ríos D, Ramírez-Malule H. Bibliometric analysis of recent research on multidrug and antibiotics resistance (2017–2018). J Appl Pharm Sci, 2019; 9:112–6. CrossRef
Gordon CJ, Tchesnokov EP, Feng JY, Porter DP, Gotte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J Biol Chem, 2020; 295:4773–9. CrossRef
Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, Ericson K, Wilkerson S, Tural A, Diaz G, Cohn A, Fox L, Patel A, Gerber SI, Kim L, Tong S, Lu X, Lindstrom S, Pallansch MA, Weldon WC, Biggs HM, Uyeki TM, Pillai SK. First case of 2019 novel coronavirus in the United States. N Engl J Med, 2020; 382:929–36. CrossRef
Huang Y, Tu M, Wang S, Chen S, Zhou W, Chen D, Zhou L, Wang M, Zhao Y, Zeng W, Huang Q, Xu H, Liu Z, Guo L. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: a retrospective single center analysis. Travel Med Infect Dis, 2020; 101606. CrossRef
Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol, 2014; 14:36–49. CrossRef
Keyaerts E, Vijgen L, Maes P, Neyts J, Ranst M Van. In vitro inhibition of severe acute respiratory syndrome coronavirus by chloroquine. Biochem Biophys Res Commun, 2004; 323:264–8. CrossRef
Kim UJ, Won EJ, Kee SJ, Jung SI, Jang HC. Combination therapy with lopinavir/ritonavir, ribavirin and interferon-a for Middle East respiratory syndrome. Antivir Ther, 2016; 21:455–9. CrossRef
Konvalinka J, Kräusslich HG, Müller B. Retroviral proteases and their roles in virion maturation. Virology, 2015; 479:403–17. CrossRef
Koren G, King S, Knowles S, Phillips E. Ribavirin in the treatment of SARS: a new trick for an old drug? CMAJ, 2003; 168:1289–92.
Kupferschmidt K, Cohen J. Race to find COVID-19 treatments accelerates. Science, 2020; 367:1412–3. CrossRef
Lim J, Jeon S, Shin H-Y, Kim MJ, Seong YM, Lee WJ, Choe KW, Kang YM, Lee B, Park SJ. Case of the index patient who caused tertiary transmission of coronavirus disease 2019 in Korea: the application of lopinavir/ritonavir for the treatment of COVID-19 pneumonia monitored by quantitative RT-PCR. J Korean Med Sci, 2020; 35:1–6. CrossRef
Liu F, Xu A, Zhang Y, Xuan W, Yan T, Pan K, Yu W, Zhang J. Patients of COVID-19 may benefit from sustained lopinavir-combined regimen and the increase of eosinophil may predict the outcome of COVID-19 progression. Int J Infect Dis, 2020. CrossRef
Liu J, Cao R, Xu M, Wang X, Zhang H, Hu H, Li Y, Hu Z, Zhong W, Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov, 2020; 6:16. CrossRef
Lokugamage KG, Hage A, Schindewolf C, Rajsbaum R, Menachery VD, Sprott D. SARS-CoV-2 is sensitive to type I interferon pretreatment. bioRxiv, 2020; 21. CrossRef
Loutfy MR, Blatt LM, Siminovitch KA, Ward S, Wolff B, Lho H, Pham DH, Deif H, LaMere EA, Chang M, Kain KC, Farcas GA, Ferguson P, Latchford M, Levy G, Dennis JW, Lai EK, Fish EN. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. J Am Med Assoc, 2003; 290:3222–8. CrossRef
Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, Song H, Huang B, Zhu N, Bi Y, Ma X, Zhan F, Wang L, Hu T, Zhou H, Hu Z, Zhou W, Zhao L, Chen J, Meng Y, Wang J, Lin Y, Yuan J, Xie Z, Ma J, Liu WJ, Wang D, Xu W, Holmes EC, Gao GF, Wu G, Chen W, Shi W, Tan W. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet, 2020; 395:565–74. CrossRef
McNab F, Mayer-Barber K, Sher A, Wack A, O’Garra A. Type I interferons in infectious disease. Nat Rev Immunol, 2015; 15:87–103. CrossRef
Morgenstern B, Michaelis M, Baer PC, Doerr HW, Cinatl J. Ribavirin and interferon-β synergistically inhibit SARS-associated coronavirus replication in animal and human cell lines. Biochem Biophys Res Commun, 2005; 326:905–8. CrossRef
Park SY, Lee JS, Son JS, Ko JH, Peck KR, Jung Y, Woo HJ, Joo YS, Eom JS, Shi H. Post-exposure prophylaxis for Middle East respiratory syndrome in healthcare workers. J Hosp Infect, 2019; 101:42–6. CrossRef
Park YJ, Walls AC, Wang Z, Sauer MM, Li W, Tortorici MA, BJ Bosch, F DiMaio, D Veesler. Structures of MERS-CoV spike glycoprotein in complex with sialoside attachment receptors. Nat Struct Mol Biol, 2019; 26:1151–7. CrossRef
Qin X, Qiu S, Yuan Y, Zong Y, Tuo Z, Li J, Liu J. Clinical characteristics and treatment of patients infected with COVID-19 in Shishou, China. SSRN Electron J, 2020; 12:27. CrossRef
Ramírez-Malule H, López-Agudelo VA, Gómez-Ríos D. Candida auris: a bibliometric analysis of the first ten years of research (2009–2018). J Appl Pharm Sci, 2020; 10:12–21. CrossRef
Ramirez-Malule H. Bibliometric analysis of global research on clavulanic acid. Antibiotics, 2018; 7:102. CrossRef
Sainz B, Mossel EC, Peters CJ, Garry RF. Interferon-beta and interferon-gamma synergistically inhibit the replication of severe acute respiratory syndrome-associated coronavirus (SARS-CoV). Virology, 2004; 329:11–7. CrossRef
Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis, 2003; 3:722–7. CrossRef
Shalhoub S, Farahat F, Al-Jiffri A, Simhairi R, Shamma O, Siddiqi N, Mushtaq A. IFN-α2a or IFN-β1a in combination with ribavirin to treat Middle East respiratory syndrome coronavirus pneumonia: a retrospective study. J Antimicrob Chemother, 2015; 70:2129–32. CrossRef
Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Leist SR, Pyrc K, Feng JY, Trantcheva I, Bannister R, Park Y, Babusis D, Clarke MO, Mackman RL, Spahn JE, Palmiotti CA, Siegel D, Ray AS, Cihlar T, Jordan R, Denison MR, Baric RS. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med, 2017; 9(396):pii: eaal3653. CrossRef
Sheahan TP, Sims AC, Leist SR, Schäfer A, Won J, Brown AJ, Montgomery SA, Hogg A, Babusis D, Clarke MO, Spahn JE, Bauer L, Sellers S, Porter D, Feng JY, Cihlar T, Jordan R, Denison MR, Baric RS. Comparative therapeutic efficacy of remdesivir and combination lopinavir, ritonavir, and interferon beta against MERS-CoV. Nat Commun, 2020; 11:222. CrossRef
Simmons G, Reeves JD, Rennekamp AJ, Amberg SM, Piefer AJ, Bates P. Characterization of severe acute respiratory syndrome-associated coronavirus (SARS-CoV) spike glycoprotein-mediated viral entry. Proc Natl Acad Sci U S A, 2004; 101:4240–5. CrossRef
Spanakis N, Tsiodras S, Haagmans BL, Raj VS, Pontikis K, Koutsoukou A, Koulouris NG, Osterhaus AD, Koopmans MP, Tsakris A. Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int J Antimicrob Agents, 2014; 44:528–32. CrossRef
Stockman LJ, Bellamy R, Garner P. SARS: systematic review of treatment effects. PLoS Med, 2006; 3:e343. CrossRef
Ströher U, DiCaro A, Li Y, Strong JE, Aoki F, Plummer F, Jones SM, Feldmann H. Severe acute respiratory syndrome-related coronavirus is inhibited by interferon-α. J Infect Dis, 2004; 189:1164–7. CrossRef
Tan ELC, Ooi EE, Lin CY, Tan HC, Ling AE, Lim B, Stanton LW. Inhibition of SARS Coronavirus Infection in vitro with clinically approved antiviral drugs. Emerg Infect Dis, 2004; 10:581–6. CrossRef
Tchesnokov EP, Feng JY, Porter DP, Götte M. Mechanism of inhibition of ebola virus RNA-dependent RNA polymerase by remdesivir. Viruses, 2019; 11:326. CrossRef
van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics, 2010; 84:523–38. CrossRef
Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, Seidah NG, Nichol ST. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J, 2005; 2:1–10. CrossRef
Walls AC, Park Y-J, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell, 2020; 181: 281–92.e6. CrossRef
Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, Hu Z, Zhong W, Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res, 2020a; 30:269–71. CrossRef
Wang Z, Chen X, Lu Y, Chen F, Zhang W. Clinical characteristics and therapeutic procedure for four cases with 2019 novel coronavirus pneumonia receiving combined Chinese and Western medicine treatment. Biosci Trends, 2020b; 14:64–8. CrossRef
World Health Organization. COVID-19. Coronavirus. 2020. Available via https://www.who.int/health-topics/coronavirus#tab=tab_1 (Accessed 10 April 2020)
World Health Organization. Cumulative number of reported suspect and probable cases (SARS). Coronavirus infections. 2003. Available via https://www.who.int/csr/sars/country/table/en/ (Accessed 10 April 2020).
World Health Organization. Middle East respiratory syndrome coronavirus maps and epicurves. Coronavirus infections. 2017. Available via https://www.who.int/csr/disease/coronavirus_infections/maps-epicurves/en/ (Accessed 10 April 2020).
Xu K, Cai H, Shen Y, Ni Q, Chen Y, Hu S, Li J, Wang H, Yu L, Huang H, Qiu Y, Wei G, Fang Q, Zhou J, Sheng J, Liang T, Li L. Management of corona virus disease-19 (COVID-19): the Zhejiang experience. Zhejiang Da Xue Xue Bao Yi Xue Ban, 2020; 49(1):0. CrossRef
Yan D, Liu X, Zhu Y, Huang L, Dan B, Zhang G, Gao Y. Factors associated with prolonged viral shedding and impact of lopinavir/ritonavir treatment in patients with SARS-CoV-2 infection. medRxiv, 2020. CrossRef
Yao T, Qian J, Zhu W, Wang Y, Wang G. A systematic review of lopinavir therapy for SARS coronavirus and MERS coronavirus-A possible reference for coronavirus disease-19 treatment option. J Med Virol, 2020; 92:556–63. CrossRef
Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, Liu X, Zhao L, Dong E, Song C, Zhan S, Lu R, Li H, Tan W, Liu D. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin Infect Dis, 2020; 2:1–25. CrossRef
Young BE, Ong SWX, Kalimuddin S, Low JG, Tan SY, Loh J, Ng OT, Marimuthu K, Ang LW, Mak TM, Lau SK, Anderson DE, Chan KS, Tan TY, Ng TY, Cui L, Said Z, Kurupatham L, Chen MI, Chan M, Vasoo S, Wang LF, Tan BH, Lin RTP, Lee VJM, Leo YS, Lye DC, Singapore 2019 Novel Coronavirus Outbreak Research Team. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA, 2020; 323:1488–94. CrossRef
Zhao Z, Zhang F, Xu M, Huang K, Zhong W, Cai W, Yin Z, Huang S, Deng Z, Wei M, Xiong J, Hawkey PM. Description and clinical treatment of an early outbreak of severe acute respiratory syndrome (SARS) in Guangzhou, PR China. J Med Microbiol, 2003; 52:715–20. CrossRef
Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet, 2020; 395:1054–62. CrossRef