INTRODUCTION
Candida auris (C. auris) is an emerging multidrug resistant yeast species attributed as the cause of nosocomial invasive infections. The first clinical case of candidiasis caused by C. auris was reported in Japan in 2009 from a patient’s external ear canal, and hence its name (auris, from latin: ear) (Satoh et al., 2009). Since this is the first report, the number of cases and outbreaks referring infection with C. auris have been increasing from year to year arising the alerts of national health institutions in several countries due to its bad prognosis, high mortality rate, and rapid spread, even under aseptic conditions in the healthcare settings. C. auris adds to the list of multidrug and antibiotics resistant infections, considered currently as one of the main public health global concerns (Gómez-Ríos and Ramírez-Malule, 2019). It has been widely reported that the invasive candidiasis caused by this yeast is resistant to the main antifungal antibiotics and it is usually incorrectly identified by the commercial biochemical methods available in the healthcare institutions (Forsberg et al., 2019; Spivak and Hanson, 2018). Therefore, the epidemiological features as the prevalence and incidence of infections are still difficult due to the lack of consistent information (Cortegiani et al., 2018; Snyder and Wright, 2019).
C. auris grows as yeast or pseudohyphae and it is a biofilm-forming strain. The reasons for the inability of C. auris to form hyphae are still unknown, although several hyphae-inhibiting metabolites and biofilm-forming metabolites have been identified as some of its secretion products (Semreen et al., 2019). The ability of this fungus to form biofilms is of special importance in the context of nosocomial diseases, since the presence of contaminated surfaces in healthcare settings contributes to the extensive transmission of infectious diseases. Pathogens embedded in dry surface biofilms, including C. auris, are able to persist on surfaces for weeks, even after rigorous surface decontamination procedures (Ledwoch and Maillard, 2018; Vickery, 2019).
This pathogen fungus represents a serious global health threat due to its persistence in the healthcare settings and opportunistic ability to cause infection via colonization of skin and bloodstream with high mortality rates (around 60%), especially in hospitalized patients with concomitant conditions (recent surgery, invasive medical devices, or admission to intensive care facilities) or risk factors (early and elderly age, intensive use of antifungal antibiotics, or immunosuppression) (Snyder and Wright, 2019). Despite the issues in its identification, e.g., by using traditional phenotypic and molecular techniques, the number of first-case and outbreak reports is increasing, revealing the virulence of the organism; which it has been already detected in five continents in more than single-case patients (Jeffery-Smith et al., 2018; Kathuria et al., 2015; Snyder and Wright, 2019). Besides, one also can consider that better detection methods might actually the reason why more outbreaks are described. In this regard, confirmed outbreaks have been reported in Europa, America, and Asia (Calvo et al., 2016; Sana et al., 2019; Schelenz et al., 2016), multiple cases in 23 countries and single cases in at least 11 countries. Nevertheless, an undetermined number of cases might be misidentified as the phylogenetic related species Candida haemulonii (C. haemulonii) or other, hence retrospectively the number of cases and countries with emergent outbreaks of C. auris could be considerably higher (Cortegiani et al., 2018; Govender et al., 2018). C. auris has been not detected out from healthcare environments and the mechanisms explaining the rapid spread of this pathogen are not completely understood; moreover, omics techniques allowed to determine the simultaneous emergence of independent clonal populations on different areas (Cortegiani et al., 2018; Lockhart et al., 2017).
In addition to its difficult detection, treatment options are reduced due to its resistance to azole, polyenes, and echinocandins antifungal antibiotics, allowing it to cause widespread spectrum infections, and even death (Chowdhary et al., 2014; Khillan et al., 2014). Furthermore, C. auris exhibits resistance to some common disinfection agents such as the quaternary compounds and it is able to survive on dry and moist surfaces up to two weeks, showing a notable persistence in the healthcare environments after disinfection (Cortegiani et al., 2018; Ledwoch and Maillard, 2018; Spivak and Hanson, 2018; Vickery, 2019). Given the increasing interest on this pathogen yeast and the associated public health concerns, several reviews related to C. auris have been identified in the recent literature (Jeffery-Smith et al., 2018; Ku et al., 2018; Navalkele et al., 2017; Spivak and Hanson, 2018). However, a bibliometric analysis regarding the characteristics and impact of those studies is still missing. In this regard, bibliometric analysis constitutes a systematic tool for monitoring the research efforts on the field, offering to veterans and new scientists an overview of the scientific panorama concerning this emerging pathogen (Gómez-Ríos and Ramírez-Malule, 2019; Ramirez-Malule, 2018). In this contribution, a bibliometric analysis of the studies on C. auris published in the timespan from January 2009 to December 2018 is presented.
METHODS
Data search and collection were performed from Scopus database. In this study, the systematic search strategy included the terms in the title of the article, abstract, and keywords. Additionally, the ‘document type’ was not constrained. Thus, the resulting search was as follows:
(TITLE-ABS-KEY ("Candida auris") for general information search. The search was done on 11 May 2019.
TITLE-ABS-KEY (("Candida auris" OR "C. auris") AND ("resistance" OR "drug resistance" OR "multidrug resistance")) for specific drug resistance search. The search was done on 16 June 2019.
Timespan: 2009–2018.
The information retrieved from the Scopus database included: (i) citation information, (ii) bibliographical information, (iii) abstract and keywords, (iv) funding details, and (v) other information. The software VOSviewer 1.6.11 was used for visualization and data analysis (van Eck and Waltman, 2010).
RESULTS AND DISCUSSION
Evolution of research on C. auris between 2009 and 2018
In the period from January 2009 to December 2018, 227 documents were identified fulfilling the search criteria. Figure 1 shows the evolution of the number of publications per year. Notice that only nine documents were published between 2009 and 2014 and all of them consist in reports of identification and isolation from patients with candidemia in Japan (Satoh et al., 2009), Korea (Lee et al., 2011; Oh et al., 2010), India (Chowdhary et al., 2013; 2014; Khillan et al., 2014; Sarma et al., 2013), and South Africa (Magobo et al., 2014). However, a slight increase in the number of publications was observed between 2015 and 2016, but more important is the fact that the first reports of hospital outbreaks in Europe and America were published during these two years. Those outbreaks occurred in United Kingdom and Venezuela in 2015–2016 and 2012–2013, respectively (Calvo et al., 2016; Schelenz et al., 2016). The first reports of hospital outbreaks of C. auris in Europe and America are included in the list of the most cited documents (Table 1) evidencing its relevance in the field.
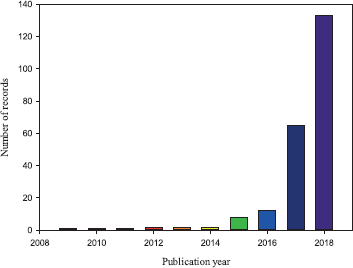 | Figure 1. Evolution of number of publications related to C. auris between 2009 and 2018. [Click here to view] |
A sharp increase in indexed documents was observed in 2017 and 2018, probably triggered by the alerts of disease control organisms, better detection protocols in addition to surveillance programs. Additionally, during the last years the number of first-case, multiple-case, and outbreaks reports increased significantly (Cortegiani et al., 2018; Osei Sekyere, 2018; Snyder and Wright, 2019). Interestingly, the notable increase of publications in the field during 2017 and 2018 evidences the accumulation of new knowledge about C. auris, which is also demonstrated by the emergence of a considerable number of review publications dealing with the topic in this period (Bidaud et al., 2018; Chowdhary et al., 2017; Cortegiani et al., 2018; de Cássia Orlandi Sardi et al., 2018; Forsberg et al., 2019; Jeffery-Smith et al., 2018; Navalkele et al., 2017; Osei Sekyere, 2018; Rossato and Colombo, 2018; Sears and Schwartz, 2017; Spivak and Hanson, 2018).
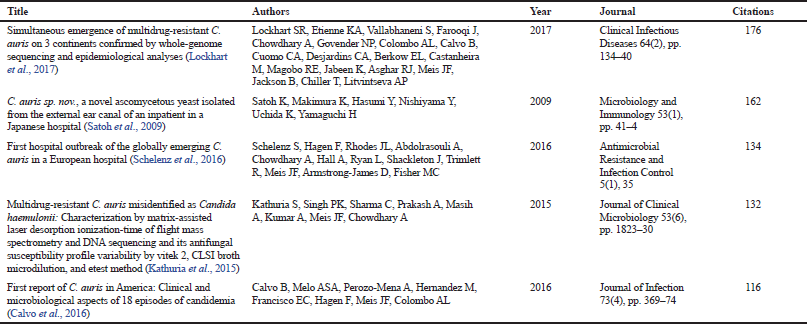 | Table 1. Most cited documents in the global research regarding C. auris. Note: the results are based on Scopus reports. [Click here to view] |
Table 1 presents the most cited research related to C. auris between 2009 and 2018 based on Scopus reports. Interestingly, the contribution with the highest impact in the field was an international collaboration between research centers of United States, Pakistan, India, South Africa, Brazil, Venezuela, and Netherlands (Lockhart et al., 2017). In that study, antifungal susceptibility test and Whole Genome Sequencing (WGS) methodologies were used to characterize 56 clinical isolates of C. auris from Africa, America, and Asia. The WGS results together with epidemiological observation confirmed the simultaneous emergence of different C. auris clonal populations on the three continents of the study (Lockhart et al., 2017). The analysis of the 56 isolates demonstrated that 93% of isolates were resistant to fluconazole, 54% to voriconazole, 35% to amphotericin B, and 41% were resistant to ≥ 2 antifungal classes; highlighting the global emergence of this multidrug resistance phenomenon (Lockhart et al., 2017).
Table 2 shows the top-5 sponsors funding researches related to the fungus C. auris. These sponsors were involved in 21.1% of all publications between 2009 and 2018. In this regard, pharmaceutical companies and disease control organisms clearly lead the sponsorship of research activities in this field.
In addition to sponsors, the knowledge generation activities led by the researchers are crucial to face the health concerns arisen from the emergence of C. auris and its multiresistant and invasive characteristics. Table 3 displays the most relevant authors, their affiliation, and the number of publications that were published in the period between 2009 and 2018, based on Scopus reports. Tables 1–3 provide relevant information for further identification of funding sources, networking, and collaboration opportunities with the leading institutions and researchers of this field.
.png) | Table 2. Top-5 sponsors funding researches related to C. auris between 2009 and 2018. [Click here to view] |
Figure 2 shows the areas of knowledge related to the studies of C. auris published between 2009 and 2018. In this regard, (i) Medicine, (ii) Immunology and Microbiology, and (iii) Pharmacology, Toxicology and Pharmaceutics contributed with 60.3%, 14.8%, and 10.9% of the indexed documents, respectively. Medicine was ranked first on this list since the most of publications consisted in cases and outbreaks reports, identification, and isolation of populations in hospitals of five continents (Ben-Ami et al., 2017; Borman et al., 2017; Calvo et al., 2016; Chowdhary et al., 2013; 2014; Emara et al., 2015; Satoh et al., 2009). The surveillance program of the Center for Disease Control and Prevention (CDC) updates monthly the new cases in the United States (https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html). Since its discovery in 2009, 725 cases (up to June 30, 2019) have been confirmed in the United States, most of them in the area of New York–New Jersey.
The leading countries in studies related to C. auris were United States, India, and the United Kingdom, which contributed with 90, 45, and 32 documents, respectively (see Table 4). Those countries had also presented the largest number of detected cases up to date (Osei Sekyere, 2018). In this study, three additional indicators were considered to rank the impact of the research: (i) ratio between the number of citations and number of publications, (ii) ratio between the number of publications and gross domestic product (GDP), and (iii) ratio between the number of citations and GDP. In this regard, a redistribution of the top-10 leading countries was observed. Netherlands ranked first in all scenarios, evidencing the impact of the research performed in this country concerning C. auris. India and the United Kingdom were always within the top-5; in contrast, China occupied the last place. These findings are coincident with the information reported in Table 3, i.e., the most productive authors and the countries where their institutions are located. When productivity was stratified by calculating the ratio between either number of publications or number of citations and GDP, Colombia and Denmark were included in top-5. Colombia is the only Latin American country with a reported multicenter hospital-associated outbreak of C. auris occurred in 2016 (Armstrong et al., 2019; Escandón et al., 2018; 2019; Parra-Giraldo et al., 2018).
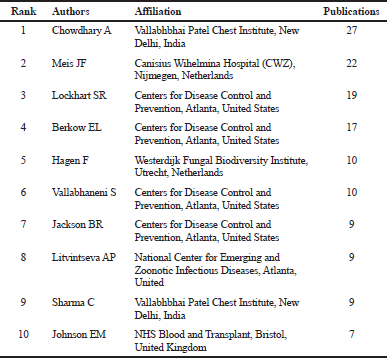 | Table 3. Top-10 leading authors of studies related to C. auris between 2009 and 2018. Note: the results are based on Scopus reports. [Click here to view] |
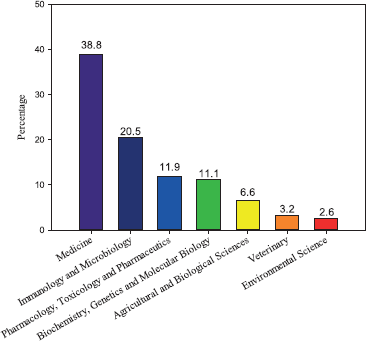 | Figure 2. Evolution of number of publications related to C. auris between 2009 and 2018. [Click here to view] |
Figures 3 and 4 show the research-topic map of C. auris studies between 2009 and 2018. The network visualization contains 26 items grouped in four clusters (Figure 3). In this regard, the biggest node, which corresponds to the keyword with the highest occurrences, was C. auris (Figure 3 and Table 5). The first isolates of this fungus from patients were initially misidentified as C. haemulonii, and both are within the same cluster (the red one) (Kathuria et al., 2015). Here, it is clear the special interest on antifungal resistance and multidrug resistance of C. auris. In fact, C. auris has been reported to be resistant to fluconazole, amphotericin B, and to a lesser extent, to echinocandins (Lockhart et al., 2017). Lockhart et al., (2017) reported that 41 isolates from C. auris infection patients from Pakistan, India, South Africa, and Venezuela between 2012 and 2015, showed 93%, 35%, and 7% of resistance to fluconazole, amphotericin B, and echinocandins, respectively.
Figure 4 shows how the research topics moved from candidemia/outbreak/fungemia (end of 2016), passing by identification/candida/azoles/echinocandins (beginning of 2017), to infection control/invasive candidiasis/multidrug resistance/antifungal resistance (end of 2017). Future studies should be focused on cheminformatics and molecular applications for the design of either new or modified antifungal antibiotics as therapeutic alternative and control measures for future outbreaks of C. auris (Mas et al., 2019).
A total of 673 organizations were involved in 227 publications between 2009 and 2018. Seventeen organizations reached the threshold of three published articles but only thirteen of these institutions were interconnected. For a threshold of five published articles, only five of these institutions were interconnected. The collaboration between institutions seems to be highly specialized and centralized, probably due to the low diffusion of the methods and complexity of identification techniques, which can be rarely applied in the moment and place of the outbreaks, especially if those occur in non-developed countries. The incipient and specialized collaboration between organizations suggest that further and stronger alliances are needed to deal with this emerging pathogen by proposing methodologies for successful and fast diagnosis, epidemiologic surveillance, clinical management and pharmacologic alternatives to face the resistance phenomenon associated to C. auris.
C. auris: antifungal antibiotics and resistance mechanisms
Figures 5 and 6 help us to visualize the panorama of drug and multidrug resistance of C. auris. The number of studies related to drug and multidrug resistance of C. auris increased substantially during 2016–2018 (Figure 5). The keywords network analysis identified “Candida auris”, “candida”, “antifungal resistance”, “fluconazole”, and “multidrug resistance”, as the nodes with more occurrences (Figure 6). Interestingly, around thirteen antifungal compounds used in the sensitivity tests of C. auris appears in the network, sharpening the problem of drug resistance.
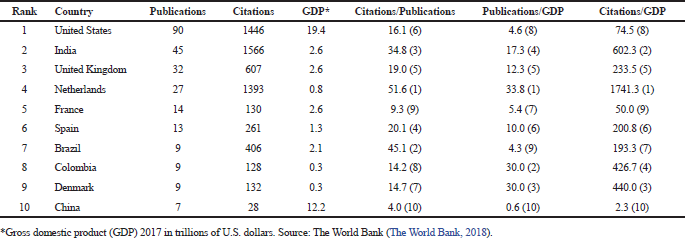 | Table 4. Top-10 leading countries of studies related to C. auris between 2009 and 2018. [Click here to view] |
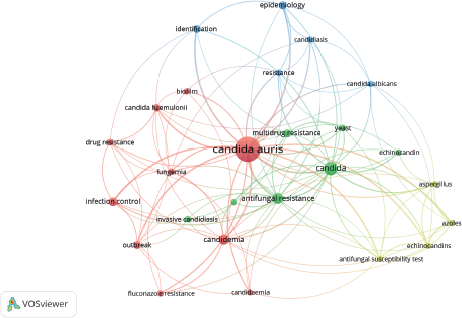 | Figure 3. Network visualization of the research-topic map of studies related to C. auris between 2009 and 2018. Note: the minimum number of occurrences of a keyword is 5. [Click here to view] |
.png) | Figure 4. Overlay visualization of the research-topic map of studies related to C. auris between 2009 and 2018. Note: the minimum number of occurrences of a keyword is 5. [Click here to view] |
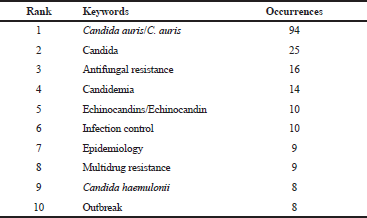 | Table 5. Top-10 keywords of C. auris studies in the period of 2009–2018. [Click here to view] |
 | Figure 5. Evolution of number of publication of C. auris related to drug and multidrug resistance. [Click here to view] |
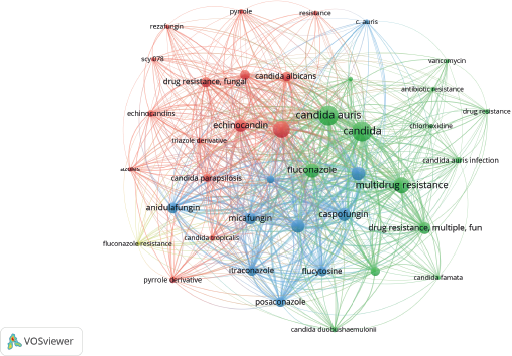 | Figure 6. Research-topic map of studies of C. auris related to drug and multidrug resistance. Note: the minimum number of occurrences of a keyword is 5. [Click here to view] |
Table 6 shows, in order of occurrences, the antifungal compounds tested on C. auris. These compounds are grouped in the antifungal classes of Azoles, Polyenes, Echinocandins, and Nucleoside analogs. A better knowledge of the mechanisms of antifungal inhibition and drug resistance in C. auris is required to propose pharmacological alternatives that help to the emergence of this multiresistant pathogen.
Currently, there is scarce knowledge about the physiology of C. auris; however, it shares similarities with other Candida species such as C. haemulonii, C. pseudohaemulonii, and C. duobushaemulonii that also causes bloodstream, invasive, and superficial infections with notable acquired drug resistance (Muñoz et al., 2018).
Most of the antifungals used against Candida species acts on components of fungi membrane and cell wall synthesis (Figure 7). Azole antifungals, such as imidazoles (clotrimazole, econazole, miconazole, ketonazole), triazoles (fluconazole, itraconazole, and voriconazole), and posaconazole are inhibitors of the Lanosterol 14-α-sterol demethylase enzyme, an enzyme involved in ergosterol biosynthesis, the main sterol of the fungus membrane (Krishnasamy et al., 2018). The disruption of this enzyme usually leads to the accumulation of toxic intermediates such as 14-α-methyl-3,6-diol, reducing the ergosterol content and affecting the membrane integrity. Polyenes such as nystatin, natamycin, and amphotericin B also act on the membrane, by binding to ergosterol and disrupting the fungal cell membrane resulting in pore formation. Echinocandins act on the cell wall of fungi, specifically inhibiting the 1,3-β-D-glucan synthase enzyme causing malformations on the fungal cell wall. Finally, nucleoside analogs as flucytocine affect replication and transcriptions by the inhibition of DNA and RNA synthesis.
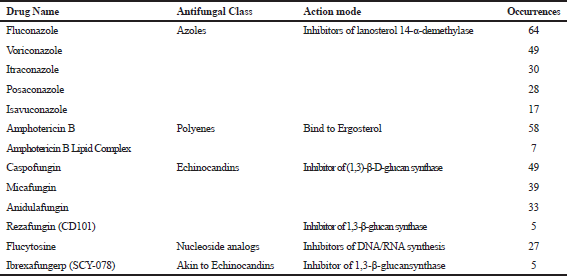 | Table 6. Most studied antifungals in sensitivity tests against C. auris. [Click here to view] |
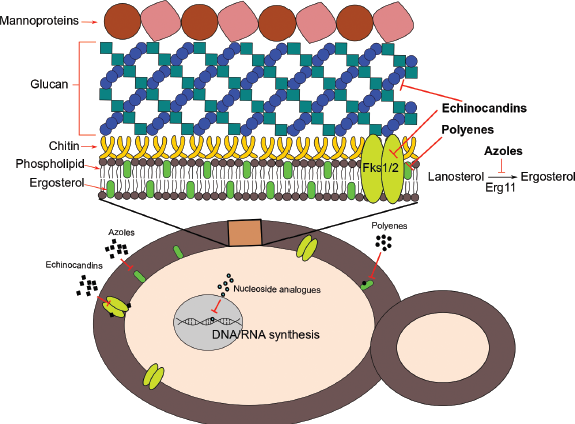 | Figure 7. Scheme of action mechanisms of the antifungal classes tested in C. auris. [Click here to view] |
Candida species, including C. auris, have developed resistance mechanisms against most of these classes of antifungals (Mas et al., 2019). Different Azole resistance mechanisms have been previously described for Candida species (Krishnasamy et al., 2018; Mishra et al., 2007; Morschhäuser 2002). Candida species can reduce the binding capacity between azoles and the enzyme 4-alpha-sterol demethylase due to alterations of the enzyme by mutations in ergosterol biosynthesis (ERG11) genes (Krishnasamy et al., 2018; White et al., 2002). In addition, it is known that those fungi can overexpress ERG11 genes in order to synthesize a higher amount of 14-α-sterol demethylase and therefore improving its survival (Krishnasamy et al., 2018; White et al., 2002). Likewise, the overexpression of efflux pump systems such as ATP-binding cassette (ABC) or major facilitator superfamily proteins diminishes the azole or drug concentration in the membrane of the fungi (Kanafani and Perfect, 2008; Krishnasamy et al., 2018).
Three mechanisms of antifungal resistance have been associated with polyenes (Peyron et al., 2002). As polyenes have a high affinity for ergosterol, some Candida species have developed resistance mechanisms by inhibiting the ergosterol biosynthesis and replacing ergosterol by other biosynthetic precursors such as fecosterol, lanosterol, and episterol in the membrane (Peyron et al., 2002). Additionally, it has been demonstrated that some Candida species can change the permeability of the membrane to polyenes hence reducing its effect. Finally, resistance to amphotericin B has been associated with mutations in genes involved in ergosterol biosynthesis (ERG2 and ERG3) (Arikan and Rex 2010).
Resistance to echinocandins is known by triggering two resistance mechanisms (Beyda et al., 2012; Perlin, 2007). The first resistance mechanisms responsible for decreasing susceptibility to echinocandins are some specific mutations on genes encoding the subunits of 1,3 β-D-glucan synthase enzyme FKS (Glucan synthase genes) (Perlin, 2007). Furthermore, it has been shown that hotspot mutations on FKS1 and FKS2 genes induce the Minimum inhibitory concentration (MIC) in C. albicans and C. glabrata species (Katiyar et al., 2006; Park et al., 2005).
Finally, there exist two documented resistance mechanisms for nucleoside analogs like flucytosine (Vermes et al., 2000). The first one is the emergence of point mutations on cytosine deaminase gene (FCY1), purine-cytosine permease (FCY2), and uracil phosphoribosyl transferase (FUR1), which prevents transport and uptake of flucytosine. The second mechanism is the overexpression of pyrimidine biosynthesis generating a high demand of this antifungal to get elevated inhibitory effects. It is necessary to highlight that Candida species can grow by forming biofilms, which constitutes an additional diffusional and chemical barrier to all the existent antifungal drugs (Kean et al., 2018; Ledwoch and Maillard, 2018; Vickery, 2019).
Our bibliometric analysis showed new efforts to identify compounds with high antifungal activity (Table 6); specifically the inhibitor of glucan biosynthesis SCY-078 and the echinocandin CD101 (Larkin et al., 2017a; 2017b). Although those compounds are still in clinical development, they have shown high susceptibility on a panel of sixteen C. auris isolates, with MIC90 of around 1 mg/L for SCY-078 and an MIC50 of 0.125 μg/L for CD101 compound.
FUTURE PERSPECTIVES
C. auris outbreaks are of global concern because of its high rate of patient mortality (Calvo et al., 2016; Chowdhary et al., 2013). Up to June 30, 2019, CDC reported 725 confirmed and 30 probable clinical cases in twelve U.S. states and 1474 patients have been found to be colonized with C. auris in ten states with previously reported clinical cases. Furthermore, the CDC also recorded single and multiple cases of C. auris in several countries:
- Single cases of C. auris: Austria, Belgium, Chile, Iran, Malaysia, the Netherlands, Norway, Switzerland, Taiwan, and the United Arab Emirates.
- Multiples cases of C. auris: Australia, Canada, China, Colombia, France, Germany, India, Israel, Japan, Kenya, Kuwait, Oman, Pakistan, Panama, Russia, Saudi Arabia, Singapore, South Africa, South Korea, Spain, the United Kingdom, the United States, and Venezuela. Note: in some of these countries, the extensive transmission of C. auris has been documented in more than one hospital.
These data and our bibliometric analysis suggest that higher investment efforts from government institutions, pharmaceutical companies, universities, and research centers are urgent to design either new or modified drug combinations in addition to new, accurate, and faster identification techniques of C. auris aimed to control further outbreaks of candidemia especially in countries with lower income, where the sanitary conditions could enhance the spreading capacity of this pathogen.
CONCLUSION
A total of 227 articles related to C. auris between 2009 and 2018 were published with United States, India, and the United Kingdom as the research-leading countries in the field. Nevertheless, Netherlands ranked first when additional indicators were considered: (i) number of citations and (ii) GDP, showing the high impact of the research performed in this country. A significant increase in studies related to drug and multidrug resistance of C. auris during 2016–2018 was found. The incipient collaboration between institutions and the lack of knowledge regarding the physiology and drug-resistance mechanisms of C. auris demand further and stronger collaboration networks for dealing with this global health problem and improving the diagnostic and identification methodologies, as well as assure development and implementation of new antifungal antibiotics for treatment and outbreak control. Furthermore, the research was focused in the following areas: (i) Medicine, (ii) Immunology and Microbiology, and (iii) Pharmacology, Toxicology and Pharmaceutics with a participation of 60.3%, 14.8%, and 10.9% of the indexed documents, respectively. Finally, even though only ten years have passed since the first case and isolation of C. auris was reported, the knowledge of this pathogen in terms of genetics, transcriptomics, and antifungal resistance have increased faster than in previously discovered pathogens with considerably more researchers and institutions involved such as Mycobacterium tuberculosis.
ACKNOWLEDGMENTS
Víctor A. López-Agudelo acknowledges the economic support provided by the Administrative Department of Science, Technology and Innovation – COLCIENCIAS, Colombia, during his Ph.D. studies (National Ph.D. scholarship Conv. 727–2015).
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
ETHICAL APPROVAL
Not required.
REFERENCES
Arikan S, Rex JH. Resistance to antifungal agents. In: Topley & Wilson’s (Eds.), Microbiology and Microbial Infections. John Wiley & Sons, Ltd., Chichester, UK, 2010. CrossRef
Armstrong PA, Rivera SM, Escandon P, Caceres DH, Chow N, Stuckey MJ, Díaz J, Gomez A, Vélez N, Espinosa-Bode A, Salcedo S, Marin A, Berrio I, Varón C, Guzman A, Pérez-Franco JE, Escobar JD, Villalobos N, Correa JM, Litvintseva AP, Lockhart SR, Fagan R, Chiller TM, Jackson B, Pacheco O. Hospital-associated multicenter outbreak of emerging fungus Candida auris, Colombia, 2016. Emerg Infect Dis J, 2019; 25:1339. CrossRef
Ben-Ami R, Berman J, Novikov A, Bash E, Shachor-Meyouhas Y, Zakin S, Maor Y, Tarabia J, Schechner V, Adler A, Finn T. Multidrug-resistant Candida haemulonii and Candida auris, Tel Aviv, Israel. Emerg Infect Dis, 2017; 23:195–203. CrossRef
Beyda ND, Lewis RE, Garey KW. Echinocandin resistance in Candida species: mechanisms of reduced susceptibility and therapeutic approaches. Ann Pharmacother, 2012; 46:1086–96. CrossRef
Bidaud AL, Chowdhary A, Dannaoui E. Candida auris: an emerging drug resistant yeast – A mini-review. J Mycol Med, 2018; 28:568–73. CrossRef
Borman AM, Szekely A, Johnson EM. Isolates of the emerging pathogen Candida auris present in the UK have several geographic origins. Med Mycol, 2017; 55:563–7. CrossRef
Calvo B, Melo ASA, Perozo-Mena A, Hernandez M, Cristina E, Hagen F, Meis JF, Lopes A. First report of Candida auris in America: clinical and microbiological aspects of 18 episodes of candidemia. J Infect, 2016; 73:369–74. CrossRef
Chowdhary A, Anil Kumar V, Sharma C, Prakash A, Agarwal K, Babu R, Dinesh KR, Karim S, Singh SK, Hagen F, Meis JF. Multidrug-resistant endemic clonal strain of Candida auris in India. Eur J Clin Microbiol Infect Dis, 2014; 33:919–26. CrossRef
Chowdhary A, Sharma C, Duggal S, Agarwal K, Prakash A, Singh PK, Jain S, Kathuria S, Randhawa HS, Hagen F, Meis JF. New clonal strain of Candida auris , Delhi, India. Emerg Infect Dis, 2013; 19:1670–3. CrossRef
Chowdhary A, Sharma C, Meis JF. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog, 2017; 13:e1006290. CrossRef
Cortegiani A, Misseri G, Fasciana T, Giammanco A, Giarratano A, Chowdhary A. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J Intensive Care, 2018; 6:1–13. CrossRef
de Cássia Orlandi Sardi J, Silva DR, Soares Mendes-Giannini MJ, Rosalen PL. Candida auris: Epidemiology, risk factors, virulence, resistance, and therapeutic options. Microb Pathog, 2018; 125:116–21. CrossRef
Emara M, Ahmad S, Khan Z, Joseph L, Al-Obaid I, Purohit P, Bafna R. Candida auris Candidemia in Kuwait, 2014. Emerg Infect Dis, 2015; 21:1091–2. CrossRef
Escandón P, Cáceres DH, Espinosa-Bode A, Rivera S, Armstrong P, Vallabhaneni S, Berkow EL, Lockhart SR, Chiller T, Jackson BR, Duarte C. Notes from the field: surveillance for Candida auris - Colombia, September 2016–May 2017. MMWR Morb Mortal Wkly Rep, 2018; 67:459–60. CrossRef
Escandón P, Chow NA, Caceres DH, Gade L, Berkow EL, Armstrong P, Rivera S, Misas E, Duarte C, Moulton-Meissner H, Welsh RM, Parra C, Pescador LA, Villalobos N, Salcedo S, Berrio I, Varón C, Espinosa-Bode A, Lockhart SR, Jackson BR, Litvintseva AP, Beltran M, Chiller TM. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in Amphotericin B resistance. Clin Infect Dis, 2019; 68:15–21. CrossRef
Forsberg K, Woodworth K, Walters M, Berkow EL, Jackson B, Chiller T, Vallabhaneni S. Candida auris: the recent emergence of a multidrug-resistant fungal pathogen. Med Mycol, 2019; 57:1–12. CrossRef
Gómez-Ríos D, Ramírez-Malule H. Bibliometric analysis of recent research on multidrug and antibiotics resistance (2017–2018). J Appl Pharm Sci, 2019; 9:112–6. CrossRef
Govender NP, Magobo RE, Mpembe R, Mhlanga M, Matlapeng P, Corcoran C, Govind C, Lowman W, Senekal M, Thomas J. Candida auris in South Africa, 2012–2016. Emerg Infect Dis J, 2018; 24:2036. CrossRef
Jeffery-Smith A, Taori SK, Schelenz S, Jeffery K, Johnson EM, Borman A, Manuel R, Brown CS. Candida auris: A review of the literature. Clin Microbiol Rev, 2018; 31. CrossRef
Kanafani ZA, Perfect JR. Resistance to antifungal agents: mechanisms and clinical impact. Clin Infect Dis, 2008; 46:120–8. CrossRef
Kathuria S, Singh PK, Sharma C, Prakash A, Masih A, Kumar A, Meis JF, Chowdhary A. Multidrug-resistant Candida auris misidentified as Candida haemulonii: Characterization by matrix-assisted laser desorption ionization–time of flight mass spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CL. J Clin Microbiol, 2015; 53:1823–30. CrossRef
Katiyar S, Pfaller M, Edlind T. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob Agents Chemother, 2006; 50:2892–4 CrossRef
Kean R, Delaney C, Sherry L, Borman A, Johnson EM, Richardson MD, Rautemaa-Richardson R, Williams C, Ramage G. Transcriptome assembly and profiling of Candida auris reveals novel insights into biofilm-mediated resistance. mSphere, 2018; 3:e00334–18 CrossRef
Khillan V, Rathore N, Kathuria S, Chowdhary A. A rare case of breakthrough fungal pericarditis due to fluconazole-resistant Candida auris in a patient with chronic liver disease. JMM Case Reports, 2014; 1:e003707. CrossRef
Krishnasamy L, Krishnakumar S, Kumaramanickavel G, Saikumar C. Molecular mechanisms of antifungal drug resistance in Candida species. J Clin Diagnostic Res, 2018; 12:DE01–6. CrossRef
Ku TSN, Walraven CJ, Lee SA. Candida auris: disinfectants and implications for infection control. Front Microbiol, 2018; 9:726. CrossRef
Larkin E, Hager C, Chandra J, Mukherjee PK, Retuerto M, Salem I, Long L, Isham N, Kovanda L, Borroto-Esoda K. The emerging pathogen Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother, 2017a; 61:e02396–16. CrossRef
Larkin EL, Long L, Ghannoum MA. Susceptibility of recent Candida auris isolates to the novel echinocandin CD101 and comparator antifungal agents. Abstr ECCMID, 2017b.
Ledwoch K, Maillard J-Y. Candida auris dry surface biofilm (DSB) for disinfectant efficacy testing. Materials (Basel), 2018; 12:18. CrossRef
Lee WG, Shin JH, Uh Y, Kang MG, Kim SH, Park KH, Jang H-C. First three reported cases of nosocomial fungemia caused by Candida auris. J Clin Microbiol, 2011; 49:3139–42. CrossRef
Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis, 2017; 64:134–40. CrossRef
Magobo RE, Corcoran C, Seetharam S, Govender NP. Candida auris – associated candidemia, South Africa. Emerg Infect Dis, 2014; 20:1250–1. CrossRef
Mas CD, Rossato L, Oliveira EB, Pedro I, Junior S, Meis JF, Colombo AL, Hayashi MAF. Effects of the natural peptide crotamine from a south american rattlesnake on Candida auris, an emergent multidrug antifungal resistant human pathogen. Biomolecules, 2019; 9:1–10. CrossRef
Mishra N, Prasad T, Sharma N, Payasi A, Prasad R, Gupta D, Singh R. Pathogenicity and drug resistance in Candida albicans and other yeast species. Acta Microbiol Immunol Hung, 2007; 54:201–35. CrossRef
Morschhäuser J. The genetic basis of fluconazole resistance development in Candida albicans. Biochim Biophys Acta - Mol Basis Dis, 2002; 1587:240–8. CrossRef
Muñoz JF, Gade L, Chow NA, Loparev VN, Juieng P, Berkow EL, Farrer RA, Litvintseva AP, Cuomo CA. Genomic insights into multidrug-resistance, mating and virulence in Candida auris and related emerging species. Nat Commun, 2018; 9:5346. CrossRef
Navalkele BD, Revankar S, Chandrasekar P. Candida auris: A worrisome, globally emerging pathogen. Expert Rev Anti Infect Ther, 2017; 15:819–27. CrossRef
Oh BJ, Shin JH, Kim MN, Sung H, Lee K, Joo MY, Shin MG, Suh SP, Ryang DW. Biofilm formation and genotyping of Candida haemulonii, Candida pseudohaemulonii, and a proposed new species (Candida auris) isolates from Korea. Med Mycol, 2010; 49:98–102. CrossRef
Osei Sekyere J. Candida auris: A systematic review and meta-analysis of current updates on an emerging multidrug-resistant pathogen. Microbiologyopen, 2018; 7:1–29. CrossRef
Park S, Kelly R, Kahn JN, Robles J, Hsu M-J, Register E, Li W, Vyas V, Fan H, Abruzzo G. Specific substitutions in the echinocandin target Fks1p account for reduced susceptibility of rare laboratory and clinical Candida sp. isolates. Antimicrob Agents Chemother, 2005; 49:3264–73. CrossRef
Parra-Giraldo CM, Valderrama SL, Cortes-Fraile G, Garzón JR, Ariza BE, Morio F, Linares-Linares MY, Ceballos-Garzón A, de la Hoz A, Hernandez C, Alvarez-Moreno C, Le Pape P. First report of sporadic cases of Candida auris in Colombia. Int J Infect Dis, 2018; 69:63–7. CrossRef
Perlin DS. Resistance to echinocandin-class antifungal drugs. Drug Resist Updat, 2007; 10:121–30. CrossRef
Peyron F, Favel A, Calaf R, Michel-Nguyen A, Bonaly R, Coulon J. Sterol and fatty acid composition of Candida lusitaniae clinical isolates. Antimicrob Agents Chemother, 2002; 46:531–3. CrossRef
Ramirez-Malule H. (2018). Bibliometric analysis of global research on clavulanic acid. Antibiotics, 2018; 7:102. CrossRef
Rossato L, Colombo AL. Candida auris: what have we learned about its mechanisms of pathogenicity? Front Microbiol, 2018; 9:1–6. CrossRef
Sana F, Hussain W, Zaman G, Satti L, Khurshid U, Khadim MT. Candida auris associated outbreak report from Pakistan: a success story of infection control in ICU of a tertiary care hospital. J Hosp Infect, 2019; In press. CrossRef
Sarma S, Kumar N, Sharma S, Govil D, Ali T, Mehta Y, Rattan A. Candidemia caused by amphotericin B and Fluconazole resistant Candida auris. Indian J Med Microbiol, 2013; 31:90–1. CrossRef
Satoh K, Makimura K, Hasumi Y, Nishiyama Y, Uchida K, Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol Immunol, 2009; 53:41–4. CrossRef
Schelenz S, Hagen F, Rhodes JL, Abdolrasouli A, Chowdhary A, Hall A, Ryan L, Shackleton J, Trimlett R, Meis JF, Armstrong-James D, Fisher MC. First hospital outbreak of the globally emerging Candida auris in a european hospital. Antimicrob Resist Infect Control, 2016; 5:35. CrossRef
Sears D, Schwartz BS. Candida auris: an emerging multidrug-resistant pathogen. Int J Infect Dis, 2017; 63:95–8. CrossRef
Semreen MH, Soliman SSM, Saeed BQ, Alqarihi A, Uppuluri P, Ibrahim AS. Metabolic profiling of Candida auris, a newly-emerging multi-drug resistant Candida species, by GC-MS. Molecules, 2019; 24:399. CrossRef
Snyder GM, Wright SB. The epidemiology and prevention of Candida auris. Curr Infect Dis Rep, 2019; 21:1–13. CrossRef
Spivak ES, Hanson KE. Candida auris: An emerging fungal pathogen. J Clin Microbiol, 2018: 56. CrossRef
The World Bank. World development indicators, 2018. Available via https://datacatalog.worldbank.org/dataset/gdp-ranking (Accessed 4 December 2018).
van Eck NJ, Waltman L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics, 2010; 84:523–38. CrossRef
Vermes A, Guchelaar H-J, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother, 2000; 46:171–9. CrossRef
Vickery K. Microbial biofilms in healthcare: formation, prevention and treatment. Materials (Basel), 2019; 12:1–5. CrossRef
White TC, Holleman S, Dy F, Mirels LF, Stevens DA. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob Agents Chemother, 2002; 46:1704–13. CrossRef