INTRODUCTION
In cases where a patient with an existing prescription seeks a physician’s consultation, most physicians either add, omit, or change patients’ medications which results in a new list of medications. If both lists are not compared, reviewed, and updated, medication discrepancies can occur [1]. These discrepancies may happen upon hospital admission, discharge, during the transfer from one department to another, or at transfer from hospital to other healthcare facilities. The discrepancies include the following errors: omission, addition, duplication, dosage, and frequency [2]. Medication error (ME) is a serious problem in each healthcare system. The Joint Commission reported that ME is considered as one of the highest types of errors in medical practice [3]. By adopting the World Health Organization (WHO) statement, “all MEs are potentially avoidable,” it is possible to reduce or even prevent ME by developing more effective healthcare systems and implementing patient safety initiatives [4]. Proper comparison and reviewing of patients’ medication lists in addition to appropriate patient history taking can assure fewer medication discrepancies and better clinical outcomes. This is simply what medication reconciliation (MedRec) means. In definition, MedRec is “the process of creating the most accurate list possible of all medications a patient is taking—including drug name, dosage, frequency, and route—and comparing that list against the physician’s admission, transfer, and/or discharge orders, with the goal of providing correct medications to the patient at all transition points within the hospital” [5]. The process of MedRec was initiated to overcome medication discrepancies that can occur during care transition, and it has been recommended as a patient safety initiative by the WHO and the IHI [2,4]. Many hospitals implemented MedRec as it is required for hospital accreditation and addressed as a key performance indicator (KPI) [6–8]. Despite this, many countries including Gulf Cooperation Council are not implementing it properly. The objective of this review was to assess the current evidence about MedRec practices and barriers to implementing MedRec by hospitals in GCC.
METHODOLOGY
Search strategy
An organized search strategy was followed to detect the relevant research. The following databases were retrieved: SCOPUS, EBSCO, and PUBMED. In addition, Google Scholar was searched to identify further eligible studies.
The search terms (title, abstract, keyword, text) were “MedRec” OR “medication discrepancies” AND (GCC OR Saudi Arabia OR United Arab Emirates (UAE) OR Kuwait OR Bahrain OR Oman OR Qatar). All searches spanned from database inception until December 10th, 2021 and all publications available until that date were included. The review included journal articles, review papers, letters to editors, conference papers, and conference reviews published in English language only. Including research outside English, even if existed, was not within the scope of the study.
Selection criteria
Preferred reporting items for systematic reviews and meta-analyses statement served as the basis for the selection criteria [9]. The search focused on finding existing publications on MedRec in different hospital settings. Studies conducted on community pharmacies were excluded. Articles from any other country were not included because the search was exclusively limited to the GCC countries. A total of 2,549 search articles were excluded whereas 191 records were included at this stage.
Quality assessment
The review included journal articles, review papers, case reports, letters to editors, conference papers, and conference reviews. All duplications were checked carefully to sustain the quality of the review.
All abstracts of the included studies were checked thoroughly to decide if the articles were relevant and a deep evaluation of full articles was conducted later.
After checking the duplicate records and other ineligibility criteria, 27 articles met our inclusion and exclusion criteria, and they were selected for this review. Figure 1 displays the inclusion and exclusion criteria for the search at each stage (PRISMA statement). At this stage, the researchers registered the protocol of this review in the PROSPERO database [ID: CRD42022310577].
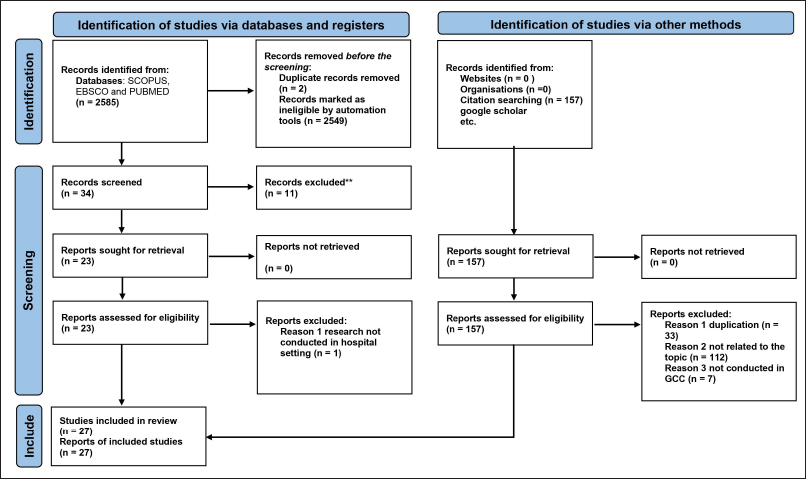 | Figure 1. PRISMA 2020 flow diagram for systematic reviews. [Click here to view] |
Data analysis
The initial classification of studies was based on which GCC country they were conducted. Then, they were classified based on their outcomes into (1) process measures and (2) outcome measures including clinical parameters measurements and healthcare utilization. Studies were also grouped based on the main element of intervention into (1) pharmacist-related and (2) other types of interventions including implementing electronic MedRec tools and educating staff about MedRec.
RESULTS
Search results
As illustrated in Figure 1, the search generated a total of 2,585 records from SCOPUS, EBSCO, and PUBMED databases; an additional 157 were identified via Google Scholar search to give a total of 2,745. Records marked as ineligible by automation tools were 2,549, while the screened ones were 34 from the 3 mentioned databases and only 22 studies were selected after reviewing the full articles. For records yielded from Google Scholar search, a manual screening was done where some were excluded because of irrelevant topics (n = 112), studies were not conducted in GCC (n = 7), and duplicated articles (n = 33) resulted in including only five studies from Google Scholar database. Finally, the total number of studies included in this review was 27.
Description of included studies
Twenty-six of these included studies were journal articles and one case report. A total of 20 studies were quantitative [prospective cross-sectional (n = 9); retrospective cross-sectional (n = 4); descriptive cross-sectional (n = 3); analytical cross-sectional (n = 1); randomized control studies (n = 2); case report (n = 1)]. Other research designs were a mixed-method research design (n = 3), an exploratory qualitative design (n = 1), and quality improvement projects (n = 3). The study designs reflected that 74% of the studies were quantitative in nature with a lack of interventional studies (Table 1).
The type of hospitals varies between private and governmental hospitals: tertiary, secondary, and primary healthcare centers. The studied hospital departments included the hospital ward, pediatric ward, oncology ward, internal medicine ward, surgical ward, cardiology ward, ambulatory dialysis department, emergency department, and intensive care unit. The most common type of ward chosen was internal medicine.
An overview of the included studies
The included studies’ overview is shown in Table 2. The period of data collection of these studies ranged from 1 day to 1 year and 8 months. The average of the study duration of all included studies is 6 months. Three studies did not state the duration. Most studies were conducted in tertiary hospitals (n = 19, 70%) while few of them were done in teaching or academic hospitals (n = 4, 15%). Two studies (7.4%) were performed in primary care hospitals, and the remaining were completed in general and secondary care hospitals (n = 1 each, 3.7%).
 | Table 1. Types of study design and their frequencies. [Click here to view] |
Out of 27 included studies, 15 studies implemented MedRec during one or all of the following stages: admission, transfer, and discharge. Nine studies did the implementation during patient admission, three studies at the discharge stage, and one study at the transfer stage. One study did the intervention during the admission and discharge stages, while only one study investigated the MedRec process during the three stages: admission, transfer, and discharge.
Subjects recruited in the included studies
The recruited subjects in the included studies vary as most studies recruited patients (n = 13, 48%), followed by healthcare professionals (n = 11, 41%), and fewer studies were conducted by reviewing patients’ prescriptions and medications from the systems (n = 3, 11%).
Out of the 11 studies that were performed on healthcare professionals, 7 of them were concentrated only on pharmacists, and the remaining 4 included physicians, pharmacists, and nurses. The total number of pharmacists that have been recruited was 994 (69%) in comparison with 285 (20%) nurses and 164 (11%) physicians.
Practice of MedRec in GCC
The review showed that more than half of the studies originated in Saudi Arabia (n = 15, 55.5%) followed by Kuwait (n = 4, 15%), then Qatar and the UAE (n = 3 each, 11%), and Oman (n = 2, 7.4%). The review did not find any MedRec study conducted in the Kingdom of Bahrain (Fig. 2). The first-ever study on MedRec was conducted in Saudi Arabia in 2009, and it was published in 2011 by Abuyassin et al. [10] One study conducted in Bahrain which focused on the need to improve the medical curriculum by incorporating more medication safety courses and assessments was excluded since it was not related to MedRec practice in the hospital [11]. Figure 2 illustrates the distribution of studies by year.
Analysis of keywords using the word cloud generator
A word cloud generator was used to analyze the keywords written in the included articles (Fig. 4). The larger the words appeared in the image, the more common the keywords were. For instance, the keywords “medication,” “reconciliation,” and “Saudi Arabia” were shown in a larger font as compared to other keywords, indicating these words were mentioned more frequently in the articles.
DISCUSSION
Discussion of MedRec practice in each GCC country
Kingdom of Saudi Arabia
More than half of the included studies were carried out in Saudi Arabia (n = 15, 55.5%). This reflects the persistent efforts from many researchers in the Kingdom to minimize medication discrepancies by practicing MedRec. Based on the information gathered from the Ministry of Health (MOH) website, MOH has improved the quality of care in Saudi Arabia by implementing the National e-health system in 2011. In 2015, The National eHealth Strategy and Change Management Office published a document on the MedRec process [12]. Although the flowchart of the process is mentioned, it is not stated who has to perform it. The conducted review revealed that the first study done in GCC countries on MedRec was in Saudi Arabia in 2009; however, it was published in 2011 [10]. The first research aimed at minimizing MEs by taking an accurate patient’s medication history. Subsequent studies evaluated the medication safety practice in Saudi Arabian hospitals and explored the challenges from healthcare professionals’ perspectives [13,14]. These studies had an impact on the research field in Saudi Arabia and other Gulf countries as they encouraged other researchers to conduct studies toward improving medication safety practices. It was clear that at that time there was a lack of medication safety practices in many hospitals due to the absence of pharmacists’ involvement in this process [13]. Their results were supported by another study done on all psychiatric hospitals in Saudi Arabia in 2013 which reported only a quarter of hospitals initiated MedRec-apart from almost half of the pharmacy directors believed that they do not have enough resources to manage discrepancies [14]. Aljadhey et al. [15] decided to do discussion sessions with the healthcare professionals to understand in depth the main barriers that prevent them from practicing MedRec. This study was the only qualitative study found in this review. As shown in Table 2, underreporting of MEs, workloads, and improper communication between healthcare providers and their patients were the main challenges to medication safety practice as stated by healthcare professionals, whereas miscommunication between health organizations, lack of use of technologies, and inadequate medication safety programs in hospitals were the main factors that lead to medication safety problems [14]. An interesting quality improvement project focusing on educational and monitoring programs to improve compliance with the MedRec process during admission at King Faisal Specialist Hospital and Research Centre was carried out in 2015 by Almidani et al. [6] Although this research was implemented in one department and during admission, which could be a limitation, yet, their initiative was the first of its kind in GCC. This review found that there was only one case report study conducted in Saudi Arabia by Mazhar et al. [16], who reported two serious adverse drug effects caused by MedRec failure during hospital admission. In the last 6 years in SA, there was a strong recognition of the role of pharmacists in the MedRec process and in identifying unintentional medication discrepancies [17–23]. According to the results of Al-Ghanmi and Al-Borie [24], pharmacists should have enough knowledge and training about the MedRec process to conduct it efficiently. Bawazeer et al. [25] shared their positive experience of involving pharmacy students in such a process and concluded that this involvement may reduce the pharmacists’ workload.
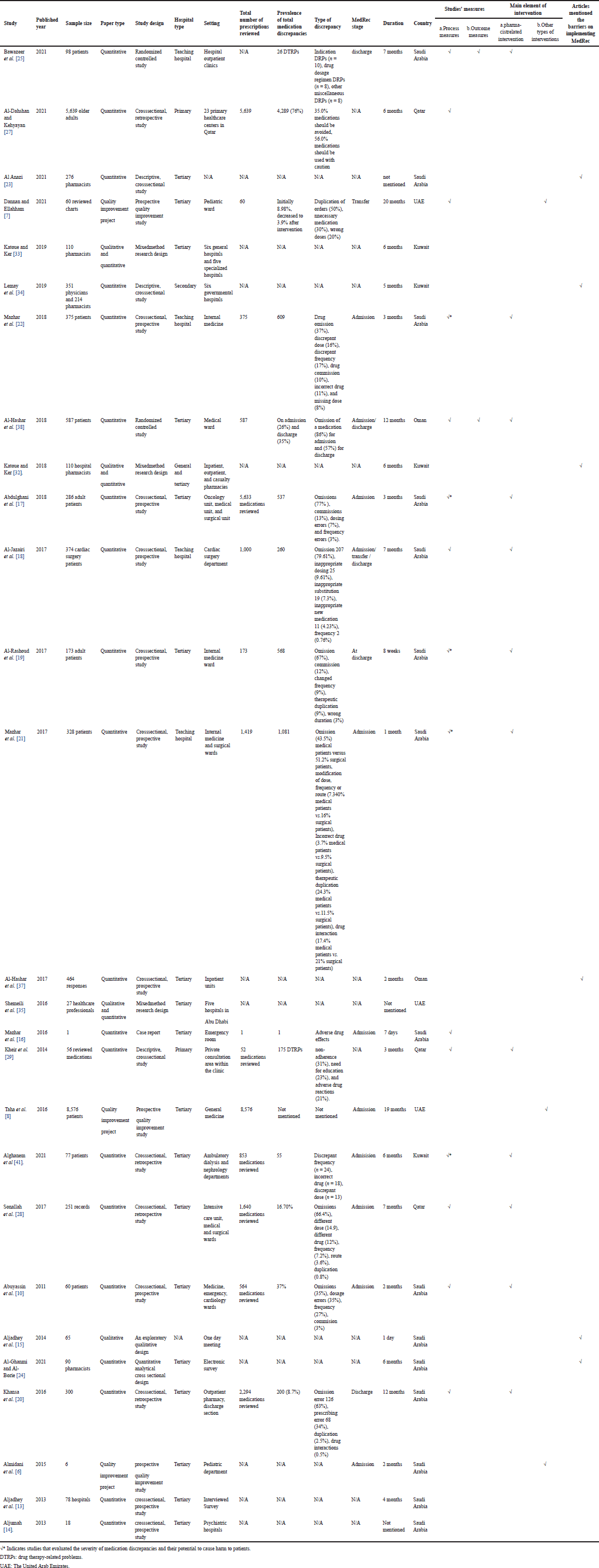 | Table 2. An overview of the included studies. [Click here to view] |
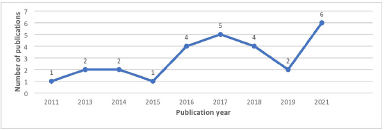 | Figure 2. Yearly-wise distribution of the included studies related to MedRec. [Click here to view] |
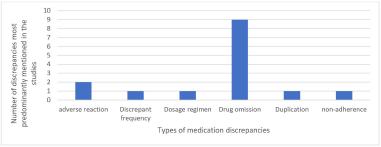 | Figure 3. Types of medication discrepancies and their rates in the included studies. [Click here to view] |
 | Figure 4. Word cloud generator of MedRec, medication discrepancies, patient safety, and GCC countries keywords. [Click here to view] |
Qatar
The hospital pharmacy sector in Qatar has developed new clinical pharmacy services including MedRec that have been implemented in many public hospitals. The Ministry of Public Health in Qatar published “The National Health Strategy 2018–2022 project” with the goals of improvement in patients’ care and plans for the “integrated model of high-quality care and delivery” [26]. This review revealed three studies conducted in Qatar related to MedRec designed as cross-sectional studies and they aimed at detecting medication discrepancies and their types [27–29].
Kuwait
The MOH in Kuwait established “The National Accreditation Program for Hospitals” in 2008, which mainly focused on improving the quality of care and patient safety by “creating, implementing, monitoring, and evaluating programs and standards of quality and safety across all departments of MOH” [30]. Our review revealed that publications about MedRec in Kuwait had started in 2018 followed by another two studies in 2019 and a very recent one in 2021. As in other developing countries, pharmaceutical care implementation in Kuwait had several barriers [31], which were divided into three categories: organizational, technical, and professional barriers. In 2018, another study was done to investigate the practice of MedRec in Kuwait hospitals and to understand in depth the main challenges in implementing it [32]. The authors concluded that MedRec was poorly practiced in hospitals and pharmacists had a limited role in it. They also adopted a mixed-method research in 2019 based on simulation-based workshops to train the pharmacists in the MedRec process in addition to surveying them to know their perceptions about such processes [33]. The participants’ preparedness to implement MedRec increased after attending the workshops which indicated that such types of workshops can enrich the pharmacists’ knowledge and enhance the skills required to implement MedRec. Another study conducted by Lemay et al. [34] indicated that the awareness level about MedRec was low among physicians compared to hospital pharmacists although physicians were the main providers involved in MedRec. This could be attributed to the MOH policy in Kuwait which put the physicians at the core of the process.
United Arab Emirates
The UAE consists of seven Emirates and its healthcare system has three health regulatory authorities. Northern Emirates (Sharjah, Ajman, Umm Al Quwain, Ras Al Khaimah, and Fujairah) follow the Ministry of Health and Prevention authority which is the federal health authority in the UAE while Abu Dhabi and Dubai have local health authorities.
Our review revealed three studies about MedRec conducted in AbuDabi hospitals. Two of them were quality improvement projects [7,8] while the third one used a mixed research design [35]. One study was done in community pharmacies in the UAE and it was excluded as the setting did not meet our inclusion criteria. The Abu Dhabi Health Authority has considered MedRec as a critical patient safety method and it is one of the KPIs for its hospitals [7,8]. In the two quality improvement projects, electronic tools were used to improve MedRec’s compliance during hospital admission and transfer, respectively. The results of the two studies were impressive as MedRec’s compliance improved and was sustained for a certain period of time. The third study was done by Shemeili et al. [35], who used a mixed research design to explore the experiences of healthcare providers on medicine management in the elderly. A discussion with a focus group revealed that healthcare providers including physicians, pharmacists, and nurses had adequate knowledge about history taking and MedRec. However, allocating these tasks was not clear among them.
Oman
The MOH in the Sultanate of Oman had issued a policy in 2019 for MedRec practice in hospitals [36].
It explains the process in detail and it is mentioned that the pharmacist is responsible for reviewing the medication history taken at the time of admission by the physicians or nurses. This review revealed two studies conducted in Oman about MedRec [37,38]. Both are quantitative studies conducted in a tertiary hospital. The first study was done in 2017 in Sultan Qaboos University Hospital before the implementation of a structured MedRec process in the hospital. The disagreement of the three healthcare providers (physicians, nurses, and pharmacists) in their roles and responsibilities to implement the MedRec process was expressed in this study although they all agreed on its importance to minimize medication discrepancies. Obstacles to implementing MedRec addressed from their perspectives include lack of time, unreliable sources of medication history, and lack of communication between healthcare providers. In 2018, the same research group conducted another study in the same hospital to evaluate the impact of MedRec on patients’ clinical outcomes after discharge. Their findings supported the importance of implementing MedRec as a medication safety practice to reduce drug related problems.
Bahrain
The National Health Regulatory Authority in Bahrain had issued a strategic plan for 2021–2025 which emphasized safe and high-quality health services. [39] Although many studies stated the high prevalence of MEs in Bahrain, none was done to examine the medication safety intervention to minimize such errors. A study by Al Khaja et al. [40] revealed that in Bahrain the percentage of incorrect prescriptions is around 90%. Our review did not find any MedRec study conducted in the Kingdom of Bahrain. However, there is one study talking about medication safety in medical education and emphasizing the need to improve the medical curriculum toward further medication safety courses and assessments. This study was published as a letter to an editorial in 2015, but it was excluded as it did not meet our inclusion criteria [11].
Studies’ measures
Process measures
Fifteen articles evaluated process measures by looking at the drug therapy-related problems (DTRPs), unintentional medication discrepancies, and rate of preventable ADEs, and all of them showed a reduction in these outcomes (Table 2), while remaining studies were exempted from this classification (n = 12). Most studies stated that drug omission was the most predominant type of discrepancy (n = 9), followed by adverse drug reaction (n = 2), frequency, dosage regimen, duplication, and nonadherence (n = 1 each) Figure 3.
Five studies evaluated the severity of medication discrepancies and their potential to cause harm to patients (Table 2). Al-Rashoud et al. [19] classified 76% of the unintentional medication discrepancies as major discrepancies while Mazhar et al. [22] and Alghanem et al. [41] reported that 60% of their discrepancies had the potential to cause moderate to severe harm compared to 52% in Abdulghani et al. [17]. In addition, Mazhar et al. [21] rated 17.7% as potentially harmful discrepancies.
Outcome measures
Two studies evaluated the outcome measures [25,38] by studying the readmission rate and impact of MedRec on clinical parameters; however, their results were not statistically significant which could be due to the short period of time and technicality in their study designs. None of the studies had accessed the cost-effectiveness of implementing the MedRec process. However, only one study estimated the medication-related cost reductions [41], which concluded that MedRec resulted in an overall reduction in medication costs of US $85.33 per patient for one month and this was attributed to the discontinuation of unnecessary medications.
Main element of intervention
Pharmacist-related intervention
The 12 studies reported on pharmacist-led intervention included mostly licensed pharmacists, clinical pharmacists, and pharmacy residents, although students with advanced pharmacy practice experience were also involved (Table 2). These studies elaborated on the multiple roles of pharmacy staff in the MedRec process and showed how their contributions significantly reduced medication discrepancies, DTRPs, and preventable adverse drug events.
It is worth mentioning that although including students in doing such comprehensive intervention could be questioned and debated, this could be quite useful in limited resources facilities to overcome this barrier after educating and training them to be highly competent. In addition, pharmacy technicians could be a useful choice in low-resource hospitals that do not have enough pharmacists to lead the MedRec process as recommended by Abdulghani et al. [17].
Other types of interventions including implementing electronic MedRec tools and educating staff about MedRec
Three articles had shared their successful quality improvement stories using electronic MedRec tools along with educating and training their staff about the MedRec process [6–8]. Taha et al. [8] used an electronic tool to perform MedRec on admission and they selected the general medicine department for the project being the busiest ward in their hospital. Their results showed a significant impact of the admission MedRec electronic tool on improving physician compliance to perform the process (from 40% to more than 85%) which was sustained for the last 4 months of the study. What was missing in their design was the absence of a system that could measure the sustainability of the improvement.
After 5 years, researchers did an almost similar project in the same hospital aiming to improve the transfer of MedRec compliance from critical care to the pediatric ward [7]. Initially, their results showed an increase in compliance from 56% to 72%, but it was not sustained. In the last phase of their project, they adopted the Irish Health Service Executive Model which yielded a sustainable improvement of 85% that lasted for 1 year of the study. The research team highlighted the importance of implementing such a model that focuses on stakeholders’ engagement and cultural change. The third study was conducted by Almidani et al. [6] to enhance the admission MedRec compliance in a pediatric ward. Their results showed an improvement in compliance from 0% to 15% to 96%. Common themes of these three successful projects included (1) providing education sessions on MedRec to staff; (2) emphasizing the importance of interprofessional collaboration; (3) continuous follow-up and reminders for the healthcare providers; and finally (4) the support from senior management. In quality improvement projects, it is very important to collectively gather all the possible ways that can lead to success.
Whose job is MedRec?
In an attempt to find a clear answer about whose responsibility is MedRec, Al-Hashar et al. [37] conducted a survey to ask the three healthcare providers (physicians, pharmacists, and nurses) this question. Their responses showed a disagreement among the three professions on who is best suited to perform this process as pharmacists and physicians considered themselves the main providers of this service, while nurses perceived physicians and pharmacists did not have a major role in this process. Interestingly, one study reported a positive acceptance of physicians not only toward pharmacists’ implementation of the process but also accepted all pharmacists’ intervention recommendations without rejecting any of them [18]. This result reflected the recognition of healthcare providers about the important role of pharmacists in this process. Another study investigated who is doing the process more accurately, pharmacists or physicians? They reported that physicians inaccurately recorded patients’ medication history during their admission and they strongly supported pharmacists being the experts in this field to be engaged in the MedRec process with physicians and nurses [17]. Their results match with Lemay et al. [34] as they assessed and compared the knowledge and perception of pharmacists and physicians toward MedRec. Their results indicated that pharmacists had more knowledge about MedRec, got more training in university about MedRec, and perceived MedRec as a valuable intervention for patient safety than physicians.
Barriers and challenges
Barriers toward implementing the MedRec process were an important area of interest in Saudi Arabia, Kuwait, and Oman with six studies devoted to this topic (Table 2). The most common barrier was the lack of policy which was mentioned in three articles [15,24,32] followed by the lack of pharmacists’ time and resources as they were mentioned in two articles [32,37]. Other obstacles that were mentioned include lack of communication among healthcare providers as well as between the patients’ and the providers, lack of management support, lack of standardized tools for MedRec, inadequate staff and workload on healthcare providers, difficulty in accessing patient information, and finally lack of agreement on roles and responsibilities. These challenges are similar to what had been addressed in the literature in other countries [42–44].
Key successful factors to improve the practice
Identifying the barriers that can render proper implementation of the MedRec process will help in suggesting key points that will enhance patients’ safety practices in hospitals. Aljadhey et al. [15] highlighted the potential areas and they mentioned studies and publications on medication safety are the key elements to improve the practice. Other factors include providing continuous education and training to healthcare providers, effective communication between healthcare providers, and organizational management support in addition to defining the roles and responsibilities of each provider. In addition, using information technology and standardized tools in implementing such intervention will facilitate the process. The tendency toward change and the fear of consequences were reported as one of the barriers. The culture of blame will prevent healthcare providers from reporting MEs. The WHO stated underreporting of MEs is a global problem and healthcare providers should be educated about the point of reporting is not to blame, but rather to learn from each other in order to enhance patient safety.
Limitations and strengths of this review
This review included all published research related to MedRec practice in GCC hospitals till December 10th, 2021, which means any recently published articles after this date were not included. Also, this review has limited the search to articles published in the English language which could be considered a limitation as publications in other languages were excluded. In addition, the heterogenicity in designing the studies led to difficulty in drawing a conclusion on the most effective MedRec approaches. The novelty of this review is that it is the first to be done to explore the practice of MedRec in GCC countries in hospital settings. Moreover, this review highlights the barriers that prevent the implementation of effective MedRec and suggests the factors that can successfully contribute to a better implementation.
CONCLUSION
The published studies showed a wide variation in the current practice of MedRec in GCC hospitals because of different policies from the MOH and the hospitals. This review outcome concluded that MedRec is likely a recognized and actively promoted process in these countries where it is comparatively more practiced in Saudi Arabia. The MedRec practice in GCC had many problems and limitations, and efforts are needed to overcome these barriers. Enhancing the practice could be achieved by sharing the experience and publishing the results and outcomes of any patient safety initiative in hospitals. Documenting these barriers is the key element to addressing the problems and resolving them. The research findings showed the need for further research to educate healthcare professionals on MedRec and to see its impact on clinical outcomes.
ACKNOWLEDGMENT
Authors would like to thank Ajman University for paying the processing fee of this article.
AUTHOR CONTRIBUTIONS
All authors made substantial contributions to the conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work. All the authors are eligible to be an author as per the International Committee of Medical Journal Editors (ICMJE) requirements/guidelines.
CONFLICTS OF INTEREST
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ETHICAL APPROVALS
This study does not involve experiments on animals or human subjects.
DATA AVAILABILITY
All data generated and analyzed are included in this research article.
PUBLISHER’S NOTE
This journal remains neutral with regard to jurisdictional claims in published institutional affiliation.
REFERENCES
1. Hughes R, editor. Patient safety and quality: an evidence-based handbook for nurses. Bethesda, MD: National Library of Medicine; 2008.
2. IHI.org [Internet]. Institute for Healthcare Improvement Online Resources, Reconcile Medications at all Transition Points. [cited 2022 Jul 9]. Available from: https://www.ihi.org/resources/Pages/Changes/ReconcileMedicationsatAllTransitionPoints.aspx
3. Joint Commission. Preventing pediatric medication errors. Sentinel Event Alert. 2008 Apr 11;39(39):1–4.
4. World Health Organization. Medication without harm (No. WHO/HIS/SDS/2017.6). Geneva, Switzerland: World Health Organization; 2017 [cited 2023 Feb 23]. Available from: https://apps.who.int/iris/bitstream/handle/10665/255263/WHO-HIS-SDS-2017.6-eng.pdf
5. Leotsakos A, Zheng H, Croteau R, Loeb JM, Sherman H, Hoffman C, et al. Standardization in patient safety: the WHO high 5s project. Int J Qual Health Care. 2014 Apr 1;26(2):109–16.
6. Almidani E, Khadawardi E, Alshareef T, Hussain IB, Almofada S, Ham AJ, et al. Improving medication reconciliation compliance at admission: a single department’s experience. Int J Pediatr Adolesc Med. 2015 Sep 1;2(3–4):141–6.
7. Dannan HE, Ellahham S. Improving transfer medication reconciliation in an Emirati tertiary hospital utilizing the Irish health service executive model. Am J Med Qual. 2021 Jan 1:1062860620920712.
8. Taha H, Luqman N, Ellahham S. Improving admission medication reconciliation compliance using the electronic tool in admitted medical patients. BMJ Open Qual. 2016 Jan 1;5(1):u209593–w4322.
9. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group*. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009 Aug 18;151(4):264–9.
10. AbuYassin BH, Aljadhey H, Al-Sultan M, Al-Rashed S, Adam M, Bates DW. Accuracy of the medication history at admission to hospital in Saudi Arabia. Saudi Pharm J. 2011 Oct 1;19(4):263–7.
11. Sequeira RP. Patient safety in medical education: medication safety perspectives. Indian J Pharmacol. 2015 Mar;47(2):135.
12. National eHealth Strategy and Change Management Office (SCMO). 2015 [Online]. [cited 2023 Feb 23]. Kingdom of Saudi Arabia: Ministry of Health, National eHealth Strategy and Change Management Office. Available from: https://www.moh.gov.sa/en/Ministry/ehealthstd/Documents/eHealth%20Standards%20Files/General/IS0302%20Saudi%20eHealth%20Project%20Glossary%20v1.0.pdf
13. Aljadhey H, Alhusan A, Alburikan K, Adam M, Murray MD, Bates DW. Medication safety practices in hospitals: a national survey in Saudi Arabia. Saudi Pharm J. 2013 Apr 1;21(2):159–64.
14. Aljumah K. CPC-083 medication reconciliation experience in psychiatric hospitals, Saudi Arabia. Eur J Hosp Pharm Sci Pract. 2013 Mar 1;20(Suppl 1):A195.
15. Aljadhey H, Mahmoud MA, Hassali MA, Alrasheedy A, Alahmad A, Saleem F, et al. Challenges to and the future of medication safety in Saudi Arabia: a qualitative study. Saudi Pharm J. 2014 Sep 1;22(4):326–32.
16. Mazhar F, Akram S, Haider N, Ahmed R. Overlapping of serotonin syndrome with neuroleptic malignant syndrome due to linezolid-fluoxetine and olanzapine-metoclopramide interactions: a case report of two serious adverse drug effects caused by medication reconciliation failure on hospital admission. Case Rep Med. 2016 Jun 28;2016:7128909.
17. Abdulghani KH, Aseeri MA, Mahmoud A, Abulezz R. The impact of pharmacist-led medication reconciliation during admission at tertiary care hospital. Int J Clin Pharm. 2018 Feb;40(1):196–201.
18. Al-Jazairi AS, Al-Suhaibani LK, Al-Mehizia RA, Al-Khani S, Lewis G, De Vol EB, et al. Impact of a medication reconciliation program on cardiac surgery patients. Asian Cardiovas Thorac Ann. 2017 Nov;25(9):579–85.
19. Al-Rashoud I, Al-Ammari M, Al-Jadhey H, Alkatheri A, Poff G, Aldebasi T, et al. Medication discrepancies identified during medication reconciliation among medical patients at a tertiary care hospital. Saudi Pharm J SPJ. 2017 Nov;25(7):1082.
20. Khansa SA, Mukhtar A, Abduljawad M, Aseeri M. Impact of medication reconciliation upon discharge on reducing medication errors. J Pharmacovigil. 2016;4(6):1–5.
21. Mazhar F, Akram S, Al-Osaimi YA, Haider N. Medication reconciliation errors in a tertiary care hospital in Saudi Arabia: admission discrepancies and risk factors. Pharm Pract (Granada). 2017 Jan–Mar;15(1):864.
22. Mazhar F, Haider N, Ahmed Al-Osaimi Y, Ahmed R, Akram S, Carnovale C. Prevention of medication errors at hospital admission: a single-centre experience in elderly admitted to internal medicine. Int J Clin Pharm. 2018 Dec;40(6):1601–13.
23. Al Anazi A. Medication reconciliation process: assessing value, adoption, and the potential of information technology from pharmacists’ perspective. Health Inform J. 2021 Jan;27(1):1460458220987276.
24. Al-Ghanmi M, Al-Borie H. Factors in?uence compliance to medication reconciliation process in King Abdullah Medical City. Am J Pharm Sci. 2021 Feb 18;9:30–5.
25. Bawazeer G, Sales I, Alsunaidi A, Aljahili S, Aljawadi MH, Almalag HM, et al. Student-led discharge counseling program for high-risk medications in a teaching hospital in Saudi Arabia: a pilot study. Saudi Pharm J. 2021 Oct 1;29(10):1129–36.
26. Ministry of Public Health—National Health Strategy 2018–2022 [Online]. [cited 2023 Feb 23]. Doha, Qatar: Gulf Publishing & Printing Company. Available from: https://www.moph.gov.qa/english/strategies/National-Health-Strategy-2018-2022/Pages/default.aspx
27. Al-Dahshan A, Kehyayan V. Prevalence and predictors of potentially inappropriate medication prescription among older adults: a cross-sectional study in the state of Qatar. Drugs Real World Outcomes. 2021 Mar;8(1):95–103.
28. Sonallah H, Ibrahim T, Sattar DA, Ahmed D, Izham M. Clinical pharmacist-led medication reconciliation initiative at a newly established hospital in Qatar: a preliminary report. Int Arch Nurs Health Care. 2017;3:083.
29. Kheir N, Awaisu A, Sharfi A, Kida M, Adam A. Drug-related problems identified by pharmacists conducting medication use reviews at a primary health center in Qatar. Int J Clin Pharm. 2014 Aug;36(4):702–6.
30. Ministry of Health Quality and Accreditation Directorate. MedRec policy. Kuwait, Kuwait: MOH; 2009 [Online]. [cited 2023 Feb 23]. Available from: https://qadkuwait.wordpress.com/
31. Katoue MG, Awad AI, Schwinghammer TL, Kombian SB. Pharmaceutical care in Kuwait: hospital pharmacists’ perspectives. Int J Clin Pharm. 2014 Dec;36(6):1170–8.
32. Katoue MG, Ker J. Implementing the medicines reconciliation tool in practice: challenges and opportunities for pharmacists in Kuwait. Health Policy. 2018 Apr 1;122(4):404–11.
33. Katoue MG, Ker J. Simulation for continuing pharmacy education: development and implementation of a simulation-based workshop on medicines reconciliation for pharmacists. J Contin Educ Health Prof. 2019 Jul 1;39(3):185–93.
34. Lemay J, Bayoud T, Husain H, Sharma P. Assessing the knowledge, perception and practices of physicians and pharmacists towards medication reconciliation in Kuwait governmental hospitals: a cross-sectional study. BMJ Open. 2019 Jun 1;9(6):e027395.
35. Shemeili SA, Klein S, Strath A, Fares S, Stewart D. An exploration of health professionals’ experiences of medicines management in elderly, hospitalised patients in Abu Dhabi. Int J Clin Pharm. 2016 Feb;38(1):107–18.
36. Sultanate of Oman pharmaceutical care 2019 [Online]. [cited 2023 Feb 23]. Oman: Directorate General of Medical Supplies, Ministry of Health. Available from: https://www.moh.gov.om/documents/71477/121151/Pharmaceutical+Care+P%26P+2019.pdf/a916dd1f-a4e0-1a69-251a-93a6712ec119
37. Al-Hashar A, Al-Zakwani I, Eriksson T, Al Za’abi M. Whose responsibility is medication reconciliation: physicians, pharmacists or nurses? A survey in an academic tertiary care hospital. Saudi Pharm J. 2017 Jan 1;25(1):52–8.
38. Al-Hashar A, Al-Zakwani I, Eriksson T, Sarakbi A, Al-Zadjali B, Al Mubaihsi S, et al. Impact of medication reconciliation and review and counselling, on adverse drug events and healthcare resource use. Int J Clin Pharm. 2018 Oct;40(5):1154–64.
39. National health Bahrain strategic plan 2021—Google Search [Internet]. [cited 2023 Feb 23]. Bahrain: National Health Regularity Authority. Available from: https://www.nhra.bh/About/Strategy/MediaHandler/GenericHandler/documents/About/Strategy/2021-2025%20NHRA%20Strategy_English.pdf
40. Al Khaja KA, Al-Ansari TM, Sequeira RP. An evaluation of prescribing errors in primary care in Bahrain. Int J Clin Pharmacol Ther. 2005 Jun 1;43(6).
41. Alghanem SS, Bayoud T, Taher S, Al-Hazami M, Al-Kandari N, Al-Sharekh M. Introduction of an ambulatory care medication reconciliation service in dialysis patients: positive impact on medication prescribing and economic benefit. J Patient Saf. 2022 Mar 1;18(2):e489–95.
42. Kennelty KA, Chewning B, Wise M, Kind A, Roberts T, Kreling D. Barriers and facilitators of medication reconciliation processes for recently discharged patients from community pharmacists’ perspectives. Res Social Adm Pharm. 2015 Jul 1;11(4):517–30.
43. Patel E, Pevnick JM, Kennelty KA. Pharmacists and medication reconciliation: a review of recent literature. Integr Pharm Res Pract. 2019;8:39.
44. Gionfriddo MR, Duboski V, Middernacht A, Kern MS, Graham J, Wright EA. A mixed methods evaluation of medication reconciliation in the primary care setting. PloS One. 2021 Dec 2;16(12):e0260882.